Abstract
Nitrogen and phosphorous compounds are significant pollutants in urban stormwater runoff. In this study, three lab-scale bioretention cells, namely a control reactor CM, and reactors M1 and M2 containing Scrap Iron Filings (SIF) with granulated brick (M1) and pumice pellets (M2), respectively, were used to evaluate the simultaneous removal of nitrate, nitrite, ammonia, total nitrogen, phosphorous, and COD using simulated runoff. Under unsaturated conditions, M1 with the ZVI-brick combination removed 91.37% TP, while M2 with the ZVI-pumice combination removed 89.76% TP. Under saturated conditions, M2 removed 72.02% TN, and M1 removed 66.1% TN. It was found that the presence of saturation zones benefitted TN removal which can be attributed to the creation of anoxic conditions within saturation zones, which favoured denitrification, as well as the prolongation of influent retention and reaction time, while it hindered TP removal. TP removal percentages for CM, M1, and M2 declined from 86.77%, 91.37%, and 89.76% in unsaturated conditions to 63.99%, 83.67%, and 71.74% in saturated conditions due to the propensity of soil-bound P to leach in anoxic environments. The media amendments were further characterized using Scanning Electron Microscopy (SEM) and X Ray Diffraction analysis (XRD), as well as adsorption and leaching tests. Significantly, the highest pollutant leaching was observed in the assessed conditions for CM, underscoring the usefulness of including media like ZVI, brick powder, and pumice pellets. This incorporation not only heightened the effectiveness of pollutant removal but also fortified their retention in potential future stormwater events. In consideration of this, M1 emerged as the preferred design option, as its non-leaching characteristics were verified through flushing with distilled water after post-stormwater influent loading cycles when compared to traditional designs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fast paced urbanization coupled with climate change has caused alarming increments in the number of cities affected by urban floods and their intensity, severity and frequency are expected to increase in the 21st century [17, 34, 36]. Phosphorous is an essential element for all life forms and nitrogen is the main mineral controlling primary production in aquatic ecosystems, but high levels of these nutrients result in eutrophication in freshwater bodies likes rivers, lakes, estuaries, and streams [9].
Bioretention cells are also called raingardens or biofilters and are in-situ stormwater treating LID structures. Conventionally, they are comprised of a top mulch layer, followed by a soil layer, and a gravel layer and may or may not be vegetated. Some studies have incorporated the use of saturation zones (also called as internal water storage zones) towards the base of the bioretention cells for enhancing the nitrogen removal rates as the anoxic zone thus created supports denitrifying microorganisms and helps buffer plant stress [10, 25]. Hsieh et al. [16] found that phosphorous retention in unvegetated bioretention cells using conventional media like 85% sand, could be exhausted in 5 years when subjected to typical stormwater runoff loadings. Lucas et al. [21] reported that even in the case of vegetation being used, bioretention cells using loamy soils as their sole media will be exhausted when a 10-year equivalent of phosphorous load from stormwater runoff is applied. Hence it becomes essential to study the effects of different media amendments for enhanced nutrient removal and their retention for longer time periods. Commonly used bioretention media amendments for phosphorous removal include coir peat, coal combustion waste products (fly ash, coal ash, bottom ash), metallurgical processing waste products (blast furnace slag, red mud, steel chips, steel slag), animal shells, iron coated sand, rocks and minerals (biotite, zeolite, limestone, olivine), local soils (iron rich soil, krasnozem soil, skye sand), expanded soils (expanded clay, shale or slate), aluminium products, iron products (iron oxide, iron hydroxide, iron modified biochar, steel wool, porous iron composite powder, iron filings), sawdust, water treatment residuals (aluminium based, calcium based or iron based), biochar, and activated carbon [23]. Commonly used media amendments in BRCs for nitrogen removal include shredded newspaper, fly ash, vermiculite, perlite, mulch, leaf compost, slate fines, wood chips, biochar, sawdust and wheat straw [18, 29].
This study uses pumice, scrap iron filings and brick granules as media additives. Pumice is a naturally occurring volcanic, highly porous rock which is suitable for adsorption since it can be powdered easily to provide increased surface area [28]. An intermittent nitrification-denitrification study for a recirculating aquaculture system was conducted using pumice stones of 3 mm diameter as biofilter substrate by Pungrasmi et al. [33]. When a carbon source was added in the form of methanol (COD:N ratio of 5:1) post the nitrification period, 169.1 ± 8.8 g-N m−3 packing volume per day of nitrate was removed by the pumice stone.
This study also uses Scrap Iron Filings (SIF), a form of zero valent iron which is a waste by-product of lathing as one of its media amendments. ZVI and ZVI based materials have been extensively studied for nitrate removal. Iron rich soil was found to enhance adsorption of NH4+ and PO43− in bioretention cells by providing microbially assimilative P for the growth of nitrifiers, denitrifiers and phosphate accumulating organisms (PAOs), and removed up to 99% of P, 97% of NH4+ and 63% of TN when combined with plant detritus and eutrophic lake sediment [48]. Rossetti [37] found that biochar supported nano ZVI reduced 86% of applied P, whereas ZVI, biochar and ZVI/biochar removed only 6%, − 23% and 17% respectively. Chiu et al. [7] studied simultaneous NO3− and PO43− removal in bioretention cells but used separate media and experimental setups for the removals, namely, batch experiments using biochar for nitrate removal and column experiments using ZVI for phosphate removal. They found that nearly 100% of P was removed in up flow columns packed with 5% ZVI and 95% sand. Tian et al. [41] also studied nitrate removal in BRCs consisting of wood biochar (18% v/v) in the vadose zone and ZVI (10% v/v) in the saturation zone. The biochar/ZVI BRC removed 30.6% to 96.7% of influent nitrate, whereas the control BRC devoid of these amendments removed -6.1% to 89.6% of influent nitrate over the 18-month testing period. A BRC amended with ZVI and activated carbon (AC) was able to achieve higher removals of TN (83.44%) and TP (97%) than a conventional BRC and one amended only with AC, when the influent contained antibiotics like sulfamethoxazole (SMX) and tetracycline (TC) [47].
Broken bricks have been used for nitrogen and phosphorous removal in wetlands [11, 24, 43] due to their adsorption capacity, support for plant growth and microbial enrichment. Broken clay bricks are easily found waste products resulting from construction activities, are inexpensive and are composed of oxides and hydroxides of Al, Fe, Ca, Mn and Si [39]. Mateus et al. [24] obtained TP, TN and COD removals of 69%, 55% and 66% respectively, in unplanted lab scale constructed wetlands when clay brick fragments were used as filling material. Dires et al. [11] used broken bricks in 8 subsurface flow constructed wetlands to treat hospital wastewater and obtained TKN removals of 73% and 74.7%, NH3 removals of 71.3% and 70.7%, NO3- removals of 79.6% and 70.9% and phosphate removals of 77.1% and 73.6% in dry and rainy seasons respectively, thereby proving that broken bricks supplied the necessary sites for the adsorption of NH4, NO3− and PO43−. Selvaraju et al. [39] conducted lab scale experiments using bricks and sand to treat water containing detergent and other inorganic salts and found that the maximum adsorption capacity of bricks was higher than sand because of its higher specific surface area and wettability. At the time of this study, no literature citing the use of granulated bricks in bioretention cells was found.
The aim of this study was to quantify the efficiency of scrap iron filings (SIF) a form of zero valent iron, brick granules (BP) and pumice pellets (PP) in simultaneously removing Total Phosphorous (TP) and Total Nitrogen (TN) from urban runoff in bioretention cells. Urban runoff typically consists of low organic carbon and high dissolved oxygen [40], which poses challenges in denitrification, hence saturation zones are employed in this study to create an anaerobic zone in the bottom sublayers of the bioretention cells [10, 15, 30, 49]. It is to be noted that additional carbon sources aren’t added to not contribute to carbon pollution or internal phosphorous remobilisation [4, 49], since phosphorous adsorbed to sediments tend to be leached under anoxic conditions [9]. Several studies have probed into the role of plants in removal of nitrogen and phosphorous from bioretention systems, and though this pathway accounts only for a fraction of the total nutrient uptake its contribution cannot be overlooked [2, 30, 31, 35, 38, 45]. However, this study eliminated the use of vegetation to avoid external interferences in gauging the pollutant removal by the media additives.
2 Materials and Methods
2.1 Experimental Setup
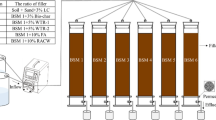
The bioretention cells were constructed using HDPE pipes of 150 mm diameter and 750 mm height using appropriate plumbing fixtures for drainage as shown in Fig. 1.
Each reactor comprised of a mulch layer made of coconut shells on the top to prevent soil erosion and alleviate weed growth. The bottom 150 mm was allocated to a well graded gravel layer to facilitate cloggage free drainage of effluent through the outlet pipes. Right above the gravel layer, C33 sand was filled for a depth of 200 mm in all the reactors with differing media amendments. This layer was topped with a 200 mm deep locally sourced soil layer and 10% by dry volume of coir peat for increased water retention and hydraulic conductivity in addition to other media amendments. The top 300 mm was used as ponding zone. The control reactor CM comprised of gravel, C33 sand and a soil-coir mixture (6:1 by volume). In reactors M1 and M2, the C33 sand layer was amended with brick granules (17:2 by volume) and pumice pellets (7:1 by volume) respectively. These ratios were chosen to keep the weight of the sand (3.8 kg) and its amendments constant (0.6 kg of brick granules and 0.6 kg of pumice pellets) for a fair comparison of nutrient removal properties. The soil-coir mixture was further amended with Scrap Iron Filings (SIF) in reactors M1 and M2 (soil: coir: SIF ratio by volume 9:2:1). The bioretention cells were un-vegetated to avoid interference from plant uptake. Prior to packaging in the columns, all the media used were washed several times until clear water was discharged. Additionally, the bioretention cells were flushed with water 24 times prior to experimentation to remove any inherent pollutants from the media since soil saturated with pollutant compounds tends to leach in bioretention cells.
2.2 Adsorption and Leaching Tests for Media
Batch sorption tests were carried out as per the methodology mentioned by Chiang et al. [6]. For the adsorption tests, unused samples of scrap iron filing, brick granules and pumice pellets are used. Conical flasks of 250 ml volume were fed with 100 ml of influent containing 20 mg/L of TN and 1 mg/L of TP. The following 7 combinations were added into the conical flasks, namely: 20 g of soil, 20 g soil+5 g pumice pellets, 20 g soil+10 g pumice pellets, 20 g soil+5 g brick granules, 20 g soil+10 g brick granules, 20 g soil+5 g SIF, and 20 g soil+10 g SIF. Post shaking in a rotary shaker at 100 rpm, the supernatants were collected and analysed for TN and TP concentrations.
Leaching tests were carried out as per the standard procedure mentioned in DIN 38414-S4 Norm [27]. SIF, brick granules and pumice pellet samples extracted from the bioretention cells post experimentation are used. 10g of each of the above samples is added to separate sealed conical flasks of 250 ml capacity containing 100 ml of dilute salt solution (0.01M CaCl2) and rotated in a rotary shaker at 160 rpm for 24 hours post which the supernatants are tested for TN and TP concentrations.
2.3 Runoff Simulation Experiments
Keeping in mind human and ecological well-being, it becomes necessary to treat stormwater to adhering standards before releasing it into water bodies or using it to improve water resilience [3]. Simultaneous removal of nitrogen and phosphorous compounds in stormwater treatment facilities is necessary to protect the health of receiving waters and prevent eutrophication [15] therefor, a synthetic runoff was used as influent throughout the study to eliminate the influence of fluctuations by pollutant concentrations. The influent concentration is constant, and the pollutant concentrations were kept within the average pollutant concentration range of urban rainwater runoff in New Delhi, India [32]. Pollutant concentrations were prepared using CH3COONa for a COD concentration of 150 mg/L, NaNO3 for a NO3–N concentration of 6 mg/L, NH4Cl for a NH4+–N concentration of 3.5 mg/L (accounting to a Total Nitrogen concentration of 9.5 mg/L) and KH2PO4 for a TP concentration of 1 mg/L. The experiment was conducted in 2 phases to simulate varying precipitation conditions. The bioretention cells were loaded twice a week for a period of 4 weeks to simulate a wet period during phase 1. To simulate a dry period the bioretention cells were loaded once a week during a period of 2 weeks during phase 2. A similar loading pattern was followed for the next 6 weeks but a saturation zone was introduced in all the BRCs to simulate phase 3 and phase 4. Effluent samples were procured and analysed for COD, NO3−–N, NH3–N, NO2–N, TN, TP and COD. On completion of the experimentation cycles, fresh and used media amendments were subjected to Field Emission Scanning Electron Microscopy (FESEM; Carl Zeiss Ultra Plus), Energy Dispersive Spectroscopy (EDS) and X-ray Diffraction analysis (XRD) to obtain the elemental compositions.
3 Results and Discussion
3.1 Adsorptive Potential of Media
Adsorption tests were conducted for the media additives and Fig. 2 depicts the concentration of the pollutants absorbed by the raw samples in different combinations. Results showed that SIF, brick granules and pumice pellets had good adsorption capacities. In the case of TN, the maximum adsorptive capacity of pumice was higher than that of brick under 5 g and 10 g dosages which is consistent with its specific surface area and porosity. However, SIF had the highest adsorption of TN and exceeded the adsorption rates of other combinations of brick and pumice (both 5 g and 10 g dosages) even when only 5 g of SIF was added, which is consistent with its high cation exchange complex (CEC).
It is to be noted that a major improvement of adsorption quantities wasn’t achieved when the SIF dosage was increased from 5 to 10 g in case of TN removal. In fact, higher TP was adsorbed when 5 g of pumice was used when compared to the 10 g dosage. With brick and SIF, increased dosage led to increased adsorption. This is acceptable since adsorption is a surface phenomenon and increased dosage resulted in increased availability of sites of adsorption and facilitated greater adsorption in most cases. TP removal was highest in SIF followed by brick granules which was then followed by pumice. In aqueous solutions, Fe0 tended to form Fe2+, Fe3+ and other iron hydroxides which are reported to be most effective in P sorption because of their high surface charge and high specific surface area per unit mass [13]. The primary phosphorous removal mechanism in bricks is its adsorption and precipitation within the sites of the brick particles [43]. Some researchers have stated that phosphate adsorption on pumice is a reversible reaction [28] and the agitation in the shaker could have caused the release of the adsorbed phosphate molecules which explained its lower TP adsorption.
3.2 Leaching Characteristics of Media
The media additives were subjected to leaching tests and Fig. 3 shows the leaching characteristics of these media extracted from the bioretention cells post experimentation.
TN was the primary contaminant with concentrations ranging from 0.93 mg/L in brick to 1.93 mg/L in pumice. TP concentrations ranged between 0.1 mg/L in brick to 0.54 mg/L in SIF. The concentrations of TP and TN were in agreement with the respective quantities added in the influent. Overall brick leached the least amount of the contaminants (TN and TP) while pumice leached the most TN. Previous studies have also observed that brick acted as a conducive media for nitrogen removal [11] and were capable of removing over 80% of applied phosphorous [43]. SIF leached the most TP in this study because the primary phosphorous removal pathway in iron was through the formation of Fe3(PO4)2 precipitates which may have been released during the rigorous agitation of this leaching test.
3.3 Removal of COD in the Bioretention Cells with and Without Saturation Zones
The bioretention cells were subjected to 4 loading phases and the removal of COD in them is shown in Fig. 4. M1 and M2 removed more COD than CM under all conditions. In the unsaturated conditions, CM removed an average of 48.19% COD, while M1 and M2 removed 64.96% and 72.79%, respectively. The average removal rates were higher during the dry phases of weekly loadings with CM, M1 and M2 achieving average COD removals of 54.9%, 70.38% and 76.8%, respectively. These removal rates during weekly loading increased further in the presence of saturation zones, and COD removals of 75.5%, 81.77% and 90.4% were achieved by CM, M1 and M2, respectively. Likewise, the average removal of COD was higher during saturated conditions with CM, M1 and M2 exhibiting 68.5%, 76.82% and 87.7% removal efficiencies. COD is primarily removed through filtration and adsorption, hence reactors M1 and M2 removed only comparably higher COD than the control. COD removal was improved in the presence of saturation zones because it may have indirectly supported enhanced denitrification by acting as an electron donor and metabolic source for heterogenotrophic denitrifiers.
3.4 Removal of Nitrate, Ammonia and TN in the Bioretention Cells with and Without Saturation Zones
Nitrogen removal happens through a combination of ammonification, nitrification and denitrification, where the ideal end product is nitrogen gas and it can also be biologically assimilated by vegetation and microorganisms [42]. The removal of N compounds in this study is shown in Fig. 5. The removal of NH3–N is shown in Figs. 5e and f. Throughout this study the control removed higher NH3–N than M1 and M2 because the SIF in the upper layers of the latter two BRCs generated NH3–N during their breakdown of NO3−–N.
Removal of nitrogen compounds in the bioretention cells with and without saturation zones (unshaded areas represent biweekly loading and shaded areas represent weekly influent loading): a TN concentration; b TN removal efficiency; c NO3−–N concentration; d NO3−–N removal efficiency; e NH3–N concentration; f NH3–N removal efficiency
In unsaturated conditions, CM removed an average of 44.96% NH3–N, whereas M1 and M2 generated NH3–N giving rise to −29.78% and −20.34% efficiencies. It is clear that the SIF in the upper layers was the contributing factor as the NH3–N generated in M1 and M2 were comparable, and studies have shown that NH3–N is mainly removed in the upper layers of BRCs where NH3–N is converted to NO3−–N through nitrification [8]. This also explains why NH3–N removal efficiencies were higher during weekly loading conditions when compared to biweekly loading under both saturated and unsaturated conditions. NH3–N was removed in the upper layers of the BRCs and weekly loadings allowed for higher dissolved oxygen levels in these strata, facilitating enhanced removal when compared to biweekly loadings. In the presence of saturation zones, NH3–N removal efficiencies increased for all 3 BRCs. CM removed an average of 76.26% NH3–N while M1 and M2 removed 59.47% and 52.12%, respectively. A couple of possible explanations for this increased efficiency are explained herewith. Firstly, it is speculated that anaerobic ammonia oxidizing (annamox) bacteria may have been present in the saturation zones. In this reaction, ammonium (NH4+) combines with nitrite (NO2−) to produce nitrogen gas (N2) and water (H2O). These bacteria use ammonium as an electron donor and nitrite as an electron acceptor to generate energy for their metabolic processes. However, the presence of annamox bacteria wasn’t verified in these BRCs as it was beyond the scope of this study. Secondly, the presence of saturation zones increased the retention and reaction times allowing for further breakdown of the NH3–N compounds into N2 in reactions involving zero valent iron and its derivatives from the SIF in the upper layers through processes such as adsorption, chemical reduction and surface complexation. ZVI has a high affinity for ammonia molecules, and it can adsorb or physically bind with ammonia on its surface. In the presence of ZVI, ammonia can undergo reductive processes, leading to the formation of ammonium
(NH4+) or even nitrogen gas (N2), depending on the reaction conditions. This reduction occurs as ZVI provides electrons for the conversion of ammonia into less toxic forms as shown in the Eqs. (1) and (2)
Reduction of ammonia using ferric and ferrous ions could have ocurred as shown in equation (3)
ZVI can also form surface complexes with ammonia (NH3 + Fe0 → NH3–Fe), where ammonia molecules bind to the iron surface through chemical interactions. These surface complexes can subsequently undergo reactions and transformations, resulting in the conversion of ammonia to other nitrogen species. Thirdly, reactions between ammonia and the pumice and brick present in the lower layers may be enhanced due to increased reaction times in the presence of saturation zones further promoting adsorption, ion exchange and chemical reactions as represented in Eq. (4), (5) and (6), respectively [33, 39].
In Eq. (4), g represents the gaseous form of ammonia, ad represents the adsorbed form of ammonia onto the brick/pumice surface, and (s) represents the solid state of the bricks/pumice. The equation indicates the physical adsorption of gaseous ammonia onto the surface of the bricks /pumice.
In Eq. (5), (aq) represents the aqueous phase, (s) represents the solid state of the bricks /pumice, NH4+ represents the ammonium ion, and X+ represents another ion released by the bricks /pumice through ion exchange. The equation demonstrates the ion exchange process in which the bricks /pumice adsorb the ammonium ions from the water and release other ions in exchange.
In Eq. (6), (aq) represents the aqueous phase, (s) represents the solid state of the bricks, NH3(aq) represents dissolved ammonia in water, NH4X(aq) represents an ammonium compound formed through a chemical reaction with the bricks, and X represents the counterion associated with the ammonium compound. The equation represents the chemical reaction between ammonia and the brick's constituents (e.g., lime or cement), resulting in the formation of an ammonium compound. Another plausible explanation is that the anoxic zone created by the saturation zone in the latter parts of the study contained denitrifiers which also aid the complete denitrification of NH3–N particles.
The removal of NO3−–N in the BRCs is shown in Figs 5c and d. It can be observed that M1 and M2 removed more NO3−–N than the control throughout the study. NO3− is removed in aqueous solutions through reduction to nitrite which reacts with Fe2+ ions in the upper layers to form ammonia or nitrogen gas [20, 26]. Lin et al. [19] also found that scrap iron fillers added to a “well” shaped base layer of permeable brick pavements (also a LID) could enhance nitrate reduction. The removal rates improved for all 3 BRCs in the presence of saturation zones which was also observed in other studies [41]. The highest removal in this study was observed in M2 which removed 67.98% NO3−–N in unsaturated conditions and 92.94% NO3––N in saturated conditions. M1 followed closely by eliminating 63.95% NO3−–N in unsaturated conditions and 78.73% NO3−–N in saturated conditions. The presence of oxides and hydroxides of Al in addition to other metals in brick was found to be beneficial in providing increased adsorption of nitrate [39]. These values were higher than the removal values of the control CM which removed 46.04% and 60.16% NO3−–N under saturated and unsaturated conditions, respectively. NO3−–N removal was slightly higher during weekly loading but this may be due to the fact the weekly loading were simulated in the end of the unsaturated and saturated phases when the system was most stable. SIF was strategically placed in the upper layers of BRCs M1 and M2 so that the NO3−–N trapped in this region which gets denitrified to form NH3−–N may enter the lower parts of the BRC containing either brick or pumice in M1 and M2 to undergo further reactions. This was essentially beneficial in the saturated zone simulations where this NH3−–N underwent further denitrification in this anoxic zone and resulted in lowered NO3−–N rates. The presence of brick and pumice in M1 and M2 respectively, also provided suitable growth and attachment conditions for microbes which were beneficial to the denitrification process. Studies have shown that microbial denitrification by soil communities play a more important role in NO3−–N reduction than adsorption and chemical precipitation [46]. Having different media zones within bioretention cells was beneficial because of the creation of a boundary layer between the upper ZVI and lower brick or pumice layers in the case of this bi-layered BRC. These boundary zones acted as transition layers between aerobic and anaerobic environments during the saturated experiments which increased the population of denitrifying bacteria [22]. The NO3−–N removal in M2 was further amplified in saturated conditions because of the promotion of redox conditions by pumice which was beneficial to denitrification.
The TN removal in the bioretention columns can be observed in Figs. 5a and b. In unsaturated conditions, the control outperformed M1 and M2 whereas in the presence of saturation zones M1 and M2 performed better. The average TN removal rates in CM, M1 and M2 during unsaturated conditions were 41.21%, 26.22% and 29.5%, respectively. In the presence of saturation zones, 58.35%, 66.1% and 72.02% TN removal was obtained in CM, M1 and M2, respectively, making the SIF-pumice combination retrofitted with saturation zones the best BRC as per this study. Saturation zones are recommended to create anaerobic environments to support heterogenotrophic denitrification in bioretention cells [15]. Pungrasmi et al. [33] showed that the denitrification rates in pumice was enhanced by the presence of COD and gas exchange limiting conditions (which was provided by the saturation zone). TN removal was greater in the presence of a saturation zone because the anoxic condition in the lower layer promoted denitrification as stated in the discussions of NO3−–N and NH3–N removal. NH3–N generation was the limiting factor to the TN removal in this study and its generation in M1 and M2 during the unsaturated phase, and its lower removal when compared to CM in the saturated phase contributed to this trendline. Overall, these reactors achieved higher NO3−–N removal than TN removal.
3.5 Removal of Total Phosphorous in the Bioretention Cells with and Without Saturation Zones
Phosphorous compounds which are present as particulate matter are generally removed through physical filtration and straining, whereas the dissolved phosphorous compounds are removed through sorption, precipitation and biological assimilation [15]. Figs 6a and b depict TP removal in the 3 BRCs of this study. Contrary to the TN removal trends, TP removal was negatively affected by the presence of a saturation zone. Saturation zones are known to have a negative impact on phosphorous removal because soil or media bound phosphorous tends to leach in anaerobic settings [10]. During unsaturated conditions, CM, M1 and M2 removed an average of 86.77%, 91.37% and 89.76% of TP, whereas the removal rates dropped to 63.99%, 83.67% and 71.74% in the presence of saturation zone. Such high TP removals were obtained by both pumice and brick containing systems because their composition of clay minerals, aluminium, iron and calcium was favourable for the precipitation of aqueous phosphorous [1]. However, the ZVI-brick combination in reactor M1 was the highest TP remover (91.37%) and it can be observed that the removal graphs tend to be more stable when compared to those of CM and M1. This aligns with the results obtained by Wang et al. [43] who achieved a stable 90% phosphorous removal in a vertical subsurface flow constructed wetland (VSSF) packed with broken bricks. M1 containing brick granules removed the highest TP in this study because it increased water retention and reaction times owing to its “wettability” [39]. The chemical composition of bricks has been attributed to its success in the precipitation of phosphorous [43]. Selvaraju et al. [39] also reported that a mixture of sand and brick is an ideal mixture for pollution uptake owing to the permeability offered by the sand and adsorption brought about by the brick whose acid sites exhibit greater affinity for phosphate over sand. Phosphate gets adsorbed to the surface of iron oxides such as rust which accounts for its main removal pathway in its interactions with SIF [44]. Iron filings are reported to be suitable for stormwater treatments as they do not cause fouling [14]. Since the co-existence of nitrate did not have negatively impact phosphate adsorption on iron, it is suitable for simultaneous TN and TP removal systems [12].
3.6 Surface Chemistry of Media from SEM and XRD Tests
The surface chemistry of the media amendments prior and after use was visualized and their compositions were investigated. Figure 7 shows SEM images, energy spectrums and elemental compositions of the media discussed in this study. Figs. 7a and b show photomicrographs of fresh and used brick granules respectively. It can be observed that the fresh brick was more undulated showing that it had sites available for adsorption of contaminants, whereas the used brick sample appeared clogged and compacted by the adsorbents from the study. The energy spectrum of the raw and used brick granules can be seen in Figs. 7c and d and their elemental compositions can be seen in Figs. 7e and f, respectively. The composition underwent a minor shift, with lesser quantities of Si, O, Fe, Al, Ca and Mg in the used sample when compared to the unused sample, as the primary pollutant removal mechanism of brick granules was surface adsorption. This composition is in agreement with that reported by Selvaraju et al. [39].
SEM–EDS analysis of unused and used media: a SEM imagery of unused brick; b SEM imagery of used brick; c energy spectrum of unused brick; d energy spectrum of used brick; e elemental composition of unused brick; f elemental composition of used brick; g SEM imagery of unused pumice; h SEM imagery of used pumice; i energy spectrum of unused pumice; j energy spectrum of used pumice; k elemental composition of unused pumice; l elemental composition of used pumice; m SEM imagery of unused SIF; n SEM imagery of used SIF; o energy spectrum of unused SIF; p energy spectrum of used SIF; q elemental composition of unused SIF; r elemental composition of used SIF
A similar pattern was observed in pumice pellet samples where the unused pumice (Fig. 7g) was visibly highly porous in comparison to the used sample (Fig. 7h) whose surface appeared cemented with adsorbed particles. However, there wasn’t a major shift in the EDS curves or elemental compositions between the 2 samples (Figs. 7i–l). The major elements of pumice are Si, O, Al, Na and K which was also confirmed by other studies [5]. In the case of SIF, the SEM image of the raw sample (Fig. 7m) appeared to be relatively smooth and uniform whereas the used sample (Fig. 7n) was corrugated with undulations filled with adsorbed and precipitated particles. There was a marked difference between the EDS curves in the unused (Fig. 7o) and used samples (Fig. 7p). The peak of Fe in the fresh samples was lowered in the used samples and was accompanied by the peaking of oxygen. This denoted that while unused SIF was made of zero valent iron, post usage samples had oxidised to form iron oxides [26]. This also agreed with the elemental compositions of fresh and used ZVI as seen in Figs. 7q and 7r, respectively. While unused ZVI was made up of 61.88% Fe and 7.45% O by weight, the used samples had a lowered weight of Fe (41.57%) and an increased weight of O (38.62%) in addition to the appearance of previously absent elements like Mg, Si and Ca. The surface morphology of SIF changed because of adsorption and its elemental composition was altered due to precipitate formation when in contact with water during the operation of the BRCs.
3.7 Leaching or Flushing Test for the Bioretention Cells Post Experimental Loading
Post the nutrient loading cycles of the study, the BRCs were flushed with distilled water, the effluents were collected and analysed for the pollutants listed in Table 1. This exercise was done to assess the possible leaching of previously captured pollutants in the BRCs. It can be observed that CM and M1 did not leach any COD whereas M2 leached 112 mg/L of COD. All 3 BRCs leached TP and the leaching was negligible in CM (0.009 mg/L), low in M1 (0.073 mg/L) and higher in M2 (0.293 mg/L). This is in agreement with the results obtained by Onar et al. [28] which showed that 80% of total dissolved phosphate was removed through adsorption on pumice and that certain conditions, such as the use of dilute NaOH solution, could regenerate the used pumice. CM leached 3.999 mg/L of TN and M1 leached 0.62 mg/L of TN with NO3−–N being the major contributing factor. Overall, the leaching characteristics of reactors CM and M2 were observable, while M1 exhibited almost no leaching. CM leached the highest quantity of pollutants under the following study conditions which proved that the addition of media such as ZVI, brick granules and pumice pellets were beneficial in not just enhancing pollutant removals, but also in their retention during future stormwater events.
4 Conclusions
The effects of adding scrap iron filings (zero valent iron), brick granules (BP), and pumice pellets (PP) in the simultaneous removal of total phosphorous (TP) and total nitrogen (TN) from urban runoff in bioretention cells were studied under the presence and absence of saturated zones. TP removal was found to be higher in unsaturated conditions, with M1 and M2 achieving 91.37% and 89.76% efficiencies, respectively. Conversely, TN removal was drastically improved in the presence of saturation zones, with average removal efficiencies of 58.35%, 66.10%, and 72.02% achieved by the bioretention cells CM, M1, and M2, respectively. The presence of Scrap Iron Filings (SIF) in the upper layers of M1 and M2 caused limitations in the form of NH3–N generation, which were mitigated by employing a saturation zone. The presence of saturation zones slightly improved COD removal and may have positively supported heterotrophic denitrification due to the high C/N ratio of the influent. Overall, the SIF-brick combination in M1 marginally performed better for TP removal, while the SIF-pumice combination in M2 performed better for TN removal. The roles of these media amendments were evident from SEM images and XRD results. In conclusion, the addition of ZVI, BP, and PP proved to have beneficial effects in the simultaneous removal of nitrogen and phosphorous compounds and could be viable amendments to the conventional media used in bioretention cells. The ZVI-brick granules combination was considered a better fit due to its non-leaching characteristics when flushed with distilled water post-study.
Data availability
All the data used is explicitly depicted through tables, graphs, and figures within the manuscript itself. Given that there isn't any data that was used that isn't included in the manuscript, a data availability statement may appear redundant.
References
Albalawneh A et al (2016) Efficiency of a horizontal sub-surface flow constructed wetland treatment system in an arid area. Water 8(2):51
Barrett ME et al (2013) Effects of media and plant selection on biofiltration performance. J Environ Eng 139(4):462–470
Biswal BK et al (2022) Biological nitrogen removal from stormwater in bioretention cells: a critical review. Crit Rev Biotechnol 42(5):713–735
Boström B et al (1988) Exchange of phosphorus across the sediment-water interface. Phosphorus in freshwater ecosystems. Springer, Berlin, pp 229–244
Cekova, B., et al. (2013). Structural examinations of natural raw materials pumice and trepel from Republic of Macedonia. Proceedings of the XV Balkan mineral processing congress, Sozopol.
Chiang YW et al (2012) Adsorption of multi-heavy metals onto water treatment residuals: Sorption capacities and applications. Chem Eng J 200–202:405–415
Chiu, P. C., et al. (2016). "Simultaneous Removal of Nitrogen and Phosphorus from Stormwater by Zero-Valent Iron and Biochar in Bioretention Cells (Part 1)."
Cho KW et al (2009) Removal of nitrogen by a layered soil infiltration system during intermittent storm events. Chemosphere 76(5):690–696
Correll D (1999) Phosphorus: a rate limiting nutrient in surface waters. Poult Sci 78(5):674–682
Dietz ME, Clausen JC (2006) Saturation to improve pollutant retention in a rain garden. Environ Sci Technol 40(4):1335–1340
Dires S et al (2019) Use of broken brick to enhance the removal of nutrients in subsurface flow constructed wetlands receiving hospital wastewater. Water Sci Technol 79(1):156–164
Du X et al (2017) The behavior of phosphate adsorption and its reactions on the surfaces of Fe–Mn oxide adsorbent. J Taiwan Inst Chem Eng 76:167–175
Elliott HA et al (2002) Influence of water treatment residuals on phosphorus solubility and leaching. J Environ Qual 31(4):1362–1369
Erickson AJ et al (2012) Capturing phosphates with iron enhanced sand filtration. Water Res 46(9):3032–3042
Glaister BJ et al (2014) Co-optimisation of phosphorus and nitrogen removal in stormwater biofilters: the role of filter media, vegetation and saturated zone. Water Sci Technol 69(9):1961–1969
Hsieh C, h, et al (2007) Bioretention column studies of phosphorus removal from urban stormwater runoff. Water Environ Res 79(2):177–184
Kumar N et al (2021) A Systematic Review Comparing Urban Flood Management Practices in India to China’s Sponge City Program. Sustain 13(11):6346
LeFevre GH et al (2015) Review of dissolved pollutants in urban storm water and their removal and fate in bioretention cells. J Environ Eng 141(1):04014050
Lin Z et al (2019) Influence of fillers on the removal of rainwater runoff pollutants by a permeable brick system with a frame structure base. Water Sci Technol 80(11):2131–2140
Liu Y, Wang J (2019) Reduction of nitrate by zero valent iron (ZVI)-based materials: A review. Sci Total Environ 671:388–403
Lucas WC, Greenway M (2008) Nutrient retention in vegetated and nonvegetated bioretention mesocosms. J Irrig Drain Eng 134(5):613–623
Ma W et al (2017) Enhanced nitrogen removal from coal gasification wastewater by simultaneous nitrification and denitrification (SND) in an oxygen-limited aeration sequencing batch biofilm reactor. Biores Technol 244:84–91
Marvin, J. T., et al. (2020). State-of-the-art review of phosphorus sorption amendments in bioretention media: A systematic literature review, American Society of Civil Engineers.
Mateus DMR et al (2016) The Potential Growth of Sugarcane in Constructed Wetlands Designed for Tertiary Treatment of Wastewater. Water 8(3):93
Muerdter CP et al (2018) Emerging investigator series: the role of vegetation in bioretention for stormwater treatment in the built environment: pollutant removal, hydrologic function, and ancillary benefits. Environ Sci: Water Res Technol 4(5):592–612
Narayanasamydamodaran S et al (2021) Scrap Iron Filings assisted nitrate and phosphate removal in low C/N waters using mixed microbial culture. Front Environ Sci Eng 15:1–14
Norm, D. (1984). " Deutsche Norm DIN 38414-S4 ".
Onar AN, Öztürk B (1993) Adsorption of phosphate onto pumice powder. Environ Technol 14(11):1081–1087
Osman M et al (2019) A review of nitrogen removal for urban stormwater runoff in bioretention system. Sustain 11(19):5415
Payne EG et al (2014) Biofilter design for effective nitrogen removal from stormwater–influence of plant species, inflow hydrology and use of a saturated zone. Water Sci Technol 69(6):1312–1319
Payne EG et al (2018) Which species? A decision-support tool to guide plant selection in stormwater biofilters. Adv Water Resour 113:86–99
Pipil H et al (2022) Spatio-temporal variations of quality of rainwater and stormwater and treatment of stormwater runoff using sand–gravel filters: case study of Delhi, India. Rend Lincei Sci Fisiche e Nat. https://doi.org/10.1007/s12210-021-01038-5
Pungrasmi W et al (2016) Nitrogen removal from a recirculating aquaculture system using a pumice bottom substrate nitrification-denitrification tank. Ecol Eng 95:357–363
Qi W et al (2022) A comprehensive analysis method of spatial prioritization for urban flood management based on source tracking. Ecol Ind 135:108565
Read J et al (2009) Plant traits that enhance pollutant removal from stormwater in biofiltration systems. Int J Phytorem 12(1):34–53
Rosenzweig BR et al (2018) Pluvial flood risk and opportunities for resilience. Wiley Interdiscip Rev Water 5(6):e1302
Rossetti, M. S. (2017). "Evaluation of phosphorous removals by biochar supported nano-scale zero-valent iron."
Rycewicz-Borecki M et al (2017) Nitrogen and phosphorus mass balance, retention and uptake in six plant species grown in stormwater bioretention microcosms. Ecol Eng 99:409–416
Selvaraju N, Pushpavanam S (2009) Adsorption characteristics on sand and brick beds. Chem Eng J 147(2):130–138
Sun Y et al (2017) The potential of using biological nitrogen removal technique for stormwater treatment. Ecol Eng 106:482–495
Tian J et al (2019) A pilot-scale, bi-layer bioretention system with biochar and zero-valent iron for enhanced nitrate removal from stormwater. Water Res 148:378–387
Vymazal J (2007) Removal of nutrients in various types of constructed wetlands. Sci Total Environ 380(1–3):48–65
Wang Z et al (2012) Study on phosphorus removal capability of constructed wetlands filled with broken bricks. Huan jing ke xue= Huanjing kexue 33(12):4373–4379
Werner S (1981) Aquatic chemistry: an introduction emphasizing chemical equilibria in natural waters. Johns Wiley, New York
White SA et al (2011) Phosphorus retention in lab and field-scale subsurface-flow wetlands treating plant nursery runoff. Ecol Eng 37(12):1968–1976
Xu H-J et al (2014) Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environ Sci Technol 48(16):9391–9399
Xu Y et al (2021) Enhanced removal of sulfamethoxazole and tetracycline in bioretention cells amended with activated carbon and zero-valent iron: system performance and microbial community. Sci Total Environ 797:148992
Zhou Z et al (2016) Efficiency promotion and its mechanisms of simultaneous nitrogen and phosphorus removal in stormwater biofilters. Biores Technol 218:842–849
Zinger Y et al (2013) Optimising nitrogen removal in existing stormwater biofilters: Benefits and tradeoffs of a retrofitted saturated zone. Ecol Eng 51:75–82
Acknowledgements
We are grateful to acknowledge that this work is supported by the Shenzhen Science and Technology Innovation Commission (Project number: KCXFZ20211020163556020).
Funding
Shenzhen Science and Technology Innovation Commission,KCXFZ20211020163556020,Zuo Jiane
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Narayanasamydamodaran, S., Zuo, J. & Kumar, N. Simultaneous Nutrient Removal from Urban Runoff Using Bi-layer Bioretention Cells with Novel Media Additives. Top Catal 67, 1038–1051 (2024). https://doi.org/10.1007/s11244-023-01894-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-023-01894-5