Abstract
Electrochemical techniques have been applied to detect the redox behaviour of the antiwear additive zinc dithiophosphate (ZDDP) in di-ethylhexyl sebacate (DEHS) base fluid solution and to study the interaction of ZDDP with ferrous surfaces. Using cyclic voltammetry it has been shown that the ZDDP is oxidised in a chemically-irreversible process at applied potentials above 1.2 V vs. Pt with an iron electrode. The influence of applied electrode potential on friction and wear characteristics of ZDDP in DEHS has also been investigated. Reciprocating rubbing tests have shown that ZDDP is effective in reducing wear only under oxidising conditions. This electrochemical approach can be used to explore the operating conditions and mechanism of action of antiwear additives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc dialkyldithiophosphates [(RO)2PS2]2Zn (ZDDPs) have been widely used as antioxidant and antiwear additives in engine lubricants for over 60 years [1,2]. There have been many investigations reported in the literature aimed at elucidating the nature of their antiwear and antioxidant activity [3].
Electrochemical techniques have been used to investigate the electrochemical behaviour of ZDDP [4–6]. These studies have examined the behaviour of ZDDP in the relatively polar, non-aqueous solvents, acetonitrile [4] and dimethylformamide (DMF) [5,6]. Work by Blankespoor [4] showed that ZDDPs were oxidised at ca. +1.5 V vs. standard calomel electrode (SCE) in a diffusion-controlled, chemically-irreversible, two-electron transfer process. Chemical irreversibility was ascribed to dimerisation of the ligand species with the formation of the disulfide [(RO)2PS2]2, as shown by the equation:
A study by Stezeryanskii et al. [6] elucidated the nature of iso-octyl-ZDDP and n-butyl-ZDDP electro-oxidation in greater depth using cyclic voltammetry, chronopotentiometry and chronoamperometry techniques. Oxidation showed a single peak at ca. +0.81 V vs. Ag/AgCl and +0.8 V vs. SCE in DMF. These workers assumed a one-electron transfer process and reported kinetic hindrance with dissociation of the complex in the preceding chemical stage. The oxidation signal was chemically irreversible, consistent with the findings of Blankespoor [4]. The proposed reaction pathway was:
Ozimina et al. [7] carried out cyclic voltammetry in acetonitrile solutions containing ZDDP, with platinum mesh as an auxiliary electrode, [Ag+ (0.1 M) in acetonitrile/Ag] as a reference electrode, and commercial copper, bronze or 100Cr6 as a working electrode. The authors found the first cathodic peak to lie at about −0.6 V and the second one at about −1.0 V up to −1.2 V, independent of the electrode material. After multi-cycle treatment, the curves stabilised to reproducible form. This was ascribed to the underpotential deposition of the metal (Zn) on the electrode surface in the first cycle, followed by bulk metal electrodeposition in subsequent cycles.
All the work reported above was limited to relatively polar fluids, unrealistic of most lubricant base fluids, and the findings obtained from such systems may not be applicable to real lubricants. The key practical problem in applying electrochemical techniques to the study of lubricant additive behaviour is that lubricating oils usually have very high electrical resistance, which makes it very difficult to control the potential of the lubricant with respect to the solid surfaces.
Recently, we reported the use of electrochemical techniques to study the redox behaviour of ZDDP in a low viscosity ester solvent, diethyl adipate (DEA) and to investigate the influence of applied electrode potential on its friction and wear properties [8]. A supporting electrolyte, LiClO4, was used to enhance the conductivity of the ester sufficiently to enable electrochemical measurements to be made. Cyclic voltammetry showed that the ZDDP was oxidised in a chemically-irreversible, two-electron transfer process at applied potentials above +0.5 V vs. Pt. High frequency reciprocating (HFRR) tests indicated that ZDDP was effective in reducing both friction and wear only under oxidising conditions, but not at the rest potential or in reducing conditions.
In this previous work, it was not possible to study high viscosity esters typical of lubricating base oil since existing supporting electrolytes were not soluble in such fluids, which have relatively large alkyl groups and thus low polarity. In the current paper, an oil-soluble supporting electrolyte, tetra-decylammonium-tetrakis-(4-fluorophenyl)-borate, TDATPhFB is synthesised and employed to overcome this limitation [9]. The behaviour of a range of ZDDPs in solution in the ester lubricant, diethylhexyl sebacate containing TDATPhFB is studied by electrochemical techniques. The methodology used by Zhu is employed [10]. First cyclic voltammetry is used to characterise the electrochemical response of ZDDP solutions. Then the influence of applied potential on the friction and wear properties of the ZDDP solutions is determined and related to their electrochemical behaviour.
Experimental
Cyclic voltammetry
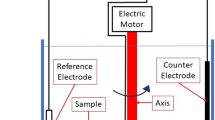
Cyclic voltammetry experiments were carried out with a PGSTAT 100 potentiostat (Ecochemie, Holland) using the microelectrode apparatus as shown in figure 1. A 10 μm diameter Pt disc microelectrode and a 0.8 mm diameter iron rod macroelectrode were studied as working electrodes. The working electrode is mounted on a micrometer so that it can be accurately positioned close to the flat face of a 1 mm diameter Pt pseudo-reference electrode and thus minimise the electrical resistance of the cell. A Pt wire is employed as a counter-electrode. These electrodes are immersed in the test lubricant, contained in a temperature-controlled bath. As shown in the figure, the size of the test cell is small so as to minimise the amount of supporting electrolyte needed, since the latter is synthesised from expensive starting materials [9]. Dissolved oxygen is removed from the electrolyte solution by bubbling high purity nitrogen gas (99.99+%). The whole test set-up is contained in a Faraday cage to reduce electrical noise.
In the current study, the electrode surfaces were polished with 0.05 μm alumina slurry on a microcloth, and then treated in 0.6 M HNO3 solution for at least 12 h prior to each measurement. The distance between the working and reference electrodes was set to 200 μm and all tests were carried out at 60 °C.
Friction and wear measurement
Friction and wear properties were determined using a high frequency reciprocating rig (HFRR) with added electrochemical instrumentation [8,10]. This is shown in figure 2.
A 6 mm diameter steel ball is loaded and reciprocated against the flat face of a 10 mm diameter steel disc at constant stroke length and frequency. The ball and disc contact is fully immersed in test lubricant at a controlled temperature. Friction between the ball and disc is monitored continuously by a load cell attached to the lower specimen holder while wear is measured as the mean of the wear scar dimensions on the disc after a test.
In this study, the ball and disc were electrically-connected, isolated from the rest of the test rig, and together made the working electrode. Two Pt wires were immersed in the lubricant to act as reference electrode and counter electrode respectively. Tests were carried out according to the conditions listed in table 1 and a fresh AISI 52100 steel ball and disc (both cleaned ultrasonically in toluene and acetone) and a fresh oil sample were used for each test. Tests lasted 30 min and wear on the disc was measured at the end of each test.
Surface mapping techniques
Wear scars were examined after HFRR tests using a Wyko RST Plus interferometer microscopy. This surface mapping technique combines phase-measuring interferometry and vertical scanning interference microscopy to generate 2-D and 3-D topographical maps of surfaces.
Test fluids and additives
The synthetic ester, diethylhexyl sebacate (DEHS) (Fluka, AG) was employed as a base fluid and was used as received without further purification. It has viscosity of 19.8 mm2 s−1 at 25 °C and 7.0 mm2 s−1 at the test temperature of 60 °C. The supporting electrolyte was 0.03 M tetradecylammonium tetrakis-(4-fluorophenyl)-borate, ((C10H21)4N(C6H5F)4B), (TDATPhFB). This was employed to increase the electrical conductivity of the base fluid [8]. The ZDDPs examined in this study are listed in table 2. The ZDDPs were stated by the supplier to be 99.99% pure. Two of the ZDDPs, the di-isooctyl- and the di-2-ethylhexyl- were primary, while the di-isopropyl ZDDP was secondary. In the text that follows, “di-” will be omitted from the ZDDP descriptors for brevity, so, for example, “di-isooctyl ZDDP” will be shortened to “isooctyl ZDDP”.
Results and discussion
Non-rubbing electrochemical results
Figure 3 shows a cyclic voltammogram obtained for 1% wt. isooctyl-ZDDP solution in DEHS with 0.03 M TDATPhFB electrolyte on a Pt microelectrode. One complication with using TDATPhFB supporting electrolyte is that this electrolyte is itself oxidised at very positive potential in DEHS to give a single irreversible oxidation peak. The peak current is decreased when 1% wt. iso-oct-ZDDP is present in the solution and is essentially zero during the second and subsequent potential cycles, indicating that the adsorption processes of ZDDP and/or its oxidation product compete with the reaction of the electrolyte’s oxidation on the electrode surface and then block the electrode surface. However, it was not clear whether ZDDP was oxidised in DEHS/TDATPhFB solutions, as the oxidation current of ZDDP may have been masked by the large background current due to the oxidation of the electrolytes.
These voltammograms could be obtained only at very slow potential scan rates, such as 2 mV s−1. At scan rates >10 mV s−1 no discernible electrode process was observed.
The electrochemical behaviour of 2-EH-ZDDP and isopropyl-ZDDP were also investigated. It was found that the cycle voltammograms of 2-EH-ZDDP and isopropyl-ZDDP showed similar electrochemical behaviour to isooctyl-ZDDP, i.e. the current response resulting from the oxidation of supporting electrolyte at 0.84 V decreased slightly during the first cycle of scanning and more significantly on subsequent scans. The decrease in oxidation current depended on the alkyl chain of the three ZDDPs, isooctyl-ZDDP having the greatest inhibiting effect and 2-EH-ZDDP the smallest effect. As will be shown later in this paper, this is consistent with the study on friction and wear properties where isooctyl-ZDDP is shown to be the best antiwear additive, followed by isopropyl-ZDDP and 2-EH-ZDDP.
Figure 4 shows a cyclic voltammogram of isooctyl-ZDDP in 0.03 M TDATPhFB/DEHS electrolyte solution with an iron electrode. This is very different from that observed with Pt electrode. In the presence of ZDDP, an oxidation current occurs at potentials >1.2 V, another oxidation current occurs at potentials from −0.44 to 0.7 V. In the absence of ZDDP in the solution, oxidation occurs at potentials >0.5 V, while on the negative-going potential sweep from the positive potential limit, reduction occurs at potentials <−0.85 V.
In all tests, nitrogen gas was bubbled through the electrolyte solution before testing but it is improbable that oxygen/water was removed completely. Also oxygen and water could have re-dissolved in the solution during voltammetric measurement, which was carried out without a continuous nitrogen purge. The principal features of the ZDDP-free voltammogram are believed to be due to a complex oxidation process which in the low water activity conditions of these experiments may form hydrous oxides via the following series of reactions, giving rise to the oxidation current at >0.5 V.
-
(i)
Dissociation of H2O
$$ \hbox{H}_{2}\hbox{O}\to \hbox{H}^{+}+\hbox{OH}^{-} $$(1) -
(ii)
Oxidation of iron
$$ \hbox{Fe}+\hbox{OH}^{-}\to \hbox{FeOH}_{({\rm ads})}+e^{-} $$(2)$$ \hbox{Fe}+2\hbox{OH}^{-}\to \hbox{Fe(OH)}_{2({\rm ads})}+2e^{-} $$(3)$$ \hbox{Fe(OH)}_{2({\rm ads})}+\hbox{OH}^{-}\to e^{-}+\hbox{H}_{2} \hbox{O}+\hbox{FeOOH}\to \hbox{Fe(OH)}_{3({\rm ads})} $$(4) -
(iii)
And/or hydrolysis of DEHS
$$ \hbox{R}_{1}\hbox{COOR}_{2}+ \hbox{H}_{2}\hbox{O}\to \hbox{R}_{1}\hbox{COOH}+\hbox{R}_{2}\hbox{OH} $$(5)Then
$$ \hbox{Fe}+2\hbox{R}_{1}\hbox{COOH}\to \hbox{Fe}_{{\rm II}} (\hbox{R}_{1}\hbox{COO})_{2({\rm sol})} + 2\hbox{H}^{+}+ 2{e}^{-} $$(6)$$ \hbox{Fe}+2\hbox{R}_{1}\hbox{COOH} \to \hbox{Fe}_{{\rm II}} (\hbox{R}_{1}\hbox{COO})_{2({\rm ads})} + 2\hbox{H}^{+}+2{e}^{-} $$(7)$$ \hbox{Fe}_{{\rm II}}(\hbox{R}_{1}\hbox{COO})_{2}+\hbox{R}_{1}\hbox{COOH} \to \hbox{Fe}_{{\rm III}}(\hbox{R}_{1}\hbox{COO})_{3} + \hbox{H}^{+}+ {e}^{-} $$(8)On the negative-going potential sweep from the positive potential limit, the reverse of reactions (4), (3), (2) and/or (8), (7), (6) should give rise to the reduction wave < − 0.85 V.
In the presence of ZDDP, an oxidation current occurs at potentials >1.2 V, possibly due to the oxidation of ZDDP by reaction (9)
On the negative-going potential sweep from the positive potential limit, the reverse of reaction (4), and/or (8), give rise to the reduction wave from 0 V to −1.3 V, which is followed by a second wave, due to the reverse of reaction (3) and (2) and/or (7) and (6) at more negative potentials, possibly together with:
and the deposition of Zn by reaction (12)
On the subsequent positive-going potential sweep, oxidation current densities occur at potentials from − 0.44 to 0.7 V, presumably due to the oxidation of Fe, illustrated by (2), (3), (6) and/or (7) and the reverse reactions (10) and (11).
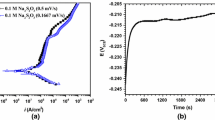
In order to investigate further the electrochemical behaviour of iron with the ZDDP solution, current transients were recorded in response to the potential being stepped from open-circuit constant values over the range −1.5 to +2.0 V. As shown in figure 5(a), current densities are quite low when the applied potential is below 1.0 V, implying that no electrode reaction occurs. When the potential is stepped to >1.0 V, such as to 1.5 V and 2 V, a large current is detected, which falls steadily with time and decays to a potential-dependent steady state value over 10 s. This arises since the magnitude of the current is controlled by the rate of diffusion of ZDDP to the electrode. The concentration gradient shortly after the potential step is large, producing correspondingly large current, but as the diffusion layer thickness increases with time, the current decreases.
In figure 5(b), it can be seen that the currents at >1.0 V were initially proportional to time(-1/2), corresponding to a diffusion-controlled process, but exhibited a non-zero intercept indicating a parallel potential-dependent, kinetically-controlled process, probably due to the oxidation of ZDDP by reaction (13)
Rubbing contact results
Figure 6 shows the wear scar diameters measured on the rubbed disc using an optical microscope after 30 min of reciprocating motion. The repeatability of the wear scar measurements was ±4%. DEHS alone gives a wear scar diameter of 0.150 mm and this is increased to 0.184 mm when electrolyte is present in solution. All of the ZDDPs reduce wear when compared to the base fluid alone and with electrolyte, indicating that the wear-reducing performance of ZDDP is largely unaffected by the presence of TDATPhFB in DEHS. From figure 6, it can be seen that there is negligible difference between the three ZDDPs with respect to their ability to reduce wear scar diameter.
The coefficient of friction was also measured by HFRR. Neither TDATPhFB nor ZDDP in DEHS had a significant effect on friction coefficient compared to the base fluid alone.
Current flow was measured during rubbing tests at controlled open circuit potential (which corresponds to the ‘rest’ potential of iron in electrolyte with no externally-applied potential). Table 3 lists the total charge which flowed during a test and the corresponding wear scar diameter for DEHS with three types of ZDDP. It was found that the charge passing through the circuit was 1.13 × 10−3 C, sixteen times higher in the absence of ZDDP, than that when ZDDP was present.
Since, the potential applied to the rubbing couple was open circuit potential, there should be no driven electrochemical reaction. However, the higher current observed in DEHS/TDATPhFB solution indicates that an electrochemical reaction did occur on the surface of the electrode during rubbing. As discussed above, some or all of the reactions from (1) to (8), involving iron’s oxidation, might contribute to the current produced in the DEHS/TDATPhFB rubbing system.
In the presence of ZDDP the much lower current observed in DEHS electrolyte solutions suggests that a protective coating is formed by ZDDP on the electrode surface. Thus the reactions from (1) to (8) are suppressed.
Figure 7 shows the effect of applied potential on wear. When potential was applied to the rubbing metals, DEHS/electrolyte alone gave a smaller wear scar at the rest potential, which was slightly increased at potentials >0.8 V. For the ZDDP-free solution, this increase in the size of wear scars at potentials >0.8 V can be attributed to corrosive wear. From the voltammogram study of iron in DEHS electrolyte solution discussed above, iron was oxidised at potential >0.5 V. The oxide product may dissolve spontaneously, as no reversible current was observed during the voltammogram study in figure 4, or in the present case by continuous rubbing. At constant positive applied potential, vulnerable bare metal would be oxidised and then dissolved away during subsequent rubbing. Hence the disc shows a bigger wear scar than that at the rest or negative potential.
When ZDDP is added to DEHS/electrolyte solution, a lower wear scar results over the whole potential range but especially at positive potentials. Iso-octyl-ZDDP reduced the wear scar still further at the highly positive potential of 1.5 V. This can be illustrated by surface mapping, as shown in figure 8, where a smaller wear scar is seen at 1.5 V than at 0.8 V. From the electrochemical study of ZDDP in DEHS showed in figure 4, the oxidation of ZDDP occurs at >1.2 V in DEHS and no electrolysis currents are found at potentials below 1.0 V. Thus this decrease in the size of wear scar at 1.5 V can be attributed to the oxidation of ZDDP on the electrode surface under potential control. The oxidation product [(RO)2 PS2 ]2 may subsequently react with H+ to form acid (RO)2 PS2H. The actual friction and wear reduction may be directly due to the adsorption of the disulphide to produce a protective layer. Alternatively it may result from a subsequent reaction of this active species at or close to the surface to generate a protective phosphate or thiophosphate coating, as reported by other studies on ZDDP [3], or by the reaction
Iso-propyl-ZDDP reduces the wear scar at 0.8 V, whereas iso-oct-ZDDP does so at 1.5 V. This suggests that ZDDP with shorter alkyl chains is more easily oxidised. The applied potential does not affect the wear scar when 1 wt. % 2-EH-ZDDP is present in the electrolyte although this additive contains the same number of carbon atoms in its alkyl chain as iso-octyl-ZDDP, indicating that molecular structure has an effect on the function of ZDDP as antiwear additives. The reason of these observed differences is unknown and requires further work.
The current was recorded during rubbing tests in DEHS/electrolyte with and without 1% wt. iso-octyl-ZDDP. As shown in figure 9, when ZDDP is absent, the current is small and approaches zero at the rest potential of 0.02 V. Under positive potential, 1.5 V, the current is quite large and fluctuates. This large current is produced by the oxidation of the iron at the oxidising potential. The repeated processes of oxidation, removal of the oxide layer and then re-oxidation may occur cyclically during the continuous sliding to produce the observed fluctuating current response. When ZDDP was present in solution, at both rest potential and oxidising potential, the current increases to a high plateau initially and then drops down to a very low level and remains steady during the remaining test period. At the open circuit potential, 0.02 V, the initial current is high, then drops suddenly to a very low level after 200 s and remains for the rest test period. Since current is measured from the test lubricants, it is believed that electrochemical reactions do occur. The oxidation of iron caused by rubbing might contribute to the higher current at the beginning of rubbing. A similar plot was obtained when the potential was controlled at 1.5 V during the HFRR test, except that the current obtained was higher than that at the open circuit potential.
This current–time plot probably reflects the formation of the protective film on the metal surface. ZDDP does not appear to form a protective layer on the metal surface at the beginning of the rubbing. Instead, the current drop, implying the formation of the film on the metal surface, occurs only after 200 s sliding. This is in agreement with the friction coefficient measurement during the HFRR test, shown in figure 10, which shows the same overall pattern with time as current response does, i.e., a higher plateau in the beginning, which decreases to a lower level after a certain time.
Since the effectiveness of ZDDP as an antiwear additive depends on the rate of formation of a surface coating, if this formation rate is proportional to the electrochemical reactivity of metal-lubricant interfaces, there should be a correlation of its effectiveness with current since the current is associated with electrochemical reactions occurring on the electrodes. Higher current at 1.5 V means that a ZDDP film forms quickly on the metal surface. This is consistent with the lower wear scar observed at 1.5 V.
When the potential is controlled at 0.02 V, where ZDDP is not supposed to be electrochemically oxidised on the electrode surface, the friction coefficient drops after 500 s rubbing, compared to 200 s under the 1.5 V potential control, as shown in figure 11. This suggests that rubbing action stimulates the decomposition of ZDDP via an oxidising process, which can be greatly accelerated by the applied oxidising potential. Recent work has shown that direct rubbing solid–solid contact is essential to form a ZDDP film at normal lubricant temperatures (at least in the absence of an applied potential) [11].
To explore further the impact of observed change in current over rubbing time, wear scars were measured after different test durations, as plotted in figure 12. The sizes of the wear scars are the same, at about 0.089 mm, during the first minute regardless of whether the potential is set 0.02 V or 1.5 V. However, when potential is controlled at 0.02 V, wear increases steadily with rubbing time over the first 5 min before stabilising at a steady value of 0.11 mm after 5 min. By contrast, when the potential is controlled at 1.5 V, wear apparently ceases after 180 s. This is in agreement with the measurement of friction coefficient discussed above and indicates the very rapid growth of the protective film under positive potential.
Conclusions
The electrochemical behaviour of zinc dithiophosphate antiwear additive has been studied with Pt and iron microelectrodes in an ester lubricant base fluid, diethylhexyl sebacate, using 0.03 M TDATPhFB as supporting electrolyte. With iron as electrode, ZDDP oxidised at potentials >1.2 V and give a sharp increase in current response. No reversible reduction current wave or peak was found and this was ascribed to the interaction between oxidation product [(RO)2S2P]S2 and Fe or H+.
High frequency reciprocating rig experiments were carried out to study the effect of electrode potential on friction coefficient and wear in a reciprocating ball on disc tester. ZDDPs were found to reduce wear in the studied base fluid at all potentials. However, with the application of oxidising potential to the rubbing metals, the size of the wear scars was found to be further reduced, showing that ZDDP action can be influenced by electrochemical potential. The fact that ZDDP reduced friction even at strongly reducing potential suggests that the mechanism of ZDDP film formation is not primarily electrochemically driven.
The application of a controlled potential can produce a number of changes to adsorption and reaction. The adsorption of ZDDPs can occur at any potential. The adsorbed ZDDP can be decomposed by the continuously rubbing and a protective coating hence formed on the metal surface. This decomposition of the ZDDPs appears to be via an oxidation process since it can be accelerated by the application of oxidising potential.
From electrochemical study of ZDDP, it is known that at positive potential, ZDDP is oxidised to disulphide [(RO)2PS2]2. The actual friction and wear reduction may be directly due to adsorption of the disulphide to produce a protective layer. Alternatively it may result from a subsequent reaction of this active species at or close to the surface to generate a thicker, protective phosphate or thiophosphate coating. The nature of the film formed by ZDDP under electrochemically-applied oxidising conditions has not yet been chemically characterised and further work needs to be carried out in this area. However the study suggests that ZDDPs may function best under oxidising conditions, as might be expected to occur in engine oils. This is in consistent with previous work in the diethyladipate/LiClO4 system [8].
This study has demonstrated that it is possible to apply electrochemical techniques, coupled with friction and wear measurements, to explore the friction and wear properties of ZDDP (and potentially other additives) in a real ester lubricating base fluid. These techniques enable the conditions under which the ZDDP is effective to be explored and also provides some information about the nature of the chemical processes occurring.
References
J.C. Bell, in: Engine Tribology, ed. C.M. Taylor, (Elsevier, London, 287, 1993)
A. Cairns, and R. Haycock, Automotive Lubricants Reference Book, (Mechanical Engineering Publication, London, 49, 1996)
Spikes H.A. (2004) The history and mechanisms of ZDDP. Tribol. Lett. 17:465
Blankespoor R.L. (1985) Electrochemical oxidation of zinc bis(O,O-dialkyl phosphorodithioates-S,S’). Mediation by 1,1′-bis(methoxycarbonyl) ferrocene. Inorg. Chem. 24:1126
Jacob S.R., Compton R.G. (1999) Electrochemical studies of the automotive lubricant additive zinc n-dibutyldithiophosphate. J. Electrochem. Soc. 146 (7): 2598
Stezeryanskii EA, Litovchenko KI, Kublanovsky VS (1995) Electro-oxidation of zinc diisooctyldithiophosphate. J. Electroanal. Chem. 390:143
Ozimina D., Scholl H., Plaza S (1997) Electrochemical simulation of friction interactions on the basis of anti-wear behaviour of metallo-dibutyldithiophosphate complexes [(DTP)2Mz+ A ] in Non-aqueous media. Tribol. Lett. 3:147
X. Xu, N. Brandon, and H. Spikes, “Study of zinc dialkyldithiophosphate using electrochemical techniques”, boundary and mixed lubrication: science and applications”, Proceedings of the 28th Leeds-Lyon Symposium on Tribology, Vienna, Sep. 2–7, 2001, edited by D. Dowson, M. Priest, G. Dalmaz, A.A. Lubrecht
X. Xu, H. Spikes, and N. Brandon, “New electrolytes for electrochemical study in hydrocarbon solution, Proc. NATO Course, Keszthely, Hungary, August 2000, Fundamentals of Tribology and Bridging the Gap between Macro- and Micro/Nanoscales, 663, edited by B. Bhushan, Kluwer Academic Publishers, Netherlands
Zhu Y., Ogano S., Kelsall G. H., Spikes H. A. (2000) The study of lubricant additive reactions using non-aqueous electro-chemistry. Tribol. Trans. 43:175
Fujita H., Spikes H. A. (2004) Formation of zinc dithiophosphate antiwear films. Proc. I. Mech. E. J218:265
Acknowledgments
The authors wish to thank Professor Geoff Kelsall of Imperial College for his helpful discussions and Dr Steve Hsu of NIST for the kind provision of ZDDP samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, X., Spikes, H. Study of zinc dialkyldithiophosphate in di-ethylhexyl sebacate using electrochemical techniques. Tribol Lett 25, 141–148 (2007). https://doi.org/10.1007/s11249-006-9132-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11249-006-9132-6