Abstract
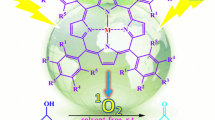
The aerobic oxidation of a variety of aromatic aldehydes to the corresponding carboxylic acids by molecular oxygen in the presence of 4-carboxyl tetraphenylporphyrin (H2TCPP), methylene blue (MB), cobalt(II) phthalocyanine sulfonate (CoPcS) and FeTCPPCl as water-soluble photosensitizers in organic-water biphasic media at room temperature under either visible light or sunlight is described. The products were obtained with 25–100% conversion and 100% selectivity. This method has a wide range of applicabilities, has a straightforward workup procedure, is chemoselective and proceeds under mild reaction conditions. The resulting products were obtained in good yields in reasonable times.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidation is one of the most essential reactions in synthetic organic chemistry, and for this purpose, a very wide variety of oxidants have been developed. The oxidation of aldehydes to carboxylic acids is of abiding interest in synthetic organic chemistry [1,2,3,4]. The popular conventional route to carboxylic acids involves the use of Jones reagent [5,6,7,8,9,10]. This is a stoichiometric reaction using highly acidic conditions which may not be tolerated by acid-sensitive functionalities in the substrate. Furthermore, the production of Cr-based side products may be viewed as a potential environmental hazard. Other efficient reagents that have been reported in the literature to achieve this transformation include oxone [11], calcium hypochlorite [12] and 2-hydroperoxyhexafluoro-2-propanol [13]. Some interesting methodologies involving metal-mediated transformation of aldehydes to the corresponding carboxylic acids have also been recently reported [14,15,16,17,18]. However, the development of catalytic systems for hydrocarbon oxidation by O2 under mild conditions is still a challenging problem. Iron porphyrins have been used as catalysts for alkane hydroxylation by O2 while consuming a stoichiometric amount of a reducing agent [19]. The oxidation of alkanes to alcohols and ketones has been accomplished at 80 °C under 10 atm pressure of oxygen [20]. A few examples of photochemical photooxidation using O2 and a metalloporphyrin catalyst have been reported [21, 22]. The photosensitized production of singlet oxygen is relevant to the photooxidation of organic compounds, as well as to DNA damage and photodynamic therapy [23, 24]. Consequently, a variety of photosensitizers have been developed and their photochemical and photophysical properties have been studied extensively [25]. Among such compounds, tetrapyrrolic compounds such as porphyrins and phthalocyanines are promising candidates for photosensitizers due to their special photophysical properties [26,27,28,29,30]. In particular, cobalt phthalocyanine has been widely used as a photosensitizer in photocatalytic reactions [31,32,33,34]. In our previous studies, we introduced efficient systems for the aerobic oxidation of alcohols and alkenes in organic solvents under visible light or sunlight [35,36,37,38]. The current study develops the photocatalytic oxygenation of aldehydes using air under visible light or sunlight irradiation with water-soluble photosensitizers in organic-water biphasic media at room temperature (Scheme 1). The products were obtained with 25–100% conversion and 100% selectivity.
Experimental
Materials and methods
Benzaldehyde, 4-methoxybenzaldehyde, 2,3-dimethoxybenzaldehyde, 3,4-dimethoxybenzaldehyde, 3-chlorobenzaldehyde, 4-chlorobenzaldehyde, anthracene, anthracene-9-carbaldehyde, cinnamaldehyde, 2,3,4,5,6-pentafluorobenzaldehyde, iron(II) chloride and solvents were purchased from Fluka or Merck and used without further purification. MB was purchased from Fluka. H2TCPP, FeTCPPCl and CoPcS were synthesized according to the literature procedures [39,40,41,42].
For generation of the carboxylic acids, 5 × 10−4 mol of the required aldehyde and 8 × 10−6 mol of water-soluble photosensitizer were added to acetonitrile (7.5 ml) plus water (7.5 ml) in a test tube. The samples were irradiated under visible light (288 power LED lamps, 1 W, 2.3 V, 59,660 Lux for 150 h at room temperature while bubbling air through the mixture (20 cm3 min−1). To prevent evaporation of the solvent, the photoreactor was equipped with a strong fan that kept the reaction temperature low and the test tube was closed with parafilm. The stability of the sensitizer was monitored by UV–Vis spectroscopy (Cintra 101) during the reaction, by following the Q band intensity of CoPcS. At the end of the reaction, the solvent was removed under vacuum, and the residue was separated by column chromatography (silica gel, n-hexane/EtOAc, 13:1) to give the corresponding carboxylic acid products. The identities of the products given in Table 3 were confirmed from their melting points and 1H NMR spectra (NMR (BRUKER DRX-300 AVANCE spectrometer). Benzoic acid (1): colorless crystals, mp 120–123 °C. 1H NMR (300 MHz, CDCl3) δ 8.17 (s, 2H), 7.60 (s, 3H) ppm, OH not observed. Cinnamic acid (2): colorless crystals, mp 132–135 °C. 1H NMR (300.13 MHz, CDCl3) δ 10.80 (brs, 1H, –COOH), 7.78 (d, J = 12.05 Hz, 1H), 7.42–7.59 (m, 5H), 6.46 (d, J = 12.05 Hz, 1H) ppm. 4-methoxybenzoic acid (3, 4): colorless crystals, mp 182–185 °C. 1H NMR (300.13 MHz, CDCl3) δ 11.85 (brs, 1H, –COOH), 8.07 (d, J = 8.3 Hz, 2H), 6.95 (d, J = 8.3 Hz, 2H), 3.89 (s, 3H) ppm. 2,3,4,5,6-pentafluorobenzoic acid (5): colorless crystals, mp 100–103 °C. 1H NMR (300.13 MHz, CDCl3) H of aldehyde (δ 10.01 (1H, s) ppm.) not observed. 3-chlorobenzoic acid (6): colorless crystals, mp 154–157 °C. 1H NMR. (300.13 MHz, CDCl3) δ 11.30 (brs, 1H, –COOH), 8.11 (s, 1H), 8.01 (d, J = 7.6 Hz, 1H), 7.59 (d, J = 7.8 Hz, 1H), 7.42 (t, J = 7.8 Hz, 1H) ppm. Anthracene-9-carboxylic acid (7): colorless crystals, mp 214–217 °C 1H NMR (300.13 MHz, CDCl3) δ 11.53 (s, 1H, –COOH), 8.97 (d, J = 9.0 Hz, 2H), 8.7 (s, 1H), 8.05 (d, J = 7.6 Hz, 2H), 7.68 (m, 2H), 7.53(m, 2H). 4-chlorobenzoic acid (8): colorless crystals, mp 237–240 °C. 1H NMR (300.13 MHz, CDCl3) δ 8.02 (d, J = 7.9 Hz, 2H), 7.43 (d, J = 8.2 Hz, 2H) ppm, OH not observed. 3,4-dimethoxybenzoic acid (9): colorless crystals, mp 179–182 °C. 1H NMR (300.13 MHz, CDCl3) δ 7.77 (d, J = 8.3 Hz, 1H), 7.61 (s, 1H), 6.92 (d, J = 8.4 Hz, 1H), 3.96 (s, 3H), 3.95 (s, 3H) ppm, OH not observed. 2,3-dimethoxybenzoic acid (10): colorless crystals, mp 121–124 °C. 1H NMR (300.13 MHz, CDCl3) δ 7.70 (d, J = 6.9 Hz, 1H), 7.09–7.22 (m, 2H), 3.98 (s, 3H), 3.91(s, 3H) ppm, OH not observed.
Results and discussion
The photooxygenation of 4-methoxybenzaldehyde was chosen as a model reaction in order to evaluate the different factors involved in the catalytic reaction. Table 1 gives the yields of 4-methoxybenzoic acid with various photosensitizers. The yield of 4-methoxybenzoic acid was CoPcS > MB > FeTCPP > H2TCPP for the same reaction time (Table 1, entries 1–4). It is worth noting that after 150 h of photooxygenation of 4-methoxybenzaldehyde in the presence of MB, FeTCPP and H2TCPP, the color of the solution had completely disappeared. Although the amount of singlet oxygen that was produced in the presence of CoPcS was low, because of lower degradation of CoPcS compared to MB, FeTCPP and H2TCPP, it could be used to convert aldehydes to carboxylic acids over a longer period of time. It is important to note that the oxidation of substrate ceased in the absence of photosensitizer, light or air (Table 1 entries 5–7). Hence, the photosensitizer, light and O2 are all essential for oxidation of aldehydes.
According to the literature, there are two major pathways for photooxygenation in the presence of photosensitizers, designated as Type I and Type II (Scheme 2) [45]. For investigation of the Type I mechanism, we performed our reactions in the presence H2O2, OH· and O ·−2 . In the presence of these reactive oxygen species, the yield of the reaction was significantly decreased (Table 1, entries 8–10).
Singlet oxygen generation (Type II) and its subsequent reaction with the substrate is the principal mechanism under our reaction conditions, since a very characteristic reaction of singlet oxygen is the [4+2] cycloaddition to conjugated cyclic dienes and polycyclic aromatic hydrocarbons such as anthracene [46,47,48,49]. Hence, singlet oxygen generation by CoPcS is evidenced by chemical trapping of 1O2 with anthracene. The UV–Vis spectra of anthracene as function of time under photoirradiation with CoPcS as a photosensitizer are displayed in Fig. 1. A decrease in intensity of the absorption band of anthracene (λmax = 375 nm) was observed over time. This response can be attributed to anthracene-9,10-endoperoxide formation (see Fig. 1). Moreover, the oxidation reaction did not occur under dark conditions, confirming that the anthracene oxidation is mediated by singlet oxygen under visible irradiation.
a UV–visible spectra for the photooxygenation of anthracene (λmax = 375 nm) in the presence of CoPcS as a photosensitizer using simulated solar light (288 power LED lamps, 1 W, 2.3 V (59,660 Lux)) under 1Atm of bubbling air in acetonitrile. b UV–Vis spectra of CoPcS (λmax = 660 nm) before and after 180 min photooxygenation
Furthermore, in the presence of N3−, which is a well-known singlet oxygen scavenger [50], conversion was inhibited (Table 1, entry 11). The photolysis was also studied in different solvent mixtures with variable water-to-acetonitrile ratios. Optimum conversion was observed in 1:1 acetonitrile/water (Table 2). The lifetime of singlet oxygen in acetonitrile is 65 μs and 38 μs in ethanol [51,52,53], which corresponds with the results in Table 2, entries 1 and 4, again providing evidence for generation of singlet oxygen in the reaction media.
High turnover number (TON) for the photooxygenation of aldehydes was observed with CoPcS under visible light (Table 3 entries 1–10). It is clear that this reaction will also proceed under solar radiation because the spectral distribution curve of the lamp is similar to that of sunlight (Table 3 entry 4).
Figure 2 shows a plot of 4-methoxybenzoic acid formation versus time in oxygenated CH3CN under visible light irradiation in the presence of CoPcS. The Q band of CoPcS was followed at 660 nm by UV–Vis spectroscopy. The plot shows that sensitizer bleaching for CoPcS was almost complete after 150 h under our reaction conditions. After loss of the Q band of CoPcS, the oxidation reaction ceased, and the yield of product remained constant. Therefore, the reaction time was chosen as 150 h in these studies.
A plausible mechanism for this reaction is shown in Scheme 3. The catalyst is activated by visible light to give singlet oxygen. The singlet oxygen then undergoes insertion into the C–H bond of the aldehyde 1 to form a peracid 3, via the acyl and hydroperoxyl radicals as intermediates [61]. In the final stage of reaction, a second aldehyde molecule reacts with the peracid 3 to generate the adduct 4. Compound 4 then decomposes by a Baeyer–Villiger type rearrangement to the final product 5 [61].
Conclusion
In conclusion, we have developed a new approach for the aerobic oxidation of aldehydes to the corresponding carboxylic acids using water-soluble photosensitizers in the presence of white light or sunlight and O2 in an organic–water biphasic media under very mild conditions. CoPcS was identified as the optimal catalyst for the oxidation reaction. This procedure is very simple and works efficiently at room temperature, giving good to excellent yields for a range of substrates.
References
Hollingworth GJ, Katritzky AR, Meth-Cohn O, Rees CW, Pattenden G (1995) In comprehensive organic functional group transformations. Elsevier Sci, Oxford
Hudlicky M (1990) Oxidations in organic chemistry. American Chemical Society, Washington DC
Larock RC (1999) Comprehensive organic transformations: a guide to functional group preparations, 2nd edn. Wiley, New York
Smith MB, March J (2001) March’s advanced organic chemistry: reactions mechanisms and structure, 5th edn. Wiley, New York
Bowden K, Heilbron IM, Jones ERH, Weedon BCL (1946) J Chem Soc. https://doi.org/10.1039/JR9460000039
Heilbron I, Jones E, Sondheimer F (1949) J Chem Soc. https://doi.org/10.1039/JR9490000604
Bladon P, Fabian JM, Henbest H, Koch H, Wood GW (1951) J Chem Soc. pp 2402–2411
Curtis R, Heilbron I, Jones E, Woods GF (1953) J Chem Soc. https://doi.org/10.1039/JR9530000457
Bowers A, Halsall T, Jones E, Lemin A (1953) J Chem Soc. https://doi.org/10.1039/JR9530002548
Djerassi C, Engle R, Bowers A (1956) J Org Chem 21:1547–1549
Benjamin RT, Sivakumar M, Hollist GO, Borhan B (2003) Org Lett 5:1031–1034
Nwaukwa SO, Keehn PM (1982) Tetrahedron Lett 23:3131–3134
Ganem B, Heggs RP, Biloski AJ, Schwartz DR (1980) Tetrahedron Lett 21:685–688
Joseph JK, Jain SL, Sain B (2007) Catal Commun 8:83–87
Lim M, Yoon CM, An G, Rhee H (2007) Tetrahedron Lett 48:3835–3839
Sloboda-Rozner D, Neimann K, Neumann R (2007) J Mol Catal A Chem 262:109–113
Mukhopadhyay C, Datta A (2008) Catal Commun 9:2588–2592
Uyanik M, Ishihara K (2009) Chem Commun. https://doi.org/10.1039/B823399C
Han A-R, Jeong YJ, Kang Y, Lee JY, Seo MS, Nam W (2008) Chem Commun. https://doi.org/10.1039/B716558G
Ellis PE Jr, Lyons JE (1990) Coord Chem Rev 105:181–193
Haranaka M, Hara A, Ando W, Akasaka T (2009) Tetrahedron Lett 50:3585–3587
Khavasi HR, Safari N (2004) J Mol Catal A 220:127–132
DeRosa MC, Crutchley RJ (2002) Coord Chem Rev 233:351–371
Greer A (2006) Acc Chem Res 39:797–804
Redmond RW, Gamlin JN (1999) Photochem Photobiol 70:391–475
Bonnett R (1995) Chem Soc Rev 24:19–33
Weber L, Hommel R, Behling J, Haufe G, Hennig H (1994) J Am Chem Soc 116:2400–2408
Pandey R, Zheng G (2000) The porphyrin handbook. In: Kadish KM, Smith KM, Guilard R (eds), vol 6. Academic Press, Boston, pp 157–230
Pushpan S, Venkatraman S, Anand V, Sankar J, Parmeswaran D, Ganesan S, Chandrashekar T (2002) Curr Med Chem Anti-Cancer Agents 2:187–207
Nyman ES, Hynninen PH (2004) J Photochem Photobiol B 73(1–2):1–28
Sorokin AB (2013) Chem Rev 113:8152–8191
Vashurin A, Maizlish V, Kuzmina I, Znoyko S, Morozova A, Razumov M, Koifman O (2017) J Porphyr Phthalocyanines 21:37–47
Ebrahimian Pirbazari A (2015) Procedia Mater Sci 11:622–627
Wang D, Guo R, Wang S, Liu F, Wang Y, Zhao C (2016) Desalin Water Treat 57:25226–25234
Hajimohammadi M, Bahadoran F, Davarani SSH, Safari N (2010) React Kinet Mech Cat 99:243–250
Hajimohammadi M, Safari N, Mofakham H, Deyhimi F (2011) Green Chem 13:991–997
Kalajahi SSM, Hajimohammadi M, Safari N (2014) React Kinet Mech Cat 113:629–640
Hajimohammadi M, Ghasemi H (2016) J Porphyr Phthalocyanines 20:670–676
Adler AD, Longo FR, Shergalis W (1964) J Am Chem Soc 86:3145–3149
Yan GP, Bischa D, Bottle SE (2007) Free Rad Biol Med 43:111–116
Kulinich VP, Shaposhnikov GP, Badaukaite RA (2010) Macroheterocycles 3:23–29
Knör G (2001) Chem Bio Chem 2:593–596
Staicu A, Pascu A, Nuta A, Sorescu A, Raditoiu V, Pascu ML (2013) Rom Rep Phys 65:1032–1051
Sawyer DT (1991) Oxygen chemistry. Oxford University Press, Oxford
Min DB, Boff JM (2002) Compr Rev Food Sci Food Saf 1:58–72
Aubry JM, Pierlot C, Rigaudy J, Schmidt R (2003) Acc Chem Res 36:668–675
Nowakowska M (1978) Macromol Chem Phys 179:2959–2967
Nowakowska M (1980) Macromol Chem Phys 181:1021–1027
Olea AF, Wilkinson F (1995) J Phys Chem 99:4518–4524
Harbour JR, Issler SL (1982) J Am Chem Soc 104:903–905
Chen Y, Xu S, Li L, Zhang M, Shen J, Shen T (2001) Dyes Pigments 51:63–69
Bressan M, Morvillo A (1989) Inorg Chem 28:950
Toffoli DJ, Gomes L, Junior NDV, Courrol LC (2008) In: AIP conference proceedings, AIP, vol 992. p 1207
Bernini R, Coratti A, Provenzano G, Fabrizi G, Tofani D (2005) Tetrahedron Lett 61:1821–1825
Wu XA, Ying P, Liu JY, Shen HS, Chen Y, He L (2009) Synth Commun 39:3459–3470
Nield E, Stephens R, Tatlow JC (1959) J Chem Soc. https://doi.org/10.1039/JR9590000166
Donleavy JJ (1936) J Am Chem Soc 58:1004–1005
Ueda I (1975) Bull Chem Soc Jpn 48:2306–2309
Taha N, Chidambaram M, Dakka J, Sasson Y (2009) Catal Lett 129:358–362
Ferenc WI, Walkow-Dziewulska AG (2001) J Serb Chem Soc 66:543–554
Iqbal N, Choi S, You Y, Cho EJ (2013) Tetrahedron Lett 54:6222–6225
Acknowledgements
Financial support of this work by Iran National Science Foundation (INSF) no. 96005616, and Research Council of Kharazmi University are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hajimohammadi, M., Ahmadi Khamesi, Z. & Nosrati, P. Efficient aerobic photooxygenation of aldehydes to carboxylic acids using cobalt(II) phthalocyanine sulfonate as a photosensitizer in organic-water biphasic media. Transit Met Chem 44, 167–173 (2019). https://doi.org/10.1007/s11243-018-0281-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-0281-x