Abstract
Two thiocyanato-Cu(II) complexes including mononuclear dithiocyanato Cu(Me3dpt)(NCS)2 (1) and the polymeric 1D [Cu(d,l-Ala)(μN,S–NCS)(H2O)] n (2) were synthesized and structurally characterized (Me3dpt = bis(N-methyl-3-propyl)methylamine, Ala = alaninate anion). The IR spectrum of complex 1 confirmed the N-bonding coordination mode of the thiocyanate groups, and its visible spectrum revealed the square pyramidal geometry around the central Cu2+ ion. Single X-ray crystallography of 1 showed that the Cu(II) center displays square pyramidal geometry with severe distortion toward trigonal bipyramidal environment. Complex 2 forms a 1-D polymeric chain with the NCS− acting as a μN,S-ligand. A distorted SP geometry around the Cu2+ centers was achieved by the O and N atoms of alaninato anion, the aqua ligand and by the N and S atoms of the bridging thiocyanate groups. Hydrogen bonds of the type N–H···O, N–H···S and O–H···O are formed in this complex leading to the extension of the 1D chain to a supramolecular network.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The thiocyanate ion NCS− is an ambidentate ligand that has been used as a typical example for linkage isomerism in coordination chemistry. Both its N and S atoms are considered to be potentially donor sites. According to Pearson’s classification for HSAB, the SCN− ion is classified as a soft base when it binds the metal ion from the S-side and as an intermediate Lewis base when it acts as N-bound. On the other hand, Cu2+ behaves as an intermediate Lewis acid [1]. Therefore, based on this classification, Cu2+ ion is expected to bind the two sides of the SCN− ion but with higher tendency to bind to the nitrogen.
The thiocyanate anion is not biologically important but it has been pointed out that it can compete with the toxic O −2 species for binding to SOD (Cu–Zn superoxide dismutase) [2]. The interaction of Cu2+ compounds with SCN− leads to the formation of many mononuclear N-bonding complexes [3–6] as well as polynuclear species with the SCN− ligand bridging Cu2+ centers in a variety of coordination modes [7–17]. These include μN,S–NCS [7–15], μN,N-NCS [16], and μS,S-NCS [17] modes. The majority of the bridging thiocyanato complexes were obtained with bi- and tridentate coligands in the Cu skeleton.
In general, five-coordinate Cu(II) species with an intermediate stereochemical environment ranging between trigonal bipyramidal (TBP) and square pyramidal (SP) were found to be the major species that are formed in solid and in solution states [3, 4, 18–25]. The nature of the coligand around the central Cu2+ ion plays a crucial role in adopting one of the two geometries. For example, sterically hindered linear aliphatic tridendate- and tetradentate-amines (Et3dien, pmedien, EtMe4dien, Me6trien),Footnote 1 and tripodal N donors tetraamine ligands (Me3tren, TPA)1 that can form five-membered chelate rings with Cu2+ ion, most likely constitute distorted TBP geometry [3, 4, 21]. Replacing the methylenic group(s) in TPA by ethylenic group(s) (pmea, pmap, tepa) tends to stabilize SP geometries in the Cu(II) complexes [22–25]. Partial increase in the steric environment of the coligand (Medpt, Et2dien) and/or increasing the length of N donor amines around the central Cu2+ ion causes severe distortion from SP to TBP [7, 8].
The following study was undertaken to investigate the molecular structures of the thiocyanato-Cu(II) complexes derived from the tridentate amine Me3dpt and from alanine. This will allow us to shed light on the bonding mode of the coordinated thiocyanate ion (N-bonding vs. S-bonding) and the dominating geometrical feature around the central copper (SP vs. TBP) in this class of compounds.
Experimental
Materials and physical measurements
N,N′,N″-Trimethylpropylenetriamine (Me3dpt) was purchased from Aldrich. All other materials were reagent grade quality. Infrared spectra were recorded on a JASCO FT/IR-480 plus spectrometer as KBr pellets. Electronic spectra were recorded on an Agilent 8453 HP diode UV–vis spectrophotometer. Thermal decomposition of the alaninato complex 2 was measured at a heating rate of 10 °C/min using a Perkin-Elmer TGA7 thermogravimetric analyzer with aluminum pan in a dynamic atmosphere of nitrogen. Elemental analyses were carried out by the Atlantic Microlaboratory, Norcross, Georgia USA.
Caution: Salts of perchlorate as well as their metal complexes are potentially explosive and should be handled with great care and in small quantities.
Synthesis of Cu(Me3dpt)(NCS)2 (1). To a warm methanolic solution containing the Me3dpt ligand (0.50 mmol) and Cu(ClO4)2·6H2O (0.186 g, 0.50 mmol) in 20 mL MeOH, ammonium thiocyanate (0.076 g, 1.0 mmol) was added. The resulting solution was heated for 5 min on a steam-bath, filtered through Celite and then allowed to crystallize at room temperature. The resulting precipitate that separated within 2 days was collected by filtration, washed with Et2O, and air dried (overall yield 80%). Single crystals suitable for X-ray analysis were obtained upon re-crystallization of the complexes from H2O. Characterization: Found: C, 37.2; H, 6.7; N, 19.6%. Calcd for C11H23N5CuS2 (MM = 353.03): C, 37.4; H, 6.6; N, 19.8%. IR (KBr, cm−1): 2074 (vs), UV–vis. {λmax, nm (εmax, M−1 cm−1)} in CH3CN: 686 (204) and 932 (141).
Synthesis of [Cu(d,l-Ala)(SCN)(H2O)] n (2). To an aqueous solution (40 mL H2O) containing CuSO4·5H2O (2.0 g, 8.0 mmol) and d,l-alanine (1.0 g. 16 mmol), KSCN (0.4 g, 4.0 mmol) dissolved in 10 mL of H2O was added dropwise, and the resulting green solution was filtered and kept in the refrigerator. The green crystals which separated after several days were collected by filtration and air dried (overall yield: 60%). Characterization: Found: C, 20.9; H, 3.6, N 12.2%. Calcd for C4H8CuN2O3S (MM = 227.72 g): C, 21.1; H, 3.5; N, 12.3%. IR (KBr, cm−1): 3500 (br), 2170 (m), 2108 (vs), 1586 (s), 1391 (m).UV–vis. (λmax): ~650 nm (very broad).
X-ray crystal structure analysis
The X-ray single-crystal data of the two compounds were collected on a Bruker-AXS SMART APEX II CCD diffractometer at 100(2) K. The crystallographic data, conditions retained for the intensity data collection, and some features of the structure refinements are listed in Table 1. The intensities were collected with Mo-Kα radiation (λ = 0.71073 Å). Data processing, Lorentz-polarization, and absorption corrections were performed using SAINT, APEX, and the SADABS computer programs [26–28]. The structures were solved by direct methods and refined by full-matrix least-squares methods on F 2, using the SHELXTL [29] program package. All non-hydrogen atoms were refined anisotropically. The hydrogen atoms were located from difference Fourier maps assigned with isotropic displacement factors and included in the final refinement cycles by use of geometrical constraints.
Results and discussion
Synthesis of the complexes
The monomeric Cu-dithiocyanato complex Cu(Me3dpt)(NCS)2 (1) and the bridging thiocyanato polymeric 1D complex [Cu(d,l-Ala)(NCS)(H2O)] n (2) were synthesized in moderate yields. The two complexes were obtained in a straight forward manner by the reaction of a methanolic solution of Cu(ClO4)2·6H2O or an aqueous CuSO4·5H2O solution and the appropriate ligand in 1:1 M ratio with NCS− anion. The isolated complexes were characterized by elemental analyses, IR and UV–vis. spectroscopy as well as by single-crystal X-ray crystallography. The complexes are soluble in acetonitrile, methanol, and DMSO. Also, for comparison, the complex Cu(Mepea)(NCS)2 that was structurally characterized [30] was synthesized using a similar procedure as that described for 1.
Infrared spectra
The IR spectra of the complexes under investigation exhibit very strong bands over the 2,070–2,110 cm−1 region due to the asymmetric stretching vibration of the coordinated thiocyanate group, νas(C≡N) (Table 2). In this class of compounds, it has been indicated that the νas(C≡N) frequencies could be used as criteria to differentiate between S-bonded (2,110–2,140 cm−1) and N-bonded (<2,110 cm−1) complexes [3, 4, 31–33]. Thus, the observed νas(C≡N) frequency of the mononuclear Cu(II) complex 1 is most likely consistent with N-bonded thiocyanate that was also confirmed by X-ray. The stretching frequencies of νas(NCS−) of the alaninato complex 3 are located within the same range reported in 1D polymeric bridged end-to-end thiocyanato complexes derived from glycine and from N,N-dimethylamino ethanol (νas(NCS−) = 2,100 cm−1) [36, 37], and in the doubly bridging dinuclear complexes [Cu2(Medpt)2(μN,S-NCS)2](ClO4)2 and [Cu2(Et2dien)2(μN,S-NCS)2](ClO4)2 [7, 8] (see Table 2). Although one would expect the split of νas(NCS−) around 2,100 cm−1 in complexes bridged by thiocyanate into two absorption bands due to the stretching frequency of S- and N-bonded thiocyanate groups, this was not always the case, and a split was also observed in mononuclear-coordinated thiocyanato complexes 1 and [Cu(TPA)(NCS)]ClO4 [4]. This analysis clearly indicates the difficulty of reaching a solid conclusion concerning the bonding coordination mode in the thiocyanato-Cu(II) complexes based on the νas(NCS−) stretching frequency.
The IR spectrum of [Cu(d,l-Ala)(μN,S-NCS)(H2O)] n shows a broad band around the 3,500 cm−1 region due to ν(O–H) of the water molecule. The stretching absorption bands located at 1,586 and 1,391 cm−1 were assigned to νas(COO−) and ν s (COO−), respectively, of the alanine ligand. The splitting Δν(COO−) between the two carboxylate bands (Δ = 195 cm−1) was found to be comparable to that observed in [Cu(Ala)2·H2O] in which the monodentate bonding of the carboxylate group was confirmed [36].
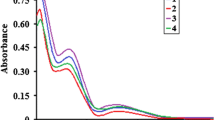
Visible spectra
The visible spectral data for the Cu(II)-thiocyanato complexes in acetonitrile are collected in Table 2 together with other related systems. The complexes display a broad absorption band in the 590–700 nm region. This spectral feature is consistent with five-coordinate Cu(II) complexes that often may be associated with a low-, or high-energy shoulder. In many cases, the low-energy shoulder indicates a square pyramidal (SP) geometry, whereas the presence of a single d–d band at λ > 800 nm (dxy, dx 2 − y 2 → dz 2) with a high-energy shoulder (spin forbidden, dxz, dyz → dz 2) is typical for trigonal bipyramidal (TBP) stereochemistry [37]. Therefore, based on the above criterion, the electronic spectral data of the complexes under investigation are consistent with SP geometry. The SP stereochemical configuration seems to be the predominant geometry in most of the Cu(II) complexes. However, it has been observed that TBP geometry was adopted by tripodal tetraamine-Cu(II) complexes that form five-membered chelate rings with Cu(II) ion [4, 7, 18, 20–25]. Increasing the steric hindrance on the coordinated linear tridentate amine in the five- or six-membered chelate rings tends to cause a severe distortion from SP to TBP geometry. This is clearly demonstrated in Cu(Me3dpt)(NCS)2 (1), [Cu2(Medpt)2(μN,S-NCS)2](ClO4)2, and [Cu2(Et2dien)2(μN,S-NCS)2](ClO4)2 [7, 8]. In most cases, the complexes may exhibit intermediate geometries that are slightly distorted from the ideal SP or TBP. The geometrical SP finding about the Cu(II) ion in solution is consistent with the X-ray structural data.
Thermal analysis of complex 2
The thermal analysis of complex 2 was investigated in order to provide insight into the coordinated water molecule. The first step of the thermogravimetric analysis corresponds to dehydration at 148 °C (mass loss: Calcd = 7.9%, Found = 8.1%). The DTA profile shows this as an endothermic step at T max = 148 °C. The following decomposition step starts just above 150 °C and completed around 300 °C. This step corresponds to the loss of alanine ligand with T max = 209 °C (mass loss: Calcd. = 39.2%, Found = 40.3%). Further heating led to reductive degradation of Cu(II) → Cu(I), which resulted in the formation of the polymeric species [Cu(SCN)] n at about 330 °C. This process was followed by another weight loss of 15.4% over the temperature range 350–525 °C (T max = 377 °C), which may indicate the release of sulfur (Calcd. = 14.1%) with the formation of another polymeric residue [Cu(CN)] n . Similar thermal decomposition behavior was reported with [Cu(Gly)(SCN)(H2O)] n [34].
Description of the structures
Cu(Me3dpt)(NCS)2 (1): The asymmetric unit of 1 consists of two crystallographic-independent Cu(Me3dpt)(NCS)2 monomers. Perspective views together with the atom numbering scheme are presented in Fig. 1a, b, respectively, and selected bond parameters are given in Table 3. Each copper center is penta-coordinated by the three N donor atoms of the amine ligand and two N atoms of the terminal thiocyanato ligands. The coordination polyhedron may be described as distorted square pyramid with τ = 0.37 (Cu1), and 0.49 (Cu2) [38]. The apical sites in CuN5 polyhedra are occupied by central N atoms of the Me3dpt ligands [Cu(1)-N(8) = 2.190(3); Cu(2)-N(4) = 2.203(3) Å]. The basal Cu–N bonds vary from 1.994(3) to 2.026(3) Å for Cu(1), and from 1.992(3) to 2.046(3) Å for Cu(2). The metal centers deviate from their CuN4 basal planes by 0.289 and 0.315 Å for Cu(1) and Cu(2), respectively. The bond parameters of the thiocyanato ligands are in the following ranges: N–C: from 1.157(5) to 1.166(5) Å; C–S: from 1.625(4) to 1.640(4) Å; Cu–N–C: from 164.4(3) to 178.2(4)°, and N–C–S: from 178.8(3) to 179.2(3)°.
In 1, the non-coordinated S atoms act as acceptors for hydrogen bonds of type N–H···S to form a supramolecular 2D system oriented along the [1 0 0] and [0 1 1] directions (Table 4; Fig. 1c).
[Cu(d,l-Ala)(μN,S–NCS)(H2O)] n (2). The principal structural features of complex 2 are illustrated in Fig. 2a, and selected bond parameters are summarized in Table 3. The copper center of [Cu(Ala)(NCS)(H2O)] n complex is penta-coordinated by O(1) and N(1) of alaninato anion, by S(1) and N(2A) of μ-N,S-bridging thiocyanato anion, and O(3) of aqua ligand, resulting in a CuN2O2S chromophore. The coordination geometry may be described as distorted square pyramid (τ = 0.154) with S(1) in the apical site. [Cu(1)–S(1) = 2.8071(13) Å]. The alaninato anion acts as a bidentate N, O-chelating ligand to form a five-membered ring around each copper center with Cu(1)–N(1) = 2.004(3) and Cu(1)–O(1) = 1.956(3) Å. The Cu(1)–O(3) bond length of the aqua ligand is 1.994(3) Å. The μ-N,S-bridging thiocyanates form 1D chains of polyhedra oriented along the a-axis of the triclinic unit cell: [Cu(1)–S(1) = 2.8071(13), Cu(1B)–N(2) = 1.946(3) Å, Cu(1)–S(1)–C(4) = 95.49(14), C(4)–N(2)–Cu(1B) = 162.9(3); N(2)–C(4)–S(1) = 178.1(4)°]. The Cu···Cu intrachain separation is 5.5929(13) Å, and the shortest Cu···Cu interchain distance is 3.6527(9) Å. Hydrogen bonds of type N–H···O and N–H···S are formed from the N(1) donor atom to acceptors O(2A) and S(1C), respectively. Further hydrogen bonds of type O–H···O are observed between aqua ligand O(3) and oxygen atoms of carboxylato groups of adjacent alaninato ligands (Fig. 2a; Table 4). These hydrogen bonds extend the 1D chains of polyhedra to a supramolecular network (Fig. 2b).
a Perspective view of [Cu(d,l-Ala)(μN,S-NCS)(H2O)] n (2) together with the atom numbering scheme. b Crystal packing view. Hydrogen bonds are indicated by broken bonds. Symmetry codes: (A) x + 1, y, z; (B) x − 1, y, z; (C) –x + 1, −y + 2, −z + 1; (D) −x, −y + 2, −z; (E) x + 1, y + 1, z; (F) x + 2, y, z
Conclusions
The Cu(II)-thiocyanato complex 1 derived from the linear tridentate amine ligands Me3dpt and Mepea [30] clearly indicates that increasing the size of the chelate rings to six tends to increase the distortion toward a TBP environment. With bidentate and less hindered linear tridentate coligands, the possibility of isolating polynuclear Cu(II) complexes with bridging NCS− is high. This was the case with [Cu(d,l-Ala)(NCS)(H2O)] n (2), and this finding is in complete agreement with previous results for complexes derived from Gly, Medpt, and Et2dien [7, 8, 34]. In general, increasing the steric hindrance at the terminal coordinated N donor atoms of the tridentate amines by incorporation of alkyl group(s) and/or heterocyclic N-ring(s) most likely suppresses the formation of bridging thiocyanato-Cu(II) complexes. The molecular dimensions and geometries of the synthesized complexes can be controlled by careful selection of the metal coligand moiety. Also, in mononuclear Cu(II)-thiocyanato complexes, the Cu2+ ion favors the N-bound coordination of the SCN− over the S-bound.
Notes
Ligand abbreviations: Me3dpt, bis(N-methyl-3-propyl)methylamine; Medpt, bis(3-aminopropyl)- methylamine; Mepea, N-(2-pyridylmethyl)-N-[2-(2-pyridylethyl)]methylamine; TPA, tris(2-pyridyl-methyl)amine; Me3tren, tris(N-methyl-2-aminoethyl)amine; Me6tren, tris(N,N-dimethyl-2-aminoethyl)-amine; Me6trien, 1,1,4,7,10,10-hexamethyltriethylenetetraamine; pmap, bis[2-(2-pyridylethyl)]-(2-pyridylmethyl)amine; pmea, bis(2-pyridylmethyl)-2-(2-pyridylethyl)amine; tepa, tris[2-ethyl-(2-pyridyl)]-amine; Et3dien,N,N′,N″-triethyldiethylenetriamine; Et2dien, N,N-diethyldiethylenetriamine; Me5dien, N,N,N′,N″,N″-pentamethyldiethylenetriamine; EtMe4dien, N′-ethyl-N,N,N″,N″-tetramethyldiethylene- triamine; pdpa, N-(2-aminopropyl)-N,N-bis(2-pyridylmethyl)amine; pzdepy, N,N′-bis[2-(2-pyridylethyl)]-piperazine; Ala, alaninate ion; Gly, glycinate ion.
References
Housecroft CE, Sharpe AG (2008) Inorganic chemistry, 3rd edn. Prentice Hall, Harlow, p 207
Bertini I, Banci L, Piccioli M, Luchinat C (1990) Coord Chem Rev 100:67
Mautner FA, Louka FR, LeGuet T, Massoud SS (2009) J Mol Struct 919:196
Mukhopadhyay U, Bernal I, Massoud SS, Mautner FA (2004) Inorg Chim Acta 357:3673
Lee Y-M, Kim B-J, Kim H-J, Kim S-Y, Kang SK, Choi S-N (2009) Polyhedron 28:3060
Wang Z-D, Han W, Bian F, Liu Z-Q, Yan S-P, Liao D-Z, Jiang Z-H, Cheng P (2005) J Mol Struct 733:125
Mautner FA, Vicente R, Massoud SS (2006) Polyhedron 25:1673
Massoud SS, Mautner FA (2005) Inorg Chim Acta 358:3334
Adhikary C, Mal D, Okamoto K-I, Chaudhuri S, Koner S (2006) Inorg Chim Acta 25:2192
Sarkar S, Mondal A, Ribas J, Drew MGB, Pramanik K, Rajak KK (2005) Inorg Chem Acta 358:641
Plieger PG, Downard AJ, Moubaraki B, Murray KS, Brooker S (2004) J Chem Soc Dalton Trans 2157
Banerjee S, Drew MGB, Lu C-Z, Tercero J, Diaz C, Ghosh A (2005) Eur J Inorg Chem 2376
Talukder P, Datta A, Mitra S, Rosair G, El-Fallah MS, Ribas J (2004) J Chem Soc Dalton Trans 4161
Gòmez-Saiz P, García-Tojal J, Arníiz FJ, Maestro MA, Lezama L, Rojo T (2003) Inorg Chem Commun 6:558
Karan NK, Mitra S, Matsushita T, Gramlich V, Rosair G (2002) Inorg Chim Acta 332:87
You Z-L, Zhu H-L (2004) Z Anorg Allg Chem 630:2754
Depree CV, Beckmann U, Heslop K, Brooker S (2003) J Chem Soc, Dalton Trans 3071
Escuer A, Font-Bardia M, Massoud SS, Mautner FA, Peñalba E, Solans X, Vicente R (2004) New J Chem 28:681
Saha S, Koner S, Tuchagues J-P, Boudalis AK, Okamoto K-I, Banerjee S, Mal D (2005) Inorg Chem 44:6379
Mautner FA, Soileau JB, Bankole PK, Gallo AA, Massoud SS (2008) J Mol Struct 889:271
Thaler F, Hubbard CD, Heinemann FW, van Eldik R, Fâbián I, Dittler-Klingermann AM (1998) Inorg Chem 37:4022
Mautner FA, Landry CN, Massoud SS, Gallo AA (2007) J Mol Struct 837:72
Schatz M, Becker M, Thaler F, Hampel F, Schindler S, Jacobson RR, Tyeklár Z, Murthy NN, Ghosh P, Chen Q, Zubieta J, Karlin KD (2001) Inorg Chem 49:2312
Vicente R, Ruiz E, Cano J, Massoud SS, Mautner FA (2008) Inorg Chem 47:4648
Massoud SS, Mautner FA, Vicente R, Louka FR (2008) Eur J Inorg Chem 3709
Bruker (2005) SAINT v. 7.23. Bruker AXS Inc., Madison, Wisconsin
Bruker (2006) APEX 2,v. 2.0-2. Bruker AXS Inc., Madison, Wisconsin
Sheldrick GM (2001) SADABS v. 2. University of Goettingen, Germany
Sheldrick GM (2008) Acta Crystallogr A64:112
Astner J, Weitzer M, Foxon SP, Schindler S, Heinemann FW, Mukherjee J, Gupta R, Mahadevan V, Mukherjee R (2008) Inorg Chim Acta 361:279
Nakamoto K (1986) Infrared and raman spectra of inorganic and coordination compounds, 4th edn. Wiley, New York
Nakamoto K (1997) Infrared and Raman spectra of inorganic and coordination compounds. Part B, 5th edn. Wiley, New York
Youngme S, Chaichit N, Pakawatchai C, Booncoon S (2002) Polyhedron 21:1279
Goher MAS, Al-Shatti LA, Mautner FA (1997) Polyhedron 16:889
Song Y, Zhu D-R, Zhang K-L, Xu Y, Duan C-Y, Yo X-Z (2000) Polyhedron 19:1461
Calvo R, Levestein PR, Castellano EE, Fabiane SM, Piero OE, Oseroff B (1991) Inorg Chem 30:216
Hathaway BJ, Wilkinson G, Gillard RD, McCleverty JA (eds) (1987) In comprehensive coordination chemistry, vol 5. Pergamon Press, Oxford, p 533
Addison AW, Rao TN, Reedijk J, Rijin JV, Verschoor GC (1984) J Chem Soc Dalton Trans 1349
Acknowledgments
This research was financially supported by the Department of Chemistry—University of Louisiana at Lafayette. S.S.M. thanks Dean B. Clark (UL Lafayette). FAM thanks Dr. J. Baumgartner (TU-Graz) for assistance.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mautner, F.A., Louka, F.R., Gallo, A.A. et al. Thiocyanato-copper(II) complexes derived from a tridentate amine ligand and from alanine. Transition Met Chem 35, 613–619 (2010). https://doi.org/10.1007/s11243-010-9371-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-010-9371-0