Abstract
The linker 1,4-bis(2-methyl-imidazole-yl)-butane (bib) was used to construct two coordination polymers, specifically [Cd(bib)(ipa)]n (1) and [Zn(bib)(tpa)]n (2), in the presence of isophthalic acid (H2ipa) and terephthalic acid (H2tpa), respectively, under solvothermal conditions. Topological analyses reveal that the crystal of complex 1 consists of a 3D threefold interpenetrating network with Schläfli symbol {65.8}, while complex 2 possesses a 2D wavelike layer structure with Schläfli symbol {66}. The photocatalytic properties of both complexes for the degradation of methyl violet have been explored, revealing that complex 2 is a better photocatalyst than 1. A mechanism for the photocatalytic properties of the complexes is proposed, based on the results of density of states (DOS) and partial DOS calculations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The photodegradation of organic dyes based on azo groups (–N=N–) as chromophore is an important objective. However, conventional semiconductor photocatalyst does not give optimal results in this application [1, 2]. Coordination polymers (CPs), as a new kind of photocatalytic materials with attractive physical properties and versatile architectures, have attracted significant attention in the purification of water by sustainable decomposition of organic dyes [1,2,3,4,5,6,7,8,9]. In CPs used as photocatalysts, the different types of interactions between their inorganic and organic moieties may lead to diverse metal–organic ligand charge transfer interactions [10]. Some important CPs [such as UIO-66, ZIF-8 and MIL-100(Fe)] have been found to possess excellent photocatalytic properties [11,12,13,14]. Therefore, the controlled construction of novel CPs offers much potential for the development of photocatalysts for the degradation of organic dyes in the presence of light. An essential consideration in the construction of CP-based photocatalysts is the selection of appropriate organic ligands [15, 16].
In continuation to our efforts to develop new CPs with photocatalytic properties [17,18,19], in this work, the linker 1,4-bis(2-methyl-imidazole-yl)-butane (bib) (Scheme 1) was used to construct CPs in the presence of two aromatic dicarboxylate co-linkers displaying configurational isomerism, namely isophthalic acid (H2ipa) and terephthalic acid (H2tpa) (Scheme 1). Two CPs, namely [Cd(bib)(ipa)]n (1) and [Zn(bib)(tpa)]n (2), were synthesized and characterized, and their photocatalytic properties for the photodecomposition of methyl violet (MV) as a representative organic dye have been investigated. The results of these investigations are presented herein.
Experimental
Materials and methods
All the chemicals were procured from commercial sources and used without further purification. The powder X-ray diffraction (PXRD) data were collected on a Bruker D8 ADVANCE X-ray diffractometer equipped with Cu-Kα radiation (λ = 1.5418 Å) at 50 kV, 20 mA with a scanning rate of 6°/min and a step size of 0.02°. The simulated powder patterns for both complexes were obtained using Mercury 2.0 software. FTIR spectra as KBr pellets were recorded using a Nicolet Impact 750 FTIR spectrometer in the range of 400–4000 cm−1. Thermogravimetric analysis (TGA) was performed under a nitrogen atmosphere from room temperature to 650 °C at a heating rate of 10 °C min−1, using a SDT Q600 thermogravimetric analyzer.
Crystal structure determination
The single-crystal X-ray diffraction data were obtained on a Bruker SMART APEX diffractometer equipped with graphite monochromated Mo-Κα radiation (λ = 0.71073 Å) by using the ω-scan technique at 100 K. The intensities were corrected for absorption effects using SADABS. The structures were solved by direct methods (SHLEXS-2014) and refined using full-matrix least-squares procedures based on F2 (SHELXL-2014) [20]. All the hydrogen atoms were generated geometrically and refined isotropically using a riding model. All the non-hydrogen atoms were refined with anisotropic displacement parameters. The disordered atoms of the propyl chain in complex 1 were not included in the models. The disordered atoms of complex 1 (C13, C14, C15, C16) were refined using C atoms split over two sites, with a total occupancy of 1. It was found the Flack parameter for the structure was 0.16(4), and hence the twin refinement was used to get the final structure. The crystallographic details and selected bond dimensions for both complexes are listed in Tables 1 and 2. CCDC numbers are 1839730 for 1 and 1839735 for 2.
Synthesis of [Cd(bib)(ipa)]n (1)
A mixture of H2ipa (0.10 mmol, 0.017 g), bib (0.10 mmol, 0.022 g), Cd(NO3)2·4H2O (0.15 mmol, 0.027 g) and 20 mL of DMF/H2O (v/v = 3:1) was stirred for 30 min and then transferred and sealed into a 25-mL Teflon-lined reactor. The mixture was heated to 120 °C for 72 h and then cooled to room temperature at a rate of 5 °C/h. Yellow block crystals of complex 1 were obtained in 46% yield based on bib. IR data (KBr, cm−1) are 3100 (m), 2943 (m), 2341 (m), 1603 (vs), 1539 (m), 1490 (m), 1375 (vs), 1269 (v), 994 (m), 737 (m).
Synthesis of [Zn(bib)(tpa)]n (2)
A mixture of H2tpa (0.10 mmol, 0.017 g), bib (0.10 mmol, 0.022 g), Zn(NO3)2·6H2O (0.15 mmol, 0.047 g) and 10 mL of DMF/H2O (v/v = 1:1) was stirred for 30 min and then transferred and sealed into a 25-mL Teflon-lined reactor. The mixture was heated to 120 °C for 72 h and then cooled to room temperature at a rate of 5 °C/h. Colorless block crystals of complex 2 were obtained in 57% yield based on bib. IR data (KBr, cm−1) are 3090 (m), 2341(m), 1615 (vs), 1501 (m), 1408 (m), 1346 (v), 1276 (m), 1147 (m), 1001 (m), 825 (v), 731 (vs), 573 (m).
Photocatalytic reactions
The photocatalytic reactions were performed as follows. A sample of complex 1 or 2 (50 mg) was dispersed in 50 mL of an aqueous solution of MV (10 mg/L) under stirring in the dark for 30 min to ensure the establishment of an adsorption–desorption equilibrium. The mixture was then exposed to UV irradiation using an Hg lamp (250 W) and kept under continuous stirring during irradiation for 100 min. Aliquots of 5 mL volume were taken out after every 10 min and separated by centrifugation for analyses by UV–Vis spectrometer. A control experiment was also performed under the same conditions but without adding the catalysts.
Computational details
The probable mechanism for the photodegradation of organic dyes in the presence of CPs as photocatalysts has been addressed by band gap calculations. The smallest unit of both CPs was geometry-optimized using the B3LYP functional [21, 22], and the 6-31G** basis set for all the atoms except the metal centers, for which the CEP-121G basis set was employed. All the calculations were carried out with the Gaussian 09 program [23]. GaussSum 3.1 was used to obtain density of state (DOS) plots [24].
Results and discussion
Structure description of complex 1
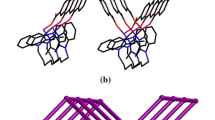
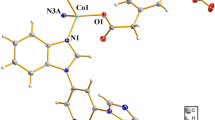
Each asymmetric unit of complex 1 comprises one independent Cd(II) center, one bib ligand and one fully deprotonated ipa ligand. The Cd(II) center has an octahedral {CdO4N2} geometry, being coordinated by four carboxylate oxygen atoms and two imidazole nitrogen atoms (Fig. 1a). The ipa2− ligand adopts a bidentate chelating coordination mode (μ2 − η1:η1) that bridges Cd(II) centers to produce chains of [Cd(ipa)]n units along the b-axis with a pitch of 17.12 Å. The bib ligand adopts a syn-anti coordination geometry to bridge the Cd(II) centers into a 1D chain of [Cd(bib)]n units. The [Cd(ipa)]n chains are further connected by the [Cd(bib)]n chains to form a 3D structure (Fig. 1b). To understand the structure more clearly, topological analysis has been used to simplify the framework. If the ipa and bib ligands are considered as connecters, the Cd(II) centers can be viewed as four-connected nodes; hence, the whole topology of the 3D structure can be simplified as a uninodal four-connected net with the Schläfli symbol {65.8}, calculated using TOPOS software (Fig. 1c). The potential voids are large enough to allow three independent identical networks, leading to a 3D threefold interpenetrating architecture (Fig. 1d). CP 1 is iso-reticular with [Co(bib)(aipa)] and [Co(bib)(glu)] (H2aipa = 5-aminoisophthalic acid and H2glu = glutaric acid) [25], which show single-crystal-to-single-crystal (SCSC) transformations.
Structure description of complex 2
CP 2 crystallizes in the monoclinic space group P2/c and can be described as a 2D network. The asymmetric unit includes one Zn(II) center, one bib ligand and one tpa ligand. Each Zn(II) center is four-coordinated by two carboxylate oxygen atoms (O1 and O3) and two imidazole nitrogen atoms (N1 and N3) (Fig. 2a). The Zn–O and Zn–N distances are 1.949(4), 1.997(6), 2.025(4) and 2.033(4) Å, respectively (Table S1). The Zn(II) centers are bridged by the carboxylic groups of the tpa2− anions to form a 1D zig-zag chain (Fig. 2b). The bib ligand also adopts syn-anti coordination to connect the Zn(II) centers, giving a 1D zig-zag chain (Fig. 2c). These [Zn(bib)]n chains are further connected by the 1D zig-zag [Zn(tpa)]n chains to generate a 2D wavelike network (Fig. 2d), which can be simplified as a four-connected network with the Schläfli symbol {66}(Fig. 2e).
IR, powder X-ray diffraction (PXRD) and thermal analyses
The IR spectra of both CPs display characteristic bands in the range of 1400–1610 cm−1 which can be attributed to the asymmetric and symmetric stretching vibrations of the carboxylato groups. The values of Δν[νas(COO) − νs(COO)] are 113 and 207 cm−1 for 1 and 2, respectively, indicating bidentate and monodentate coordination modes of the carboxylato groups, respectively (Fig. S1) [26]. A band at ca. 1500 cm−1 for complex 1 can be assigned to ν(C=N) absorption of the imidazole rings of the bib ligand. To determine whether the crystal structures are truly representative of the bulk materials used for property studies, X-ray powder diffraction (PXRD) experiments were carried out for both CPs (Fig. 3). The diffraction peaks of the experimental and simulated patterns are a good match, indicating the phase purity of the bulk samples.
To assess their thermal stabilities, thermogravimetric analyses (TGA) for both CPs were performed under a nitrogen atmosphere (Fig. S2). The frameworks of both were thermally stable up to ~ 350 °C, after which decomposition of both CPs commenced.
Photocatalysis
The photocatalytic performances of both complexes for the degradation of methyl violet as a model organic dye were investigated. The photodegradation experiments were carried out under UV irradiation after achieving adsorption equilibrium in the dark. The photocatalytic results for both CPs are presented in Fig. 4. In both cases, the UV–Vis band corresponding to MV decreases progressively with reaction time (Fig. 4a, b). The UV–Vis band intensities of MV at 506 nm against reaction time (t) were plotted for both CPs (Fig. 4c). The results indicate that approximately 46% of MV in the presence of 1 and 71% of MV in the presence of 2 were decomposed after 40 min. In addition, control experiments on the photodegradation of MV carried out under similar reaction conditions showed decomposition of MV only up to 23%. Overall, the photocatalytic results indicate that catalyst 2 is more active for the decomposition of MV than 1 [27, 28].
The structural integrity of both CPs after photodegradation of MV was assessed with the help of PXRD experiments. The PXRD results indicate that the crystallinity of both CPs was well maintained, even after degradation of MV (Fig. 3). The different catalytic activities of the two CPs can be attributed to the use of isomeric co-ligands, coordination modes of the metal centers and the differences of the final frameworks [29, 30].
To gain a better understanding of the mechanism of photocatalysis of MV by both these CPs, the band structures of both 1 and 2 have been calculated using density functional theory. As presented in Fig. 5, the valence band lying just below the Fermi levels of both complexes is mainly contributed by the aromatic centers as well as the oxygen and nitrogen atoms with negligible contributions from the Cd center in 1 and Zn center in 2. Also, the conduction band for CP 1 which lies just above the Fermi level in the range of −1.31 to −0.59 eV is derived from the aromatic moieties with admixture of the oxygen atoms. Similarly, in the case of CP 2, the conduction band above the Fermi level lying in the range of −1.38 to −0.77 eV has contributions from the aromatic carbons and oxygen atoms. Hence, as evident from the pDOS plots, the electronic transitions in both CPs are mainly operating from the aromatic center to another aromatic region (ligand-to-ligand) with no contribution from the metal centers. Hence, in the typical photocatalytic process, CPs 1 and 2 undergo excitation by light to generate electron–hole pairs. The holes then migrate to the metal center, while the electron migrates to the ligand.
It is apparent that CP 2 displays better catalytic efficiency toward the degradation of MV. This may be due to the better ability of the Zn(II) centers to facilitate formation of the hydroxyl radical (OH⋅) on the coordination network, although this feature requires further investigation [31, 32]. Although catalysts 1 and 2 possess similar ligands, their different metals may result in distinct band gap sizes, explaining the differences in their photocatalytic activities [33, 34].
Conclusion
In summary, the linker 1,4-bis(2-methyl-imidazole-yl)-butane (bib) has been utilized to construct two new coordination polymers from isomeric aromatic dicarboxylic acid ligands. We obtained two different frameworks: a 3D threefold interpenetrating network with Schläfli symbol {65.8} in the case of 1, and a 2D wavelike layer structure with Schläfli symbol {66} for 2. Both CPs show photocatalytic properties for the photodegradation of MV dye. Hence, these results provide information for the further development of designed frameworks that can provide efficient catalytic systems for the photodegradation of aromatic dyes.
References
Yoon MY, Srirambalaji R, Kim K (2012) Chem Rev 112:1196
Chen CC, Ma WH, Zhao JC (2010) Chem Soc Rev 39:4206
Wen T, Zhang DX, Zhang J (2013) Inorg Chem 52:12
Wang XL, Han N, Lin HY, Tian AX, Liu GC, Zhang JW (2014) Dalton Trans 43:2052
Zhang LN, Lu ST, Zhang C, Du CX, Hou HW (2015) CrystEngComm 17:846
Wang FQ, Wang CM, Yu JC, Xu KH, Li XY, Fu YY (2016) Polyhedron 105:49
Mu B, Li CX, Song M, Ren YL, Huang RD (2016) CrystEngComm 18:3086
Liao ZL, Li GD, Bi MH, Chen JS (2008) Inorg Chem 47:11
Guo J, Yang J, Liu YY, Ma JF (2012) CrystEngComm 14:6609
Li HX, Zhang XY, Huo YN, Zhu J (2007) Environ Sci Technol 41:4410
Wang SB, Wang XC (2015) Small 11:3097
Gao YW, Li SM, Li YX, Yao LY, Zhang H (2017) Appl Catal B Environ 202:165
Silva CG, Corma A, García A (2010) J Mater Chem 20:3141
So MC, Wiederrecht GP, Mondloch JE, Huppand JT, Farha OK (2015) Chem Commun 51:3501
Liu J, Wu J, Li F, Liu W, Li B, Wang J, Li Q, Yadav R, Kumar A (2016) RSC Adv 6:31161
Jin JC, Wu XR, Luo ZD, Deng FY, Liu JQ, Singh A, Kumar A (2017) CrystEngComm 19:4368
Wang J, Wu X, Liu J, Li B, Singh A, Kumar A, Batten SR (2017) CrystEngComm 19:3519
Lu L, Wang J, Xie B, Liu J, Yadav R, Singh A, Kumar A (2017) New J Chem 41:3537
Wang J, Bai C, Hu HM, Yuan F, Xue GL (2017) J Solid State Chem 249:87
Sheldrick GM (2015) Acta Crystallogr Sect A Found Adv 71:3
Becke AD (1993) J Chem Phys 98:5648
Lee CT, Yang WT, Parr RG (1998) Phys Rev B Condens Matter Mater Phys 37:785
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven JT, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi, J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski J W, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL,Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong WM, Gonzalez C, Pople JA (2009) Gaussian 09 revision B.01, Gaussian, Inc., WallingFord CT
O’Boyle NM, Tenderholt AL, Langner KM (2008) J Comput Chem 29:839
Zhou HF, He T, Yue KF, Liu YL, Zhou CS, Yan N, Wang Y (2016) Cryst Growth Des 16:3961
Hu JM, Guo R, Liu YG, Cui GH (2016) Inorg Chim Acta 450:418
Mitkina TV, Zakharchuk NF, Naumov DY, Gerasko OA, Fenske D, Fedin VP (2008) Inorg Chem 47:6748
Britten J, Hearns NGR, Preuss KE, Richardson JF, Bin-Salamon S (2007) Inorg Chem 46:3934
Meng JX, Lu Y, Li YG, Fu H, Wang EB (2011) CrystEngComm 13:2479
Guo J, Yang J, Liu YY, Ma JF (2012) CrystEngComm 14:6609
Mahata P, Madras G, Natarajan S (2006) J Phys Chem B 110:13759
Gong Y, Li J, Qin J, Wu T, Cao R, Li J (2011) Cryst Growth Des 11:1662
Wang CM, Wang FQ, Dong CF, Yu ZC, Wang ZC, Zhao YN, Li GD (2015) Z Anorg Allg Chem 641:1125
Wang FQ, Dong CF, Wang ZC, Cui YR, Wang CM, Zhao YN, Li GD (2014) Eur J Inorg Chem 36:6239
Acknowledgements
The authors acknowledge financial assistance from Sichuan University of Science and Engineering (Nos. 2015RC26, 2015RC29 and 2017RCL02), the Education Committee of Sichuan Province (Nos. 17ZA0264, 17ZB0312, 18ZB0422, 18ZB0425), the Project of Zigong Science and Technology (No. 2016HG06), the Opening Project of Key Laboratory of Green Catalysis of Sichuan Institutes of High Education (No. LYJ1705) and Innovative Entrepreneurial Training Plan of undergraduates in Guangdong Province (Nos. 201810571008, 201810571012, 201810571047, 201810571082, 201810571061, 201810571091).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, L., Wang, J., Chen, F. et al. Structures and photocatalytic performance of two d10 metal-based coordination polymers containing mixed building units. Transit Met Chem 44, 107–114 (2019). https://doi.org/10.1007/s11243-018-0274-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-0274-9