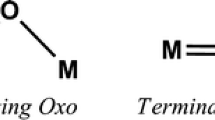Abstract
The iron tricarbonyl complex of octafluorocyclooctatetraene was synthesized by Hughes and co-workers and shown by X-ray crystallography to have a trihapto–monohapto structure (η3,1-C8F8)Fe(CO)3 in contrast to the tetrahapto structure (η4-C8H8)Fe(CO)3 formed by the non-fluorinated cyclooctatetraene. This difference has stimulated a comprehensive density functional theoretical study of the octafluorocyclooctatetraene metal carbonyl complexes (C8F8)M(CO) n (n = 4, 3, 2, 1 for M = Ti, V, Cr, Mn, and Fe; n = 3, 2, 1 for M = Co, Ni) for comparison with their hydrogen analogues (C8H8)M(CO) n . In most such systems, the substitution of fluorine for hydrogen leads to relatively small changes in the preferred structures. However, for the iron carbonyl derivatives (C8X8)Fe(CO)3 (X = H, F), the difference observed experimentally has been confirmed by theory with (η3,1-C8F8)Fe(CO)3 and (η4-C8H8)Fe(CO)3 being the lowest energy structures by 4 and 14 kcal/mol, respectively. The ligand exchange reactions C8H8 + (C8F8)M(CO) n → C8F8 + (C8H8)M(CO) n are predicted to be exothermic for almost all of the systems considered, with the (η3,1-C8X8)Fe(CO)3 system being the main exception. This suggests that the C8F8 ligand generally bonds more weakly to transition metals than the C8H8 ligand in accord with the electron-withdrawing effect of the ligand fluorine atoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclooctatetraene (COT) [1, 2] has occupied a prominent place in the historical development of organometallic chemistry in view of its flexible hapticity in bonding to transition metals, lanthanides and actinides [3]. In this connection, the first metal carbonyl complexes of cyclooctatetraene to be synthesized were the three very stable iron carbonyl complexes (η4-C8H8)Fe(CO)3, trans-(η4,η4-C8H8)Fe2(CO)6, and cis-(η5,η5-C8H8)Fe2(CO)5, first reported in 1959 as products from reaction of iron pentacarbonyl with cyclooctatetraene [4–7].
Fluorocarbon organometallics were recognized early to be frequently more stable thermally and to exhibit different bonding modes from their hydrocarbon analogues [8]. In this connection, a variety of fluoroolefins have been shown to be useful ligands for the synthesis of a variety of transition metal derivatives [9, 10]. One of the more interesting fluoroolefin ligands in metal carbonyl chemistry is octafluorocyclooctatetraene (OFCOT), which can be synthesized on a multigram scale [11]. Reactions of OFCOT with metal carbonyl derivatives provide a variety of interesting complexes such as (η5-Me5C5)Co(η4-C8F8) [12] and (η5-C5H5)Mn(η6-C8F8) [13]. Of particular interest is the reaction of OFCOT with Fe2(CO)9 in refluxing hexane, which gives an air-stable sublimable complex of stoichiometry (C8F8)Fe(CO)3 [2]. X-ray crystallography shows this complex not to have an (η4-C8F8)Fe(CO)3 structure similar to its hydrocarbon analogue (Fig. 1) but instead an (η3,1-C8F8)Fe(CO)3 structure in which three adjacent carbons of the C8F8 ring bond to the Fe(CO)3 group as a trihapto allylic ligand and a fourth isolated carbon of the C8F8 ring forms an Fe–C σ bond (Fig. 2). A dihapto iron tetracarbonyl intermediate (η2-C8F8)Fe(CO)4 was isolated by carrying out the reaction of Fe2(CO)9 with OFCOT in hexane at room temperature. Mild heating converts (η2-C8F8)Fe(CO)4 into (η3,1-C8F8)Fe(CO)3 with loss of one of the CO groups.
The different structures of the preferred mononuclear reaction products of iron carbonyls with cyclooctatetraene and with octafluorocyclooctatetraene (Figs. 1, 2) suggest that other metal carbonyls might also give complexes with octafluorocyclooctatetraene that differ from their complexes with cyclooctatetraene. For the first-row transition metals other than iron, the only example of a mononuclear cyclooctatetraene metal carbonyl complex is the chromium derivative (η6-C8H8)Cr(CO)3 [14, 15], which is significantly less stable than the iron complex (η4-C8H8)Fe(CO)3. The fluorinated analogue of this chromium complex, namely (C8F8)Cr(CO)3, is currently unknown. Thus, the experimental chemistry of (C8X8)M(CO) n derivatives (X = H, F) is very limited outside of iron. However, the preferred structures and energetics of the entire series of first-row transition metal carbonyl derivatives of cyclooctatetraene, namely C8H8M(CO) n (M = Ti, V, Cr, Mn, Fe, Co, Ni; n = 4, 3, 2, 1), have been investigated using density functional theory [16]. This paper reports the use of the same density functional theory methods to investigate the structures and energetics of the perfluorinated analogues of such derivatives, namely (C8F8)M(CO) n .
Theoretical methods
Electron correlation effects were included by employing density functional theory (DFT), which has evolved as a practical and effective computational tool, especially for organometallic compounds [17–23]. Two DFT methods were used in this study. The first method uses the hybrid B3LYP functional, which incorporates Becke’s three-parameter exchange functional (B3) with the Lee, Yang, and Parr (LYP) correlation functional [24, 25]. The second approach uses the BP86 method, which combines Becke’s 1988 exchange functional (B) with Perdew’s 1986 correlation functional [26, 27]. The BP86 method has been observed to be somewhat more reliable than the B3LYP method for the type of organometallic systems considered in this paper [28–30]. In the present paper, the B3LYP and BP86 methods agree with each other fairly well in predicting the structural characteristics of the (C8F8)M(CO) n (M = Ti, V, Cr, Mn, Fe, Co, Ni) derivatives of interest.
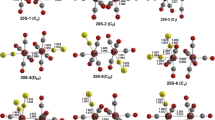
For comparison with our previous research, the same double-ζ plus polarization (DZP) basis sets were adopted in the present study. Thus, for carbon and fluorine, the double-ζ plus polarization (DZP) basis set used here adds one set of pure spherical harmonic d functions with orbital exponents α d(C) = 0.75 and α d(F) = 0.90 to the Huzinaga–Dunning standard contracted DZ sets, and is designated (9s5p1d/4s2p1d) [31, 32]. For the first-row transition metals, in our loosely contracted DZP basis set, the Wachters’ primitive sets were used, but augmented by two sets of p functions and one set of d functions, and contracted following Hood et al., and designated (14s11p6d/10s8p3d) [33, 34]. The optimized geometries from these computations are depicted in Figs. 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, and 13 with all bond distances given in Ångstroms.
The new (η6-C8H8)Cr(CO)2 structure not found in the previous study [16]
The geometries of all of the structures were fully optimized using both the DZP B3LYP and DZP BP86 methods. The harmonic vibrational frequencies were determined at the same levels by evaluating analytically the second derivatives of the energy with respect to the nuclear coordinates. The corresponding infrared intensities were evaluated analytically as well. All of the computations were carried out with the Gaussian 09 program package in which the fine grid (75 302) is the default for evaluating integrals numerically and the tight (10−8 hartree) designation is the default for the energy convergence [35]. The \( \left\langle {S^{ 2} } \right\rangle \) values are 0, 0.75, 2, and 3.75 for singlet, doublet, triplet, and quartet spin state structures, respectively, corresponding to \( \left\langle {S^{ 2} } \right\rangle \) = S(S + 1), where S is the total spin number, e.g., 0 for singlets, 1/2 for doublets, 1 for triplets, and 3/2 for quartets.
Results and discussion
Molecular structures
Titanium complexes
For (C8F8)Ti(CO)4, three stationary points with η6, η4, and η2,2 coordination of the C8F8 ring have been optimized (Fig. 3; Table 1). The global minimum is the hexahapto singlet structure (η6-C8F8)Ti(CO)4 (Ti4-S), which is analogous to the lowest energy hydrocarbon (η6-C8H8)Ti(CO)4 structure [16] with an 18-electron titanium configuration. The isomeric tetrahapto triplet structure (η4-C8F8)Ti(CO)4 (Ti4-1T) lies 10.7 kcal/mol (B3LYP) or 16.8 kcal/mol (BP86) above Ti4-S. The bis(dihapto) triplet structure (η2,2-C8F8)Ti(CO)4 (Ti4-2T) is a still higher energy structure, lying 12.9 kcal/mol (B3LYP) or 19.3 kcal/mol (BP86) above Ti4-S. In both Ti4-1T and Ti4-2T, the titanium atom has only a 16-electron configuration, accounting for the triplet spin state.
Successive removal of carbonyl groups from the (C8F8)Ti(CO)4 global minimum structure leads to stationary points for (C8F8)Ti(CO)3 (Ti3-S), (C8F8)Ti(CO)2 (Ti2-S), and (C8F8)Ti(CO) (Ti1-S) (Fig. 4; Table 2). All of these structures have octahapto η8-C8F8 rings, which leads to the favored 18-electron configuration for (η8-C8F8)Ti(CO)3. The predicted dissociation energy of one CO group from (C8F8)Ti(CO)4 (Ti4-S) to form (C8F8)Ti(CO)3 (Ti3-S) is 19.9 kcal/mol (B3LYP) or 26.0 kcal/mol (BP86). Dissociation of the next CO group from (C8F8)Ti(CO)3 (Ti3-S) is predicted to require a significantly higher energy of 26.0 kcal/mol (B3LYP) or 33.0 kcal/mol (BP86). The dissociation of CO groups from the dicarbonyl (C8F8)Ti(CO)2 (Ti2-S) is predicted to require still higher energies, indicating that (C8F8)Ti(CO)2 is reasonably stable toward further carbonyl loss.
Vanadium complexes
A stable doublet structure (η5-C8F8)V(CO)4 (V4-D) was found to be a genuine minimum without any imaginary vibrational frequencies. Structure V4-D with a pentahapto η5-C8F8 ring is analogous to the hydrocarbon (η5-C8H8)V(CO)4 structure found in the previous DFT study (Fig. 5; Table 3) [16]. The structures V3-D, V2-D, and V1-D are analogous to the corresponding hydrocarbon structures (η6-C8H8)V(CO)3, (η8-C8H8)V(CO)2, and (η8-C8H8)V(CO).
The loss of one CO group from (η5-C8F8)V(CO)4 (V4-D) gives the hexahapto structure (η6-C8F8)V(CO)3 (V3-D) with a 17-electron vanadium configuration (Fig. 5; Table 3). The predicted energy for this CO dissociation process of 9.0 kcal/mol (B3LYP) and 16.3 kcal/mol (BP86) is significantly lower than the CO dissociation from (C8F8)Ti(CO)4 discussed above. Further dissociation of a CO group from (η6-C8F8)V(CO)3 to give the octahapto structure (η8-C8F8)V(CO)2 requires a relatively high energy of 37.6 kcal/mol (B3LYP) or 42.4 kcal/mol (BP86). The next CO dissociation process, namely the dissociation of (η8-C8F8)V(CO)2 to (η8-C8F8)V(CO) + CO, requires a similar energy of 34.6 kcal/mol (B3LYP) or 41.1 kcal/mol (BP86).
Chromium complexes
A stable bis(dihapto) structure (η2,2-C8F8)Cr(CO)4 (Cr4-S), analogous to the hydrocarbon (η2,2-C8H8)Cr(CO)4 structure, was found as a genuine minimum without any imaginary vibrational frequencies (Fig. 6; Table 4). The chromium atom in Cr4-S is approximately octahedral with the favored 18-electron configuration. The hexahapto structure Cr3-S and the octahapto structures Cr2-2S and Cr1-S are analogous to the corresponding hydrocarbon structures (η6-C8H8)Cr(CO)3, (η8-C8H8)Cr(CO)2, and (η8-C8H8)Cr(CO) found in the previous DFT study [16] (Figs. 6, 7). However, a hydrocarbon analogue of the hexahapto (η6-C8F8)Cr(CO)2 structure Cr2-1S was not found in the previous work. Such a hydrocarbon analogue was obtained by replacement of fluorine with hydrogen followed by reoptimization to give a new (η6-C8H8)Cr(CO)2 structure not found in the previous study (Fig. 8). This hexahapto structure (η6-C8H8)Cr(CO)2 lies 1.7 kcal/mol (B3LYP) or 4.0 kcal/mol (BP86) in energy above the previously found isomeric octahapto structure (η8-C8H8)Cr(CO)2.
Successive loss of carbonyl groups from the bis(dihapto) structure (η2,2-C8F8)Cr(CO)4 (Cr4-S) gives first the hexahapto structure (η6-C8F8)Cr(CO)3 (Cr3-S), the two structures (η6-C8F8)Cr(CO)2 (Cr2-1S) and (η8-C8F8)Cr(CO)2 (Cr2-2S), and finally the octahapto structure (η8-C8F8)Cr(CO) (Cr1-S). The octahapto structure (η8-C8F8)Cr(CO)2 (Cr2-2S) with the favored 18-electron configuration for the central chromium atom lies 19.9 kcal/mol (B3LYP) or 14.4 kcal/mol (BP86) above the hexahapto structure (η6-C8F8)Cr(CO)2 (Cr2-1S) with only a 16-electron configuration for the chromium atom. The predicted energies required for this CO loss are 15.2 kcal/mol (B3LYP) or 18.6 kcal/mol (BP86) for the conversion of (η2,2-C8F8)Cr(CO)4 (Cr4-S) to (η6-C8F8)Cr(CO)3 (Cr3-S) where the increased hapticity of the C8F8 ring in the product retains the favored 18-electron configuration upon CO loss. However, the conversion of the 18-electron complex (η6-C8F8)Cr(CO)3 (Cr3-S) to the 16-electron complex (η6-C8F8)Cr(CO)2 (Cr2-1S) by further CO loss requires the much larger energy of 39.1 kcal/mol (B3LYP) or 47.5 kcal/mol (BP86). Further CO dissociation from (η6-C8F8)Cr(CO)2 (Cr2-1S) to give (η8-C8F8)Cr(CO) (Cr1-S) requires the very high energy of 58.8 kcal/mol (B3LYP) or 60.8 kcal/mol (BP86).
Manganese complexes
A stable dihapto doublet spin state structure (η2-C8F8)Mn(CO)4 (Mn4-D) was found as a genuine minimum without any imaginary vibrational frequencies (Fig. 9; Table 5). The manganese atom in Mn4-D is approximately square pyramidal with a 17-electron configuration.
Loss of a carbonyl group from (η2-C8F8)Mn(CO)4 (Mn4-D) gives the pentahapto structure (η5-C8F8)Mn(CO)3 (Mn3-D) with a local 18-electron environment for the manganese atom analogous to the long known [36, 37] (η5-C5H5)Mn(CO)3. Further CO loss from Mn3-D gives the hexahapto complex (η6-C8F8)Mn(CO)2 (Mn2-D) and then the octahapto complex (η8-C8F8)Mn(CO) (Mn1-D). Both Mn2-D and Mn1-D have the expected 17-electron manganese configuration for doublet spin state structures. The CO loss from (η2-C8F8)Mn(CO)4 (Mn4-D) to give (η5-C8F8)Mn(CO)3 (Mn3-D) requires the relatively small energy of 13.6 kcal/mol (B3LYP) or 10.8 kcal/mol (BP86), presumably because the pentahapto η5-C8F8 ligand in Mn3-D provides a favorable manganese environment. Further loss of a carbonyl group from (η5-C8F8)Mn(CO)3 (Mn3-D) requires a significantly higher energy of 26.2 kcal/mol (B3LYP) or 31.4 kcal/mol (BP86) and leads to a hexahapto complex (η6-C8F8)Mn(CO)2 (Mn2-D in Fig. 9) with a 17-electron configuration. The next CO dissociation process, namely that of the hexahapto complex (η6-C8F8)Mn(CO)2 (Mn2-D) to the octahapto complex (η8-C8F8)Mn(CO) (Mn1-D in Fig. 9 and Table 5), requires the much higher energy of 64.6 kcal/mol (B3LYP) or 66.0 kcal/mol (BP86). The conversion of a hexahapto η6-C8F8 ligand in Mn2-D into an octahapto η8-C8F8 ligand in Mn1-D balances the carbonyl loss so that the 17-electron manganese configuration is retained in Mn1-D. The fluorocarbon complex (η8-C8F8)Mn(CO) is analogous to the corresponding hydrocarbon complex (η8-C8H8)Mn(CO) found in the previous DFT study.
Iron complexes
A singlet dihapto (η2-C8F8)Fe(CO)4 structure (Fe4-S in Fig. 10; Table 6) is found without any imaginary vibrational frequencies analogous to (η2-C8F8)Mn(CO)4. Structure Fe4-S has the favored 18-electron configuration. The structure of the (C8F8)Fe(CO)4 product from the reaction of Fe2(CO)9 with octafluorocyclooctatetraene at room temperature appears to be Fe4-S on the basis of its infrared spectrum. However, this has not yet been confirmed by X-ray crystallography [2].
Three energetically low-lying structures were found for (C8F8)Fe(CO)3 (Fig. 10; Table 6). All three structures are genuine minima with no imaginary vibrational frequencies. The global minimum is the trihapto-monohapto structure (η3,1-C8F8)Fe(CO)3 Fe3-1S, which has been synthesized by the reaction of Fe2(CO)9 with octafluorocyclooctatetraene and structurally characterized by X-ray crystallography [2]. An analogous (η3,1-C8H8)Fe(CO)3 was not found in the previously reported DFT study on the analogous hydrocarbon system [16]. Replacing fluorine with hydrogen in Fe3-1S and reoptimizing gives a new (η3,1-C8H8)Fe(CO)3 structure (Fig. 10) [16]. However, this (η3,1-C8H8)Fe(CO)3 structure lies 14.8 kcal/mol (B3LYP) or 16.2 kcal/mol (BP86) above the experimentally known (η4-C8H8)Fe(CO)3 structure (Table 7). The tetrahapto structure (η4-C8F8)Fe(CO)3 (Fe3-2S) lies 4.2 kcal/mol (B3LYP) or 3.9 kcal/mol (BP86) above Fe3-1S. The bis(dihapto) isomer (η2,2-C8F8)Fe(CO)3 (Fe3-3S) is a still higher energy structure, lying 9.3 kcal/mol (B3LYP) or 6.6 kcal/mol (BP86) above Fe3-1S. The (C8F8)Fe(CO)3 structures Fe3-2S and Fe3-3S are analogous to the experimentally known hydrocarbon structure (η4-C8H8)Fe(CO)3 [4–6] and the (η2,2-C8H8)Fe(CO)3 structure found in the previous theoretical study [16], respectively. All three (C8F8)Fe(CO)3 structures have the favored 18-electron iron configuration.
A singlet hexahapto structure (η6-C8F8)Fe(CO)2 Fe2-S with the favored 18-electron iron configuration is predicted to be the lowest energy (C8F8)Fe(CO)2 structure (Fig. 11; Table 8). A higher energy triplet bis(dihapto) structure (η2,2-C8F8)Fe(CO)2 (Fe2-T) with a 16-electron iron configuration lies 3.9 kcal/mol (B3LYP) or 21.9 kcal/mol (BP86) above Fe2-S. The discrepancy between the singlet–triplet splitting for (C8F8)Fe(CO)2 predicted by the B3LYP and BP86 methods is not surprising since Reiher and collaborators have shown that the B3LYP method favors higher spin structures relative to the BP86 method [38, 39]. Both Fe2-S and Fe2-T are analogous to the corresponding hydrocarbon structures (η6-C8H8)Fe(CO)2 and (η2,2-C8H8)Fe(CO)2 optimized in the previous theoretical study [16].
The dissociation energy ∆Ediss for loss of a carbonyl group from (η2-C8F8)Fe(CO)4 (Fe4-S) to give (η3,1-C8F8)Fe(CO)3 (Fe3-1S) is 15.6 kcal/mol (B3LYP) or 17.9 kcal/mol (BP86). The dissociation energy ∆Ediss for loss of a carbonyl group from (η3,1-C8F8)Fe(CO)3 (Fe3-1S) to give the hexahapto complex (η6-C8F8)Fe(CO)2 (Fe2-S) is significantly higher at 31.6 kcal/mol (B3LYP) or 29.7 kcal/mol (BP86), consistent with the experimental stability of (C8F8)Fe(CO)3 derivatives. Further carbonyl loss from (η6-C8F8)Fe(CO)2 (Fe2-S) requires the rather high energy of 75.8 kcal/mol (B3LYP) or 75.2 kcal/mol (BP86) to give the singlet octahapto complex (η8-C8F8)Fe(CO) (Fe1-S), which has the favored 18-electron iron configuration. However, the singlet structure Fe1-S complex is a relatively high-energy structure, lying 48.4 kcal/mol (B3LYP) or 24.5 kcal/mol above the isomeric triplet hexahapto structure (η6-C8F8)Fe(CO) (Fe1-T). A hydrocarbon analogue of the triplet hexahapto structure Fe1-T was found in the previous theoretical study [16]. However, a singlet octahapto structure (η8-C8H8)Fe(CO) was not found in the previous theoretical study. This may relate to the relatively high energy of the singlet octahapto (η8-C8F8)Fe(CO) structure relative to the triplet hexahapto (η6-C8F8)Fe(CO) in the hydrocarbon system.
Cobalt complexes
A doublet trihapto structure (η3-C8F8)Co(CO)3 (Co3-D in Fig. 12 and Table 9) is found for the tricarbonyl. The analogous hydrocarbon structure (η3-C8H8)Co(CO)3 was found in the previous theoretical study [16]. A bis(dihapto) structure (η2,2-C8F8)Co(CO)2 (Co2-D) was found for the dicarbonyl. The cobalt atom in Co2-D can be considered to be tetrahedrally coordinated to the two C8F8 double bonds and two carbonyl groups and has the 17-electron configuration expected for a doublet structure.
Two structures for the monocarbonyl (C8F8)Co(CO) were optimized. The lowest energy structure (η6-C8F8)Co(CO) (Co1-D in Fig. 12) is a doublet hexahapto structure with a 17-electron configuration for the cobalt atom. A quartet structure (η2,2-C8F8)Co(CO) (Co1-Q), lying 14.1 kcal/mol (B3LYP) or 37.1 kcal/mol in energy above Co1-D, has the C8F8 ligand bonded to the cobalt atom as a bis(dihapto) ligand similar to that in the doublet dicarbonyl (η2,2-C8F8)Co(CO)2 (Co2-D). The cobalt atom in (η2,2-C8F8)Co(CO) (Co1-Q) has a 15-electron configuration consistent with the quartet spin state.
The energy ∆Ediss required for carbonyl dissociation from (η3-C8F8)Co(CO)3 (Co3-D) to give (η2,2-C8F8)Co(CO)2 (Co2-D) is 3.5 kcal/mol (B3LYP) or 20.3 kcal/mol (BP86). Further dissociation of a carbonyl group from Co2-D to give Co1-D requires the considerably higher energy of 31.0 kcal/mol (B3LYP) or 28.9 kcal/mol (BP86).
Nickel complexes
The dihapto structure (η2-C8F8)Ni(CO)3 (Ni3-S in Fig. 13 and Table 10) is predicted for the tricarbonyl. The nickel atom in Ni3-S is tetracoordinate similar to the well-known Ni(CO)4. Loss of a carbonyl group from Ni3-S requires 15.1 kcal/mol (B3LYP) or 15.4 kcal/mol (BP86) to give the bis(dihapto) derivative (η2,2-C8F8)Ni(CO)2 (Ni2-S in Fig. 13 and Table 10). Further dissociation of a carbonyl group from (η2,2-C8F8)Ni(CO)2 (Ni2-S in Fig. 13) requires 26.8 kcal/mol (B3LYP) or 29.6 kcal/mol (BP86) to give the hexahapto derivative (η6-C8F8)Ni(CO) (Ni1-S in Fig. 13). The nickel atoms in Ni3-S, Ni2-S, and Ni1-S all have the favored 18-electron configuration.
Thermochemistry
The carbonyl dissociation energies (∆Ediss) of the fluorocarbon derivatives by the process C8F8M(CO) n → C8F8M(CO) n−1 + CO are compared to those of the corresponding hydrocarbon derivatives [16], i.e., C8H8M(CO) n → C8H8M(CO) n−1 + CO (n = 4 for M = Ti, V, Cr, Mn, Fe; n = 3 for Co, Ni) considering the lowest energy structures (Table 11). For the vanadium, chromium, and nickel systems, the dissociation energies for the analogous fluorocarbon and hydrocarbon derivatives are very similar. For the hexahapto titanium systems (η6-C8X8)Ti(CO)4 (X = F, H), carbonyl dissociation to give the corresponding octahapto derivatives (η8-C8X8)Ti(CO)3 is ~22 kcal/mol more endothermic for the fluorocarbon derivatives than for the hydrocarbon derivatives. This suggests that octahapto η8-C8X8 coordination is less favorable for the hydrocarbon derivatives than for the corresponding fluorocarbon derivatives. For the manganese systems, the CO dissociation from (η2-C8X8)Mn(CO)4 to give (η5-C8H8)Mn(CO)3 involves conversion of a dihapto η2-C8X8 ligand to a pentahapto η5-C8X8 ligand. This process is ~8 kcal/mol more endothermic for the fluorocarbon derivatives than for the corresponding hydrocarbon derivatives. Again this increase in hapticity of the C8X8 ligand is more favorable for the fluorocarbon derivatives than for the hydrocarbon derivatives.
The thermochemistry of carbonyl dissociation from the fluorocarbon and hydrocarbon iron carbonyl systems (C8X8)Fe(CO)4 (X = F, H) is not directly comparable since the lowest energy hydrocarbon derivative is a tetrahapto structure (η4-C8H8)Fe(CO)3 whereas the lowest energy fluorocarbon derivative is a trihapto–monohapto structure (η3,1-C8F8)Fe(CO)3. This suggests a preference of carbon atoms in a fluorocarbon ligand to bond to a metal in odd-numbered groups of one or three relative to carbon atoms in the corresponding hydrocarbon ligands. In terms of the thermochemistry, carbonyl dissociation from (η2-C8F8)Fe(CO)4 (Fe4-S in Fig. 10) to give (η3,1-C8F8)Fe(CO)3 (Fe3-1S) is ~8 kcal/mol more endothermic than carbonyl dissociation from (η2-C8H8)Fe(CO)4 to give (η4-C8H8)Fe(CO)3. The CO dissociation of (η2-C8F8)Fe(CO)4 to give (η3,1-C8F8)Fe(CO)3 (Fe3-1S) has been observed experimentally [2].
Cobalt is the only one of the seven first-row transition metals for which carbonyl dissociation of the fluorocarbon derivative (C8F8)Co(CO)3 requires less energy than the corresponding hydrocarbon derivative. This dissociation involves the conversion of trihapto derivatives (η3-C8X8)Co(CO)3 to bis(dihapto) derivatives (η2,2-C8X8)Co(CO)2 for both the fluorocarbon and hydrocarbon systems. In this case, carbonyl dissociation from the fluorocarbon derivative is ~11 kcal/mol less endothermic than that from the hydrocarbon derivative.
Table 12 reports the reaction energies of the following ligand exchange reactions using the absolute energies for the (C8H8)M(CO) n complexes obtained in the previous paper [16]:
Almost all of these reactions are predicted to be exothermic, indicating that the C8H8 rings are more strongly bonded to the transition metals than the C8F8 rings. This is consistent with the high electronegativity of fluorine. This makes C8F8 rings weaker electron donors to metal atoms than C8H8 rings in otherwise equivalent metal complexes. The conspicuous anomaly is the endothermic reaction of C8H8 with (η3,1-C8F8)Fe(CO)3 to give C8F8 + η3,1-C8H8Fe(CO)3. This can be related to the relatively high energy of (η3,1-C8H8)Fe(CO)3 relative to isomeric (C8H8)Fe(CO)3 structures (Table 7) as contrasted with the low energy of the fluorocarbon analogue (η3,1-C8F8)Fe(CO)3 relative to its isomers. A previous study on (C8F8)2M complexes of the first-row transition metals also predicted the displacement of the fluorinated C8F8 ligands with the corresponding hydrocarbon C8H8 to be exothermic [40].
Mulliken atomic spin densities
A significant number of the (C8F8)M(CO) n structures have one or more unpaired electrons. Thus, all of the (C8F8)M(CO) n structures of the transition metals of odd atomic number (V, Mn, Co) are necessarily at least doublet spin states with a single unpaired electron. Also a few of the (C8F8)M(CO) n structures of the transition metals of even atomic number (Ti, Fe) are triplet spin states with two unpaired electrons. No low-energy triplet state structures were found for the Cr and Ni (C8F8)M(CO) n derivatives. The Mulliken atomic spin densities on the metal atoms in all of the doublet, triplet, and quartet spin state (C8F8)M(CO) n structures are listed in Table 13.
The doublet spin state (C8F8)M(CO) n structures of V, Mn, and Co should have a Mulliken spin density of nearly unity on the metal atom if the metal atom has a formal 17-electron configuration. This is the case for all of the doublet spin state structures except for Mn3-D with a pentahapto η5-C8F8 ring and some of the cobalt structures including particularly Co3-D with a trihapto η3-C8F8 ring. In these structures with an odd number of carbon atoms of the C8F8 ring within bonding distance of the central metal atom, the central metal approaches a formal 18-electron configuration with the spin largely on the uncomplexed ligand carbon atoms.
The atomic spin densities in the triplet (C8F8)M(CO) n structures can be interpreted in a similar manner. If the triplet spin state arises from a 16-electron configuration of the central metal atom with two unpaired electrons, then the Mulliken spin density on the metal atom should approach 2. This is clearly the case with the triplet spin state (C8F8)Fe(CO) n complexes Fe1-T and Fe2-T. However, the triplet state (C8F8)Ti(CO)4 complexes Ti4-1T and Ti4-2T with tetrahapto or bis(dihapto) C8F8 ligands have a spin density of approximately unity on the titanium atom leaving one unpaired electron for the C8F8 ligand.
Conclusion
The octafluorocyclooctatetraene metal carbonyl complexes (C8F8)M(CO) n (n = 4, 3, 2, 1 for M = Ti, V, Cr, Mn, Fe; n = 3, 2, 1 for M = Co, Ni) have been investigated by density functional theory for comparison with their hydrogen analogues (C8H8)M(CO) n . In most such systems, the substitution of fluorine for hydrogen leads to relatively small changes in the preferred structures. However, for the iron carbonyl derivatives (C8X8)Fe(CO)3 (X = H, F), the substitution of fluorine for hydrogen has a major effect, now demonstrated by both experiment and theory. Thus for (C8H8)Fe(CO)3, the experimentally observed tetrahapto structure (Fig. 1) lies more than 14 kcal/mol below the isomeric bis(dihapto) and trihaptomonohapto structures. However, for the corresponding perfluorinated derivative (C8F8)Fe(CO)3, the experimentally observed trihapto–monohapto structure lies ~4 kcal/mol below the isomeric tetrahapto structure and ~9 kcal/mol below the bis(dihapto) structure.
Thermochemical studies predict the ligand exchange reactions C8H8 + (C8F8)M(CO) n → C8F8 + (C8H8)M(CO) n to be exothermic for almost all of the systems considered, with the (η3,1-C8X8)Fe(CO)3 system being the main exception. This indicates that the C8F8 ligand bonds more weakly to transition metals than the C8H8 ligand. This can be related to the high electronegativity of fluorine relative to hydrogen.
References
Fray GI, Saxton RG (1978) The chemistry of cyclooctatetraene and its derivatives. Cambridge University Press, Cambridge
Barefoot A C III, Corcoran E W Jr, Hughes RP, Lemal DM, Saunders WD, Laird BB, Davis RE (1981) J Am Chem Soc 103:970
Deganello G (1979) Transition metal complexes of cyclic polyolefins. Academic Press, New York
Manuel TA, Stone FGA (1959) Proc Chem Soc Lond 90
Manuel TA, Stone FGA (1960) J Am Chem Soc 82:366
Rausch MD, Schrauzer GN (1959) Chem Ind 957
Nakamura A, Hagihara N (1959) Bull Chem Soc Jpn 32:880
Stone FGA (1972) Pure Appl Chem 30:551
Hughes RP (1990) Adv Organometal Chem 31:183
Hughes RP (2010) J Fluor Chem 131:1059
Lemal DM, Buzby JM, Barefoot III AC, Grayston MW, Laganis ED (1980) J Org Chem 45:3118
Hughes RP, Samkoff DE, Davis RE, Laird BB (1983) Organometallics 2:195
Hemond RC, Hughes RP, Rheingold AL (1989) Organometallics 8:1261
Kreiter CG, Maasbol A, Anet FAL, Kaesz HD, Winstein S (1966) J Am Chem Soc 88:3444
King RB (1967) J Organometal Chem 8:139
Wang H, Du Q, Xie Y, King RB, Schaefer HF (2010) J Organometal Chem 695:215
Ziegler T, Autschbach J (2005) Chem Rev 105:2695
Bühl M, Kabrede H (2006) J Chem Theory Comput 2:1282
Brynda M, Gagliardi L, Widmark PO, Power PP, Roos BO (2006) Angew Chem Int Ed 45:3804
Sieffert N, Bühl M (2010) J Am Chem Soc 132:8056
Schyman P, Lai W, Chen H, Wang Y, Shaik S (2011) J Am Chem Soc 133:7977
Adams RD, Pearl WC, Wong YO, Zhang Q, Hall MB, Walensky JR (2011) J Am Chem Soc 133:12994
Lonsdale R, Olah J, Mulholland AJ, Harvey JN (2011) J Am Chem Soc 133:15464
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Becke AD (1988) Phys Rev A 38:3098
Perdew JP (1986) Phys Rev B 33:8822
Furche F, Perdew JP (2006) J Chem Phys 124:044103
Wang H, Xie Y, King RB, Schaefer HF (2005) J Am Chem Soc 127:11646
Wang H, Xie Y, King RB, Schaefer HF (2006) J Am Chem Soc 128:11376
Dunning TH (1970) J Chem Phys 53:2823
Huzinaga S (1965) J Chem Phys 42:1293
Wachters AJH (1970) J Chem Phys 52:1033
Hood DM, Pitzer RM, Schaefer HF (1979) J Chem Phys 71:705
Frisch MJ et al (2009) G09. Gaussian, Inc., Wallingford, CT
Fischer EO, Jira R (1954) Z Naturforsch 9b:618
Piper TS, Cotton FA, Wilkinson G (1955) J Inorg Nucl Chem 1:165
Reiher M, Salomon O, Hess BA (2001) Theor Chem Acc 107:48
Salomon O, Reiher M, Hess BA (2002) J Chem Phys 117:4729
Wang H, Li R, King RB (2013) J Fluor Chem 153:121
Acknowledgments
We are grateful for financial support from the China Scholarship Council and hospitality of Center for Computational Quantum Chemistry of the University of Georgia, USA. We acknowledge financial support from the Fundamental Research Funds for the Central Universities (Grant SWJTU12CX084), the China National Science Foundation (Grant 11174237), the Sichuan Province, Applied Science and Technology Project (Grant 2013JY0035), the open research fund of the Key Laboratory of Advanced Scientific Computation, Xihua University (Grant: szjj2012-035), and the US National Science Foundation (Grant CHE-1057466).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, H., Wang, H., Die, D. et al. The hapticity of octafluorocyclooctatetraene in its first-row mononuclear transition metal carbonyl complexes: effect of perfluorination. Transition Met Chem 39, 95–109 (2014). https://doi.org/10.1007/s11243-013-9778-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-013-9778-5