Abstract
The substitution reaction of the Pt(IV) complex [PtCl4(bipy)] with guanosine-5′-monophosphate (5′-GMP) was studied by UV–Vis spectrophotometry. This reaction was investigated under pseudo-first-order conditions at 37 °C in 25 mM Hepes buffer (pH = 7.2) in the presence of 10 mM NaCl to prevent the hydrolysis of the complex. The substitution of chlorides in [{trans-Pt(NH3)2Cl}2(μ-1,2-bis(4-pyridyl)ethane)](ClO4)2 (Pt3) complex by 5′-GMP was followed by 1H NMR spectroscopy under second-order conditions. Very similar values for the rate constants of both substitution steps were obtained. The Pt(IV) complexes, [PtCl4(bipy)] and [PtCl4(dach)], as well as dinuclaer Pt(II) [{trans-Pt(NH3)2Cl}2(μ-pyrazine)](ClO4)2 (Pt1), [{trans-Pt(NH3)2Cl}2(μ-4,4′-bipyridyl)](ClO4)2 · DMF (Pt2) and [{trans-Pt(NH3)2Cl}2(μ-1,2-bis(4-pyridyl)ethane)](ClO4)2 (Pt3) complexes, displayed potent cytotoxic activity against human ovarium carcinoma cell line TOV21G and lower activity toward human colon carcinoma HCT116 cell line at the same concentrations. Our data indicate that these platinum complexes could be explored further, as potential therapeutic agents for ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of platinum coordination compounds in cancer chemotherapy has been extensively studied following the discovery of the therapeutic properties of cis-diamminedichloroplatinum(II) (cis-[Pt(NH3)2Cl2], cisplatin) by Rosenberg et al. [1, 2]. Cisplatin is one of the most widely utilized antitumor drugs, exhibiting high efficacy against solid tumors, particularly testicular and ovarian cancer [3–5]. The anticancer activity of cisplatin is based on its ability to form intrastrand covalent adducts with DNA by binding of Pt to the N7 atoms of two adjacent guanine bases [6, 7]. However, the clinical efficiency of cisplatin, cis-[PtCl2(NH3)2], is limited by toxic side effects, in particular a dose-limiting nephrotoxicity, by drug resistance in tumor cells, and by a narrow range of activity [8, 9].
Platinum(IV) complexes have greater inertness than the corresponding Pt(II) complexes. Hence, these complexes may have some advantages, such as: allowing oral administration, reduced toxicity, and decrease in the amount of the complex that is lost or deactivated on the path to the target cell [10]. Platinum(IV) complexes have enormous potential as anticancer agents in terms of both high activity and low toxicity. About 3000 Pt(IV) complexes have been synthesized and investigated in an attempt to improve the antitumor activity, lower toxicity and to design a drug that would be able to overcome resistance. Only about 30 platinum complexes have entered into clinical trials [11, 12]. However, it is generally believed that since Pt(IV) complexes are less reactive in ligand substitution reactions relative to their Pt(II) analogues, they must be reduced to Pt(II) species before binding to DNA. Upon entering into the cell, there are two metabolic pathways for Pt(IV) complexes: reduction by agents present in the cell (glutathione, ascorbic acid) or direct interaction with DNA in the nucleus. The first pathway leads to well-known interactions of Pt(II) complexes, while the second proposes the formation of adducts between Pt(IV) and DNA [13, 14].
Multinuclear complexes of platinum(II) represent a third generation of antitumor drugs the same as platinum(IV) complexes [15]. These complexes consist of either two or three platinum centers that are linked trough a flexible bridge such as an aliphatic chain [16] or a rigid bridge that consists for instance of azole molecules [17]. The reason for the increasing interest in multinuclear complexes is their ability to form DNA adducts that differ significantly from those formed by cisplatin and related complexes [18], which results in a completely different antitumor behavior. These complexes usually exist in cationic forms in solution and hence have high solubility in water; some of them are active in both cisplatin-sensitive and cisplatin-resistant cell lines [19, 20]. The biological activity of polynuclear platinum complexes may be modulated by geometry and number of leaving groups in the platinum coordination sphere as well as by the nature of the linkers connecting the platinum centers. In contrast to the mononuclear complexes, such as antitumor cisplatin and clinically ineffective transplatin, in the dinuclear case both geometries are antitumor active [21]. As the structure of the DNA adducts determines repair, protein recognition, and other downstream cellular events, an understanding of their formation and biological consequences is essential to further drug development.
We report here the cytotoxic activity of some newly synthesized platinum(IV) and dinuclear platinum(II) (see Figs. 1 and 2) complexes on two human carcinoma cell lines: TOV21G (ovarian cancer cell line) and HCT-116 (colon cancer cell line), indicating their potential therapeutic use for treatment of ovarian and colon cancer. In addiction, we studied the substitution reactions of the platinum(IV) complex [PtCl4(bipy)] (Fig. 1) and dinuclear Pt(II) complex [{trans-Pt(NH3)2Cl}2(μ-1,2-bis(4-pyridyl)ethane)](ClO4)2 (Pt3) (Fig. 2) with guanosine-5′-monophosphate (5′-GMP) using UV–Vis spectrophotometric and 1H NMR techniques to clarify the mechanism of these interactions.
Experimental
Chemicals
Potassium tetrachloroplatinate (K2PtCl4), a starting complex for the other synthesis, was purchased from Strem Chemicals, while cisplatin, cis-diamminedichloroplatinum(II), cis-[PtCl2(NH3)2], were purchased from Aldrich. The ligands (1R,2R)-1,2-diaminocyclohexane (dach) (Acros Organics), 2,2′-bipyridyl (bipy) (Aldrich) as well as nucleophile guanosine-5′-monophosphate sodium salt (5′-GMP) (Acros Organic) were used without further purification. Hepes buffer (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) was obtained from Aldrich. Transplatin, trans-[PtCl2(NH3)2], pyrazine, and 4,4′-bipyridyl,1,2-bis(4-pyridyl)ethane, which were used for synthesis of dinuclear complexes, were also obtained from Acros Organics. The complexes [PtCl4(bipy)], [PtCl4(dach)] were prepared according to the published procedures [22], while the dinuclear platinum(II) complexes, such as [{trans-Pt(NH3)2Cl}2(μ-pyrazine)](ClO4)2 (Pt1), [{trans-Pt(NH3)2Cl}2(μ-4,4′-bipyridyl)](ClO4)2∙DMF (Pt2), and [{trans-Pt(NH3)2Cl}2(μ-1,2-bis(4-pyridyl)ethane)](ClO4)2 (Pt3), were synthesized according to the literature procedures [23, 24].
Chemical analyses were performed on a Carlo Erba Elemental Analyser 1106. UV–Vis spectra were recorded on Shimadzu UV 250, Hewlett-Packard 8452A diode-array spectrophotometers and a Perkin-Elmer Lambda 35 double-beam spectrophotometer, equipped with thermostated 1.00 cm quartz Suprasil cells. The temperature was controlled throughout all kinetic experiments to ±0.1 °C. The 1H NMR measurements were recorded on a Varian Gemini-200 spectrometer.
Cell culture
Two human carcinoma cell lines, TOV21G (ovarium) and HCT-116 (colon), were purchased from the American Type Culture Collection and cultured in 1:1 mixture of MCDB 105 and Medium 199 (TOV-21G) and DMEM (HCT116), supplemented with 10–15 % fetal bovine serum, 100 units/mL of penicillin and 100 mg/mL of streptomycin. Human mesenchymal stem cells (MSC) from peripheral blood were kindly provided by Dr Diana Bugarski (Institute for Medical Research, University of Belgrade, Serbia) and maintained in DMEM with 10 % FBS. All cells were cultured and treated at 37 °C in a humidified incubator containing 5 % CO2. All reagents were purchased from Sigma Chemicals.
UV–Vis measurements
Nucleophile stock solution was prepared shortly before use by dissolving the chemicals in purified water. All other chemicals were of analytically reagent grade. Highly purified, deionized water was used in the preparation of all solutions. All kinetic measurements were performed under pseudo-first-order conditions, that is, at least a 10-fold excess of the entering nucleophile was used. The working wavelength for reaction system was determined by recording the spectra of reaction mixtures over the wavelength range between 220 and 450 nm. The reaction was initiated by mixing equal volumes of the complex and nucleophile solutions (1.5 mL) in the quartz cuvette.
The substitution reactions of platinum(IV) complex [PtCl4(bipy)] with 5′-GMP were studied spectrophotometrically by following the change in absorbance at suitable wavelengths as a function of time at 37 °C. These reactions were followed in 25 mM Hepes buffer (pH = 7.2) in addition of 10 mM NaCl to prevent the hydrolysis of complex. All reported pseudo-first-order rate constants, k obsd, represent an average value of two to four independent kinetic runs for each experimental condition (data are given in the Tables 1S in Electronic supplementary material). Rate constants were calculated using the computer programs Microsoft Excel and Origin 6.1.
1H NMR measurements
1H NMR kinetic experiment of [{trans-Pt(NH3)2Cl}2(μ-1,2-bis(4-pyridyl)ethane)](ClO4)2 (Pt3) complex with 5′-GMP was studied on a freshly prepared sample of reactants. A 10-mM solution of the complex and 20-mM solution of the ligand were prepared in 300 μL of D2O, 10 min prior to the start of the kinetic experiment. The solution of the ligand was added to the solution of the complex to initiate the reaction. NMR spectra were recorded at 22 °C, over a period of several days, until completion of the reaction was reached. No buffer was used to prevent increased activation of the complexes due to coordination of phosphate, or interfering signals in the observed peak area. The pH*(pD = pH + 0.4) changed from 7.2 to 6.7 during the reaction. All chemical shifts are referenced to TSP (trimethylsilylpropionic acid). The 1H NMR measurements were recorded on a Varian Gemini-200 spectrometer. Acquisition parameters for the 1H experiments were pulse sequences S2 pul length 21.0 degree, relaxation delay 1.0 s, acquisition time 4.0 s, 56 repetitions, and sweep width 3000.3 Hz.
Cytotoxicity assay (MTT test)
Cell viability was assessed by the MTT assay. Cells were harvested from the culture during the exponential growth phase and seeded into 96-well culture plates at 5 × 105 cells/mL (TOV-21G and HCT-116) and 1 × 106 cells/mL (MSC) in fresh medium, 100 μL/well. After a 24 h time period, cells were treated with selected concentrations of complexes for 3 days. Control wells were prepared by addition of culture medium. Wells containing culture medium without cells were used as blanks. After incubation, drug-containing medium was discarded and replaced with serum-free medium containing 15 % of MTT (5 mg/mL) dye. After additional 4 h of incubation at 37 °C in a 5 % CO2 incubator, medium with MTT was removed and DMSO (150 μL) with glycine buffer (20 μL) was added to dissolve blue formazan crystals. The plates were shaken for 10 min. The optical density of each well was determined at 595 nm using microplate multimode detector Zenyth 3100. The percentage of cytotoxicity was calculated using the formula: % of viable cells = ((TS − BG0) − E/(TS − BG0) × 100), where BG0 is for background of medium alone, TS is for total viability/spontaneous death of untreated target cells, and E is for the experimental well.
Results and discussion
Reactions of [PtCl4(bipy)] complex with 5′-GMP
The substitution reactions of Pt(IV) complexes with the biologically relevant molecule 5′-GMP have been frequently studied [22, 25–29]. It is well known that 5′-GMP can coordinate to metal ions through N1 and N7 atoms, depending on pH of the solution. At pH = 7.2, N1 is protonated (pKa = 9.3 [30–34]), and coordination of 5′-GMP to Pt(IV) and Pt(II) complexes is via N7 of the purine base. Previously, for the reactions of a few Pt(IV) complexes with 5′-GMP two reaction steps were found, one slow which was substitution and the fast one which was reduction. The final reaction product was a substituted complex of Pt(II) [22]. The suggested mechanism for the reaction of the [PtCl4(bipy)] complex with 5′-GMP could be the same (Scheme 1). The substitution reaction of one chloride ligand in Pt(IV) complex with nucleophile is characterized by a second-order rate constant (k 2).
The observed pseudo-first-order rate constants, k obsd as a function of the total concentration of nucleophile could be described by Eq. 1. The rate constant for the reduction reaction was not determined.
The obtained value of rate constant k 2 for substitution of one chloride in [PtCl4(bipy)] complex with 5′-GMP, at 37 °C in 25 mM Hepes buffer (pH = 7.2) with addition of 10 mM NaCl, is (5.9 ± 0.3) × 10−2 M−1 s−1 while the value of k 1 is (8.4 ± 2.0) × 10−5 s−1. The observed small intercept (k 1) is ascribed to the back reaction with the excess chloride present in solution to prevent the spontaneous hydrolysis of the complex.
If we compare the reactivity of the investigated complex with already published results, it could be seen that the order of reactivity of Pt(IV) complexes toward the biologically relevant nucleophile 5′-GMP is [PtCl4(bipy)] > [PtCl4(en)] > [PtCl4(dach)] [22]. The substitution behavior for [PtCl4(bipy)] depends on the π–acceptor properties of the inert ligand 2,2′-bipyridyl. As a result of these properties, there is an increase in electrophilicity of the platinum center and reactivity toward entering nucleophile (5′-GMP). The slightly greater reactivity of [PtCl4(en)] complex than [PtCl4(dach)] could be explained by comparing the structures of inert ligands ethylenediamine and trans-1,2-diaminocyclohexane [22]. A major influence on the reactivity of [PtCl4(dach)] is steric hindrance, while in the case of [PtCl4(bipy)] the most important influence arises from electronic effects.
Reactions of [{trans-Pt(NH3)2Cl}2(μ-1,2-bis(4-pyridyl)ethane)](ClO4)2 (Pt3) complex with 5′-GMP
1H NMR spectroscopy was used to investigate the substitution reactions of dinuclear Pt(II) complex [{trans-Pt(NH3)2Cl}2(μ-1,2-bis(4-pyridyl)ethane)](ClO4)2 (Pt3) with 5′-GMP in aqueous solution at pD 6.7 and 22 °C. The substitution kinetics was studied under second-order conditions, with an initial molar ratio of 1:2 (complex:5′-GMP). Considering that the initial concentrations of 5′-GMP and the complex, c a0 and c b0, respectively, can be expressed by c a0 ≠ c b0, we applied Eq. 2 for the first reaction step in order to determine k. The concentration of the 1:1 product, that is, singly substituted complex, is represented by x. The concentration x is calculated considering the area of the signals for free 5′-GMP and coordinated 5′-GMP (estimated error is 5 %, Electronic supplementary material, Table 2S).
The integral of the singlet of the H8 proton in 5′-GMP was unavailable to measure because there was a multiplicity of product signals, and we therefore used the intensity of the doublet of the H1′ proton of 5′-GMP (see Fig. 3). The intensity of the signal for the free 5′-GMP at 5.90 ppm decreases during the reaction, whereas at the same time a signal for coordinated 5′-GMP appears around 6.05 ppm and increases with time (Fig. 3). A plot of the right side of Eq. 2 versus reaction time resulted in a straight line passing through the origin (see Fig. 4). The value of the second-order rate constant k, when one 5′-GMP molecule is coordinated, was obtained from the slope and is (5.7 ± 0.1) × 10−3 M−1 s−1.
The value for the rate constant for the second substitution process (k 2) could be determined from Eq. 3. A plot of 1/c a versus time gives a straight line, from which the value for the rate constant k 2 results from the slope and the intercept is 1/c a0 (see Fig. 4). We obtained very similar values for the rate constants of both substitution steps. For the second substitution step, the obtained value of k 2 is (4.60 ± 0.02) × 10−3 M−1 s−1
The value of k, obtained for the substitution of the first chloride by 5′-GMP investigated by 1H NMR spectroscopy, is smaller than the value obtained from UV–Vis spectrophotometry [24]. The difference between the rate constants is a consequence of temperature effects (37 °C during UV–Vis measurements) and also of slightly different pH compared to pH*(pD = pH + 0.4). The pH* changed from 7.2 to 6.7 during the reaction [24]. The rate constant k 2 is the same order of magnitude as the value obtained for the second substitution step, investigated by UV–Vis spectrophotometry [24]. The two Pt(II) centers in [{trans-Pt(NH3)2Cl}2(μ-1,2-bis(4-pyridyl)ethane)](ClO4)2 (Pt3) complex act completely independent of each other and show the same thermodynamic and kinetic properties. The obtained values are in agreement with literature data for the related dinuclear platinum(II) complexes [35].
Cytotoxic activity of platinum complexes
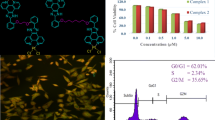
We compared the cytotoxic capacity of these platinum complexes toward TOV21G, HCT116 tumor human cell lines, and human MSC, normally dividing cells rapidly (Fig. 5).
All the complexes displayed a dose-dependent and time-dependent cytotoxicity toward the tested cell lines, but the highest cytotoxic effect was shown toward TOV21G cells (Fig. 5). The complex [PtCl4(dach)] at lower concentrations induced significantly higher cytotoxic effect toward TOV21G cells than the other four complexes.
HCT116 cells were more resistant to cytotoxic effects of the selected complexes (Fig. 5). Again, [PtCl4(dach)] was the most efficient and exerted very similar activity toward HCT116 cells as cisplatin.
The complexes Pt2 and Pt3 displayed cytotoxicity toward MSC similar to cisplatin, but the other three complexes Pt1, [PtCl4(dach)], and [PtCl4(bipy)] were more toxic.
The cytotoxicities of a series of Pt(IV) complexes such as [PtCl2(OH)2(en)], [PtCl2(OH)2(ipa)], [PtCl2(OCOCH3)2(en)], [PtCl4(en)], and [PtCl4(dach)] were studied toward L1210/0 cell line. The highest cytotoxicity toward this cell line is shown by [PtCl4(dach)], and it decreases in the order: [PtCl4(dach)] > [PtCl4(en)] > [PtCl2(OCOCH3)2(en)] > [PtCl2(OH)2(ipa)] > [PtCl2(OH)2(en)] [27]. Satraplatin (JM-216), which is structurally similar to our investigated Pt(IV) complexes, shows a high cytotoxicity toward ovarium carcinoma cell line [36]. The cytotoxicity of our [PtCl4(dach)] complex is very similar to the cytotoxicities of previously investigated Pt(IV) complexes [27].
The dinuclear azine-bridged Pt(II) complexes, for example, [{cis-Pt(NH3)2Cl}2(μ-pyrazine)](NO3)2, [{cis-Pt(NH3)2Cl}2(μ-pyridazine)](NO3)2 show significant cytotoxicity for the ovarium carcinoma cell line and a lower cytotoxicity toward colon cancer cell line, compared to cisplatin [23].
A new chiral ligand, 2-(((1R,2R)-2-aminocyclohexyl)amino)acetic acid (HL), was designed and synthesized to prepare a series of novel dinuclear Pt(II) complexes with dicarboxylates or sulfate as bridges. All compounds showed antitumor activity to HCT116 very close to the activity of oxaliplatin and better than our the dinuclear Pt(II) complexes [36]. Also, dinuclear Pt(II) complex, {[cis-Pt{NH3)2Cl]2(μ-4,4′-methylendianiline)}(NO3)2, is highly cytotoxic against the murine leukemia (P-388) and the human non-small-cell lung cancer (A-549) cell lines, and it is more cytotoxic than cisplatin at most concentrations tested [37].
Conclusion
The substitution reaction of the [PtCl4(dach)] complex with 5′-GMP is a slow process and followed by fast reduction of Pt(IV) to Pt(II) complexes. The obtained rate constant, k 2, is comparable with those obtained earlier with similar Pt(IV) complexes [22].
However, the substitution reactions of the investigated dinuclear [{trans-Pt(NH3)2Cl}2(μ-1,2-bis(4-pyridyl)ethane)](ClO4)2 (Pt3) complex with 5′-GMP proceeds via two reaction steps. The first step is substitution of one chloride by 5′-GMP (5.7 ± 0.1) × 10−3 M−1 s−1, while the second step is substitution of another one (4.60 ± 0.02) × 10−3 M−1 s−1. Both rate constants are very similar.
All tested complexes displayed cytotoxic activity against TOV21G cell line, with lower activity toward HCT116 at the same concentrations. The complex [PtCl4(dach)] at lower concentrations induced significantly higher cytotoxic effect toward TOV21G cells than the other four complexes.
These results could contribute to better understanding of the interactions of Pt(IV) complexes with some biologically important nucleophiles and their antitumor activity.
References
Rosenberg B, van Camp L, Krigas T (1965) Nature 205:698–699
Rosenberg B, van Camp L, Trosko JE, Mansour VH (1969) Nature 222:385–386
Ash DC (1980) J Clin Hematol Oncol 10:55–62
Chu GJ (1994) Biol Chem 269:787–790
Lippert B (1999) Cisplatin. Chemistry and biochemistry of a leading anticancer drug. Wiley-VCH, New York
Jamieson ER, Lippard SJ (1999) Chem Rev 99:2467–2498
Reedijk J (1999) Chem Rev 99:2499–2510
Wong E, Giandomenico CM (1999) Chem Rev 99:2451–2466
Von Hof DD, Schilsky R, Reichert CM, Reddick RL, Rozencweig M, Young RC, Muggia FM (1979) Cancer Treat Rep 63:1527–1531
Arendse MJ, Anderson GK, Majola RN, Rath NP (2002) Inorg Chim Acta 340:65–69
Jakubec MA, Galanski M, Arion VB, Hartinger CG, Keppler BK (2008) Dalton Trans 183–194
Jung Y, Lippard SJ (2007) Chem Rev 107:1387–1407
Talman EG, Kidani Y, Mohrmann L, Reedijk J (1998) Inorg Chim Acta 283:251–255
Lemma K, House DA, Retta N, Elding LI (2002) Inorg Chim Acta 331:98–108
Esposito BP, Najjar R (2002) Coord Chem Rev 232:137–149
Farrell N (1995) Comments Inorg Chem 16:373–389
Reedijk J (2003) Proc Natl Acad Sci USA 100:3611–3616
McGregor TD, Balcarova Z, Qu Y, Tran M.-C, Zaludova R, Brabec V, Farrell N (1999) J Inorg Biochem 77:43–46
Kraker AJ, Hoeschele JD, Elliott WL, Showalter HDH, Sercel AD, Farrell NP (1992) J Med Chem 35:4526–4532
Perego P, Caserini C, Gatti L, Carenini N, Romanelli S, Supino R, Colangelo D, Viano I, Leone R, Spinelli S, Pezzoni G, Manzotti C, Farrell N, Zunino F (1999) Mol Pharmacol 55:528–534
Farrell N (2004) In: Sigel A, Sigel H (eds) Metal ions in biological systems, New York. Basel Marcel Dekker Inc, New York, pp 251–296
Jovanović S, Petrović B, Bugarčić ŽD (2010) J Coord Chem 63:2419–2430
Komeda S, Kalayda GV, Lutz M, Spek AL, Yamanaka Y, Sato T, Chikuma M, Reedijk J (2003) J Med Chem 46:1210–1219
Soldatović T, Jovanović S, Bugarčić ŽD, van Eldik R (2012) Dalton Trans 41:876–884
Choi S, Mahalingaiah S, Delaney S, Neale NR, Masood S (1999) Inorg Chem 38:800–1805
Choi S, Vastag L, Larrabee JC, Personick ML, Schaberg KB, Fowler BJ, Sandwick RK, Rawji G (2008) Inorg Chem 47:1352–1360
Choi S, Filotto C, Bisanzo M, Delaney S, Lagasee D, Whitworth JL, Jusko A, Li C, Wood NA, Willingham J, Schwenker A, Spaulding K (1998) Inorg Chem 37:2500–2504
Choi S, Cooley RB, Voutchkova A, Leung CH, Vastag L, Knowles DE (2005) J Am Chem Soc 127:1773–1781
Choi S, Vastag L, Leung CH, Beard AM, Knowles DE, Larrabee JA (2006) Inorg Chem 45:10108–10114
Summa N, Schiessl W, Puchta R, Eikema Hommes N, van Eldik R (2006) Inorg Chem 45:2948–2959
Arpalahti J, Lehikoinen P (1990) Inorg Chem 29:2564–2567
Arpalahti J, Lippert B (1990) Inorg Chem 29:104–110
Martin RB (1999) In: Lippert B (ed) Cisplatin, chemistry and biochemistry of leading antitumor drugs. Wiley-VCH, Zurich, pp 193–200
Shourky M, van Eldik R (1996) J Chem Soc Dalton Trans 2673–2678
Ertürk H, Maigut J, Puchta R, van Eldik R (2008) Dalton Trans 2759–2766
Boulikas T, Pantos A (2007) Cancer Ther 5:537–583
Damin F, Xiaoliang Y, Xiaoyong W, Shouchun Z, Jiafei M, Jian D, Liping L, Zijian G (2007) J Biol Inorg Chem 12:655–665
Acknowledgments
The authors gratefully acknowledge financial support from the Ministry of Science and Technological Development, Republic of Serbia (Project No.172011 and No.175069).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arsenijević, M., Milovanović, M., Volarević, V. et al. Cytotoxic properties of platinum(IV) and dinuclear platinum(II) complexes and their ligand substitution reactions with guanosine-5′-monophosphate. Transition Met Chem 37, 481–488 (2012). https://doi.org/10.1007/s11243-012-9613-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-012-9613-4