Abstract
Functional genomics have advanced in rice with the availability of high quality and well annotated genome data, coupled with characterizing several genes by genetic manipulation. Several protocols have been put forth for the later over the years with significant improvements. Since different cultivars of rice respond differently to different protocols of genetic transformation, there is always room for further improvements. In the present study, we have attempted to highlight important factors leading to successful transformation in rice. An efficient, rapid and reproducible protocol independent of genotype specificity has also been developed to genetically transform rice using scutellum-derived embryogenic calli from mature seeds by Agrobacterium-mediated transformation. The protocol has been tested on six diverse cultivars of indica rice and one cultivar of japonica rice, achieving a total transformation efficiency up to 94%, in some cultivars. The protocol involves use of a popular surfactant, Silwet L-77, which dramatically enhances the efficiency of the Agrobacterium-mediated transformation. Combined with variety specific, high efficiency callusing and regeneration media and optimal hygromycin strength, significant improvement has been achieved in both transformation and regeneration efficiencies across different cultivars. The proposed protocol is also shorter, and shoot regeneration is achieved in around two months starting from the beginning. The protocol may be easily improvised and adapted for several other varieties of rice.
Key message
A rapid, highly efficient and reproducible protocol has been developed that can cater to diverse rice cultivars owing its success to the use of the popular surfactant, Silwet L-77, during co-cultivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is one of the most important food crop and feeds nearly 4 billion people, i.e. half of the human population. It has the largest cultivation area in the world amongst the crop plants, spanning across several climate zones, both in latitude and altitude. The world’s largest land area under rice cultivation happens to be in India, accounting for 40% of the total food grain production in the country. Rice serves as a staple food for 800 million Indians, is a source of livelihood for millions of farmers across the country, and plays a major role in the Indian economy due to its high exports (Pathak et al. 2020). Several indica rice varieties are cultivated across the country, majorly as rain-fed crops, and their cultivation is often troubled with challenges of climate change, most commonly drought, flooding, high temperature, cold, salinity, soil and water degradation, and most importantly, sustainable increase in productivity to feed the ever growing population.
Genetic manipulation of crops serves as an indispensable tool for understanding gene functions contributing to functional genomics and paving way for crop improvement by altering genetic pathways. Genetic manipulation in rice began decades ago where protoplasts were largely targeted and direct DNA delivery methods, such as electroporation, PEG based infusion or biolistics were employed successfully (Christou et al. 1991; Datta et al. 1990; Shimamoto et al. 1989). Later, Agrobacterium-mediated transformation rapidly became the method of choice for genetically transforming rice as it served most pre-requisites of a desirable genetic manipulation technique for any plant system, i.e. higher transformation rates, stable integration of the T-DNA into the plant genomes, relatively lower copy number of T-DNA integration per transformation event, relatively complete transfer of large T-DNA fragments with defined ends and finally, little rearrangements of the transferred T-DNA in the plant genome. Also, the plants regenerated via this method are generally physiologically normal and fertile (Hiei et al. 1994, 2008). Immature embryos and embryogenic calli derived from mature seeds are the two most common explants used across different rice varieties (Supplementary File 1). Transformation of calli, is the method of choice for genetically manipulating rice as mature seeds are available throughout the year in bulk, and can be easily stored. Transforming immature embryos, although more rewarding in terms of efficiency, but could be more labour intensive and expensive as healthy and good quality immature embryos are required as explants, at correct stage of their development for a successful transformation attempt. Maintenance of healthy rice plants throughout the year in greenhouses for obtaining immature embryos adds to the cost and labour (Hiei and Komari 2008). Indica rice varieties have been found to be more recalcitrant to Agrobacterium-mediated transformation, compared to their japonica counterparts. The reasons understood being; more susceptibility to stress induced during Agrobacterium infection, leading to tissue death; and low frequency or a total failure to regenerate, even from transformed calli (Hiei and Komari 2008; Karthikeyan et al. 2011; Sahoo et al. 2011).
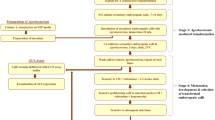
The present study is based on several years of comprehensive work on rice tissue culture, wherein numerous transgenics were generated in PB1 and Basmati 370 cultivars of indica rice. An attempt has also been made to highlight the factors deemed to be most important for successful transformation in rice and devising a general methodology for transforming diverse rice cultivars by incorporating these factors. The protocol was successfully applied to a total of six elite indica rice cultivars, viz. PB1, Basmati 370, IR64, Swarna, BPT5204 and MTU1010; and one japonica rice cultivar, Taipei-309. The above mentioned indica rice varieties were chosen due to their high economic value, i.e. because of their high yielding capabilities or large areas of cultivation. We found that use of a surfactant, Silwet L-77, during the co-cultivation step, coupled with optimal cultivar specific growth media, higher growth temperatures and an optimal concentration of the selection antibiotic led to quicker regeneration and higher transformation frequencies. A summarized flowchart of the protocol has been given in Fig. 1.
Materials and methods
Plant material
In our present study, the mature seeds of Taipei-309, Pusa Basmati-1 (PB1), Basmati 370, IR64, Swarna, MTU1000 and BPT5204 were used as explants. The seeds of all the cultivars used were sourced from the Department of Genetics, Indian Agricultural Research Institute (IARI), New Delhi, India. The seeds were stored at 4 °C, until use.
Surface sterilisation and explant preparation
The seeds (50–100) were de-hulled and surface sterilized using 20 ml 0.1% solution of mercuric chloride in a 100 ml flask, to which 2–3 drops of Tween 20 was added along with 40–50 mg Bavistin. The flask was shook vigorously for 10–15 min, followed by 5–6 rinses with surplus sterile water. The seeds were finally suspended in 10 ml sterilized distilled water and incubated at 37 °C for 4–5 h for imbibition. Post incubation the seeds were transferred to a sterile petridish, where the embryo portion were separated from the rest of the endosperm part with the help of a sterile scalpel. The incision was made behind the embryo in such a way that a small portion of the endosperm was also excised along with the embryo. The embryos, thus obtained, were used as final explants for callus induction.
Growth conditions
All tissue culture steps; such as callus induction, selection and regeneration were done in culture room condition where the temperature was maintained between 30 and 32 °C, and a day-night cycle was set where photoperiod of 16 h and a dark phase of 8 h was maintained. Whenever complete darkness was required to be maintained, such as during initial callus induction, co-cultivation, selection and initial regeneration phases, the tissue culture plates were covered in aluminium foil. Co-cultivation was performed in a growth chamber maintained at 22 °C with lights in off mode. Light intensity was maintained at 80–100 µMol m−2 s−1, using a mixture of yellow and white fluorescent tube lights, during the photoperiod. For all media description and compositions, finalised for the chosen cultivars, refer Table 1.
Optimization of callus induction/growth media, regeneration media and MIC assay
For callus induction, four different compositions of media were used, namely, C1, C2, C3 and C4 (Table S1). The excised embryo explants were inoculated on the four media and allowed to proliferate for 2 weeks, in dark. The embryogenic calli produced were compact and solid, and sub-cultured again on the same corresponding media and allowed to grow for another week, followed by scoring for size (mm) using ImageJ software, fresh weight (mg) and percentage of seeds forming calli. Similarly, four different compositions of the regeneration media; R1, R2, R3 and R4 (Table S2), were screened by placing 2 week-old calli of all chosen varieties on the test media for 3 weeks, in light. Regeneration efficiency was screened by scoring for the percentage of calli producing shoots on the regeneration media. Also, the number of shoots produced per calli were scored. MIC (Minimum Inhibitory Concentration) assay was performed for selection medium by placing 2 week-old calli on selection medium, supplemented with different concentrations of hygromycin/ Augmentin, and scoring for calli showing necrosis, in the case of hygromycin, after 3 weeks. For Augmentin, growth of calli was compared with control medium with no added antibiotic. For regeneration medium, MIC assay was performed for hygromycin similar to selection media and the scoring was done by counting the number of calli showing shoot regeneration on different antibiotic strengths.
Induction of embryogenic callus
Once the callus induction media were selected for each of the chosen cultivars, the excised embryo explants were placed on the CIM I/II and incubated in dark in the culture room conditions for 7–8 days, which were long enough to produce small, compact and solid embryogenic calli in all cultivars. These solid and compact calli masses were separated out from the rest of the unwanted parts, including seed remnants, shoot/ root outgrowths and soft/ mushy callus portions and sub-cultured on fresh CIM I/II for 5–6 days.
Vector, construct and Agrobacterium strain used for transformation
The binary vector system used in this study was a monocot specific expression vector, pB4NU (Raghuvanshi et al. Ph.D. thesis), which is a modified version of plant expression vector pCAMBIA-1301, where Ubiquitin promoter was cloned in the multiple cloning site (MCS) using the PstI site. Hence, the T-DNA region of the vector harbours a constitutive monocot specific ubiquitin promoter driving the expression of the gene of interest, along with a GUS reporter marker gene, uidA, the expression of which is driven by an independent CaMV 35S promoter (Fig. S1). The vector confers hygromycin resistance to the transformed plant cells as it harbours HPT-II (hygromycin phosphotransferase II) gene as the selectable marker gene in the T-DNA region. An over-expression construct of a rice F-box protein encoding gene, OsFBT4 (LOC_Os02g47640), amplified from the IR64 cultivar of indica rice, was made by cloning the CDS of the gene using the BamHI and SmaI restriction sites of the pB4NU vector (Fig. S1). The empty pB4NU vector and the pB4NU-OsFBT4 construct were then transformed into the EHA105 strain of Agrobacterium tumefaciens. The Agrobacterium colonies, harbouring the two plasmids, were then used for all transformation experiments.
Co-cultivation and washing of the calli
Agrobacterium culture—A freshly transformed and streaked colony of Agrobacterium was used to inoculate a primary or starter culture in a 100 ml flask containing 10 ml LB (Luria and Bertani) broth, supplemented with 10 mg/L rifampicin and 50 mg/L kanamycin. Since EHA105 is a faster growing strain of Agrobacterium, the primary culture was not allowed to exceed 24 h. The secondary culture of Agrobacterium was initiated by adding 4% of the primary culture in 50 ml LB broth in a 100 ml flask, supplemented with only 50 mg/L kanamycin and no added rifampicin. The secondary culture was allowed to grow for 5–6 h, within the log phase (O.D. 0.6–0.8). The bacterial cells were harvested by gentle centrifugation at 4000 rpm, 25 °C, in SS–34 tubes for 15 min.
Co-cultivation of Agrobacterium with rice calli—While pelleting down the Agrobacterium culture, the sub-cultured rice calli were added to 100 ml LCM, in a 250 ml flask. The pelleted Agrobacterium was added to LCM in such a way that the final OD of the suspension was close to 0.1. Keeping OD low at this point was vital for callus recovery, post-infection. To the suspension, 20 µL, i.e. 0.02% final concentration Vac-In-Stuff Silwet-L77 (Lehle Seeds) was added along with 150 µM final concentration of acetosyringone, dissolved in methanol. This suspension of Agrobacterium and calli was covered in aluminium foil and was kept on an orbital shaker at 50–60 rpm, at 28 °C, for 40–45 min. The Agrobacterium suspension was drained and the calli transferred to a sterile petridish. Without blotting, the calli were placed on SCM poured in 90 mm petridishes. Note- the calli should not be blotted dry and instead, just be directly transferred to the solid medium to avoid loss of Silwet. The plates were covered in aluminium foil and were incubated for 80–86 h, i.e. 3 days, in a growth chamber set at 22 °C, in dark.
Washing of infected calli—After the incubation period, the calli were given 3 washes of 10 min each with sterile distilled water in a 250 ml flask. An orbital shaker was employed for the purpose, set at 200 rpm and 32 °C. The first 3 washes were followed by 4–5 more washes of 15 min each, with 600 mg/L Augmentin. During all the wash steps 50–60 ml water was used so as to increase the movement of the calli and the force exerted on the calli, on the shaker. This was followed by a lysozyme treatment step, where 50 ml sterile distilled water having pH- 6.2 was added to the flask along with 100 mg lysozyme powder (Amresco) and 600 mg/L Augmentin. The calli were incubated on an orbital shaker set at 32 °C, 150 rpm for 2 h and 30 min. After the completion of this step, the calli were rinsed 6–7 times with surplus sterile distilled water. Finally, the calli were suspended in 20–30 ml water supplemented with 600 mg/L Augmentin. The excess liquid was drained and the calli were transferred to a sterile petridish. Without blotting, the calli were placed in RECM I/II plates and incubated in the culture room conditions, in dark, for 7 days.
Selection process
After 7 days of recovery post co-cultivation, the calli were transferred to SELM I/II for 7–8 days, followed by a second transfer to fresh SELM I/II for another 7–8 days. Both the times, the calli were pushed substantially into the medium for better and faster selection, and prevention of Agrobacterium infection relapse. The selection step was performed in the culture room conditions, in dark.
Shoot regeneration
After 15–16 days on the selection medium, the calli which displayed a mixture of necrotic black and whitish-cream coloured proliferating masses, were transferred as such to REGM I/II for 7–8 days, in the culture room conditions, in dark. Afterwards, only the whitish-cream coloured, rapidly proliferating calli masses (Fig. 2) were selected to be transferred to REGM III/IV, in light, and maintained till shoot regeneration took place, transferring them to fresh media every 7–8 days. Accumulation of phenolic compounds in the medium was avoided at all costs as it was found to be detrimental for regeneration.
Behaviour of calli of the chosen rice cultivars at different stages of the protocol. The figure shows the calli of the chosen rice cultivars at different stages of the protocol. Note the higher degree of necrosis at the Selection II/ Regeneration I stage of the protocol in the recalcitrant cultivars; IR64, Swarna and BPT5204
Root initiation, hardening and establishing the plants in soil
The callus masses showing shoots (Fig. 2) that were at least 1–2 cm long on the regeneration medium, were transferred to RM poured in 50 ml culture tubes. Plants showing rapidly growing shoots and roots were selected for transfer to liquid RGM (Yoshida et al. 1976), for hardening, by immersing the roots and a portion of stem in the liquid medium and letting the leaves out of the tubes in open for faster transpiration and growth. Rapid growth of roots and shoots was observed on RGM, which after 3–4 weeks, were transferred to a proper mix of soil and manure, suitable for the particular variety of rice transformed, and allowed to complete their life cycle in a green house till seed set.
Histochemical GUS assay
GUS- histochemical staining was carried out using the protocol given by Jefferson et al. (1987). The calli/ leaves were incubated at 37 °C in GUS histochemical buffer (50 mM sodium phosphate buffer pH 7.0, 10.0 mM EDTA pH 8.0, 0.5 mM K3Fe(CN)6, 0.5 mM K4Fe(CN)6.3H2O, 0.1% Triton X-100 and 1.0 mM X-gluc dissolved in DMSO) for 24 h, followed by de-staining with 3:1 volumes of acetone: ethanol.
PCR confirmation of transgenic plants
The transgenic nature of the regenerated plants was confirmed by PCR amplification of the vector-specific HPT-II gene using the genomic DNA isolated from WT as well as regenerated transgenic plants. The genomic DNA was isolated using the DNA extraction buffer (100 mM Tris–Cl pH 8.0; 500 mM NaCl; 50 mM EDTA pH 8.0; and 10 mM β-mercaptoethanol). Leaf tissue from transgenic plants were ground in presence of liquid nitrogen, to which 200 µl of extraction buffer was added and incubated at RT for 5 min. The samples were centrifuged at 13,000 rpm for 15 min, at 4 °C. Supernatant was mixed with an equal volume of iso-propanol and incubated on ice for 10 min, followed by centrifugation at 13,000 rpm for 10 min, at 4 °C. The pellet was washed with 70% ethanol, followed by air drying and re-suspending in 20–30 µl of Milli-Q (MQ) water. The genomic DNA was used for PCR amplification of HPT-II gene using a combination of primers; HPTII-F (5ʹ-TCTACACAGCCATCGGTCCAG-3ʹ) and HPTII-R (5ʹ-GATGTAGGAGGGCGTGGATAT-3ʹ).
Statistical analysis
All experiments were carried out using at-least three biological replicates and all results were displayed as the averages of the three replicates, along with their standard deviation values. Wherever applicable, statistical significance of the data was analysed using one-factor ANOVA in MS Excel program.
Results
In the present study, an attempt has been made to point out and address the various factors contributing to successful Agrobacterium-mediated transformation in rice. A summarized flow-chart of the protocol have been given in Fig. 1. The behaviour of calli, across the chosen cultivars, at different stages of the protocol have been depicted in Fig. 2.
Optimization of callus-induction/growth conditions
The excised embryos along with small portion of the adjoining endosperm were inoculated on four different compositions of callus induction media. After 2 weeks of incubation, root and shoot outgrowths were observed, along with callus formation. Each callus mass that emanated from the embryo could be divided into a hard, solid embryogenic scutellar callus which was adjoining/ proximal to the seed; and a softer, mushy non-embryogenic callus, distal to the seed. Transfer of only the compact and solid scutellar calli to fresh medium and removal of all other tissues helped in faster and more uniform growth of the callus and a significantly higher transformation efficiency. It was observed that addition of 2 mg/L 2,4-D was sufficient to induce compact callus formation from majority of seeds in all the cultivars, but the growth was slower and the calli somewhat inferior. Addition of proline and glutamine and presence of combinatorial multiple sugars, such as sucrose and maltose in the medium did marginally increase the percentage of seeds forming calli, but significantly improved the callus growth rate and quality, as reflected in callus size and fresh weight (Table S1). Additionally, most indica rice cultivars, except BPT5204, responded better to MS basal medium which contains a higher level of nitrogen, while others showed improved growth on NB basal medium. A slightly higher temperature of 30–32 °C was found to be optimal for robust callus proliferation. It was also noted, that relatively fresh and good quality seeds with a 100% germination rate significantly improved callus quality and growth time. Such calli responded better in all the downstream events and ultimately exhibited a higher transformation/ regeneration frequency. Choosing the cultivar-specific optimal medium composition, nearly 90% callusing efficiency was observed across all cultivars tested (Table S1).
Co-cultivation of calli with Agrobacterium
Once fast proliferating scutellar embryogenic calli were obtained, they were co-cultivated with Agrobacterium for 3 days. Some major modifications were done during this Agrobacterium infection step, which are as follows; use of a surfactant (Silwet L-77), low final bacterial suspension O.D. (0.1) and longer incubation time (45 min) during co-cultivation, and maintenance of lower temperatures (22 °C) and longer duration (3 days) on solid co-cultivation medium. We found that use of very minute quantities (0.02%) of Silwet L-77, remarkably improved transformation efficiency in PB1 and Basmati-370 (Fig. 3), as percentage of hygromycin resistant calli was significantly higher in both cultivars, when co-cultivated in presence of the surfactant. Moreover, the frequency of independent transformation events per callus also increased remarkably when Silwet L-77 was added during co-cultivation. Since Silwet L-77 was observed to reduce water surface tension and bring bacterial cells closer to the surface of the plant cells, it was expected to intensify the infection as the Agrobacterium cells were seen to penetrate deeper into the callus mass through extracellular spaces. We checked for any possible toxic effects of Silwet L-77 on callus growth across all cultivars. Silwet L-77, at 0.025% concentration for 60 min, did not appear to cause toxicity in any of the cultivars (Table S3), as no callus growth retardation was seen. Although, Silwet L-77 was not observed to affect the growth rate of Agrobacterium but it did seem to modify the growth characteristics of the bacteria, on the co-cultivation medium around the calli. A thicker, denser and far-more stickier layer of Agrobacterium was noted around the calli, after incubation on the co-cultivation medium. Hence, a more rigorous washing protocol was needed to get rid of this Agrobacterium growth. Simple washing protocols that have been proposed earlier by several other groups failed to contain the Agrobacterium growth in the downstream selection steps. A modified and more rigorous washing protocol was developed, based on enzymatic (lysozyme) digestion of bacterial cell wall, use of higher concentration of Augmentin and a higher speed of shaking for a longer duration (see Methods). The present protocol completely eliminated the Agrobacterium infection when coupled with maintenance of a higher growth temperatures during the downstream steps.
Effect of Silwet L-77 on transformation efficiency in PB1 and Basmati-370. A shows improved microcalli proliferation from the parent calli when Silwet was used during co-cultivation, in both cultivars; B graphically depicts the percentage of co-cultivated calli showing proliferation at 50 mg/L concentration of hygromycin. The average number of microcalli proliferating on the surface of co-cultivated calli undergoing selection is graphically presented in (C). In this experiment 2 week old calli grown on cultivar-specific callus induction media were co-cultivated with Agrobacterium for 2 days, in presence and without Silwet. The calli were selected on 50 mg/L hygromycin and observed for new microcalli outgrowths, considered to be transformation events. Three biological replicates were used in the analysis and standard deviation values are shown as error bars. The data is statistically significant as analyzed by one-way ANOVA (**P value < 0.005)
Co-cultivation using the present protocol caused greater stress to the callus cells across most cultivars as evident by greater necrosis and lesser recovery in several calli, especially in the more recalcitrant cultivars such as Swarna and IR64 which severely affected the overall transformation efficiency. So it was thought to introduce a short recovery period of 6–7 days where no hygromycin was added to the medium rather a higher strength of Augmentin (350 mg/L) was added to check the relapse of Agrobacterium infection. It was also noted that temperatures between 30 and 32 °C during the recovery and selection period was critical for effectively preventing the Agrobacterium infection and improved the growth and selection of the transformed calli. Lower temperatures during the recovery phase, especially below 27 °C, promoted development of resistance in Agrobacterium to Augmentin leading to its uncontrolled growth and loss of calli (data not shown).
Selection of transformed calli and hygromycin toxicity in recalcitrant rice varieties
The strength of selection antibiotic, hygromycin, was observed to be one of the most critical factor, governing the transformation efficiency, especially for the recalcitrant cultivars of rice, viz. Swarna and IR64. Calli of these recalcitrant cultivars were unable to tolerate higher doses of hygromycin (40–50 mg/L) for more than three selection cycles of 7–8 days each and turned black due to necrosis, even when positive for GUS activity. The few surviving and slowly proliferating white coloured calli which were certainly resistant to hygromycin were transferred to the regeneration medium where they turned necrotic after sometime and did not regenerate. Swarna was found to be most sensitive to hygromycin and did not survive the selection cycles to reach the regeneration stage at hygromycin strength of 50 mg/L and 40 mg/L. Other cultivars that were well tolerant to hygromycin and proliferated well during the selection period showed poor regeneration when full strength of hygromycin was used. To circumvent the hurdle of hygromycin toxicity, minimum inhibitory concentration (or MIC) assay was carried out at selection as well as regeneration stages. Three concentrations of hygromycin, i.e. 50, 35 and 25 mg/L were used to see the toxic effects of the antibiotic on the healthy calli of all the chosen cultivars. Two-week-old calli were sub-cultured on to the callusing medium supplemented with different strengths of hygromycin. After 3 weeks of growth the cultivars were scored based on percentage of calli showing more than 50% necrosis. As expected, 80–100% calli across all cultivars showed necrosis at full strength (50 mg/L) of hygromycin, that dropped in the range of 50–80% at 35 mg/L and further fell to 30–55% at the concentration of 25 mg/L (Table 2). In all three cases, Swarna was found to be most sensitive followed by IR64 and then BPT5204, as they not only showed higher percentages of necrosis and cell death but also faster necrosis. On the other hand, PB1 and Taipei-309 were found to be the most tolerant amongst all. Hence, according to the result of the MIC assay, it was most appropriate to use hygromycin strength at 45 mg/L for the lesser sensitive PB1 and Taipei-309; 40 mg/L for moderately sensitive MTU, BPT5204 and Basmati-370; and 35 mg/L for more sensitive Swarna and IR64. Two selection cycles of 7–8 days each were found to be sufficient for effectively selecting the transformed calli.
We checked for the toxic effects of hygromycin on the regeneration stage, for which 2 week old calli were similarly transferred to regeneration medium supplemented with the above three concentrations of hygromycin, in addition to a fourth concentration of 20 mg/L. After 3 weeks, the cultivars were scored for hygromycin toxicity by counting the number of calli showing shoot regeneration. Interestingly, none of the seven varieties regenerated shoots on 50, 35 and 25 mg/L strengths of hygromycin, even after 4 weeks on cultivar-optimized, regeneration media (Table 2). Moreover, even at 20 mg/L none of the cultivars, except Taipei-309, showed regeneration. It was therefore concluded that regeneration from callus was especially sensitive to hygromycin selection and higher doses of the antibiotic was unnecessary and should be avoided, at the regeneration stage, as it could be detrimental in most cultivars. Since a lower concentration of 25 mg/L hygromycin was sufficient to completely inhibit shoot emergence from the untransformed calli the same was used as the preferred antibiotic strength for the regeneration stage for all cultivars except Swarna. An even lower strength of 20 mg/L was preferred in case of Swarna for regeneration. We further investigated the poor response of Swarna and IR64 on selection and regeneration media in presence of antibiotics. MIC assay was also performed for Augmentin to rule out the potential toxic effects of higher doses of the antibiotic. The results showed no apparent toxic effects of the antibiotic on callus growth or regeneration, even at a higher concentration of 350 mg/L, when compared to the control medium, in any of the 7 cultivars tested (Table S4).
Dark conditions during recovery, selection and first phase of regeneration, when hygromycin concentration was higher in the medium, was also observed to be a major contributor to callus survival and better regeneration after the co-cultivation process. A much higher necrosis rate was noted, especially in more recalcitrant cultivars such as Swarna, IR64 and BPT5204, when light was present during the selection phase which severely affected the regeneration potential of the calli (data not shown).
Optimization of regeneration medium, regeneration frequency and plant establishment
The factors that were observed to be most important for shoot regeneration from the transformed calli were; cultivar specific optimized regeneration medium composition; optimized minimal strength of selection antibiotic; transfer of healthy and rapidly proliferating transformed calli to the regeneration medium; external conditions, especially higher temperature (30–32 °C); and finally, periodic sub-culturing of calli on fresh medium every 7–8 days. Regeneration is the most crucial stage of any transformation protocol as frequency of regeneration greatly affects the overall transformation efficiency. It was observed that maximum shoot regeneration happened within a month post transfer from the selection medium to the regeneration medium in all cultivars. Afterwards, the calli starts to lose their vigour and shall never regenerate which makes it important to provide them with optimal regeneration conditions from the beginning. The optimal balance between auxin and cytokinins, and mild to moderate dehydration provided by the medium has been reported in several previous protocols to enhance regeneration from calli. Based on extensive literature survey, four different media compositions were tested across the chosen cultivars (Table S2). Regeneration was seen on all four media tested, but higher percentage was seen when a combination of auxin (NAA) and cytokinin (BAP + kinetin) was used. The addition of sorbitol for inducing osmotic stress to the calli was seen to have profound effects on speeding up somatic embryogenesis, greening and finally, shoot initiation. The regeneration medium optimized for each cultivar in this study promoted faster as well as higher frequency of regeneration and resulted in more number of shoots per calli.
Post-selection, the whole calli (except completely necrotic tissue) were transferred from selection medium to the regeneration medium, REGM I/II, for 7–8 days in dark. During this phase the calli of all cultivars were observed to proliferate as translucent to pure white callus masses from multiple points. These small, proliferating calli masses were transferred to light on REGM III/IV, which differed from the earlier medium in having a lower strength of hygromycin, and were maintained on the same until greening and shoot regeneration occurred. On REGM III/IV, the calli began to proliferate rapidly and a notable difference in colour was observed from whitish to creamish followed by greening and formation of somatic embryos and finally shoot regeneration. A higher temperature (30–32 °C) and weekly transfer to fresh medium greatly enhanced the speed and frequency of regeneration.
Some of the proliferating calli after the second round of selection were employed for GUS- histochemical assay. The results showed that in case of PB1 and Taipei-309, 100% of the calli that were co-cultivated showed proliferation in presence of hygromycin and were positive for GUS staining, hence 100% Agrobacterium infection efficiency was achieved for the two cultivars (Table 3). Likewise, the infection efficiency for the other cultivars were close to 90% or above. The real transformation efficiency, defined as the percentage of transgenic shoots (independent events) obtained per number of calli co-cultivated, was certainly lower due to regeneration constraints. The maximum regeneration efficiency was obtained in Taipei-309 (94%), closely followed by PB1 (91%) (Table 3). The other cultivars also showed satisfactory results with Basmati-370 showing 79% efficiency, MTU (72%), BPT5204 (63%), IR64 (62%) and Swarna showing the lowest transformation efficiency of 23%. Swarna was found to be the most recalcitrant to transformation due to necrosis of the calli post co-cultivation. Time taken from seed inoculation to regeneration is yet another factor that makes a protocol more lucrative and a method of choice. The current protocol produced transgenic shoots, beginning from seed inoculation, as early as 43 days; Taipei-309 regenerated in 42–44 days and PB1 in 47–49 days. Other cultivars took a little longer to start regeneration; Basmati-370 (53–57 days), MTU (57–61 days), IR64 (58–64 days), BPT5204 (59–63 days) and Swarna (62–67 days).
Once the regenerated shoots reached nearly 2 cm in length, they were transferred to the rooting media (RM), poured in jam bottles or culture tubes for better aeration (Fig. 2), and allowed to grow till the plants reached 10–15 cm in height and had profusely branched roots. Addition of 30 mg/L hygromycin in the rooting medium ensured that no escape shoots matured into complete plants, and effectively circumvented lower strength of hygromycin in selection/ regeneration media. No escapees were ever observed when the above recommended hygromycin concentrations were used. The established plants were eventually transferred to liquid rice growth medium (RGM) for hardening for at least 2 weeks before they were transferred to pots in soil. The transgenic nature of the regenerated plants was confirmed by performing GUS-histochemical staining of the hardened plants (Fig. S2) before transferring them to green house, producing a more than 90% positive result across all cultivars. The result was also supported by PCR confirmation, by amplifying the HPT-II gene from the isolated genomic DNA, which produced bands corresponding to the size of the gene. All samples across the chosen cultivars were positive for the PCR-test, producing a 100% result (Fig. S2). Also, a comparable transformation efficiency was obtained when the over-expression constructs of OsFBT4 was used in place of the empty pB4NU vector, in the PB1 cultivar.
Discussion
Agrobacterium-mediated transformation is an indispensable tool for genetic manipulation of rice. Numerous protocols have been proposed in the past for several rice cultivars (see Supplementary File 1), with variable success rates, duration of the protocols and ease of performance. The indica rice varieties have been previously reported to be difficult to transform as compared to their japonica counterparts (Hiei et al. 1997; Karthikeyan et al. 2011) and no single protocol in the past has been able to universally cater to diverse rice varieties. In the present study, we have attempted to highlight the various factors important to successful rice transformation, and incorporate them into a simple, shorter and effective protocol using embryogenic scutellar calli, derived from mature seeds, and can be applied to diverse rice varieties. We also demonstrate the effectiveness of a popular surfactant, Silwet L-77, in transforming rice calli with much higher efficiency.
We understood several factors that need to be addressed for successful transformation of rice, using Agrobacterium. These factors include (i) good quality and healthy seed lot as a starting material; (ii) optimized cultivar-specific callus induction and regeneration media; (iii) freshly sub-cultured rapidly proliferating calli; (iv) high efficiency Agrobacterium strain that is freshly transformed with the desired binary construct; (v) use of a surfactant, such as Silwet L-77, during co-cultivation, coupled with prolonged co-cultivation period and a robust Agrobacterium elimination protocol; (vi) introduction of a short recovery phase with no selection antibiotic, post co-cultivation; (vii) maintenance of higher temperature (30–32 °C) during all stages, except co-cultivation; (viii) maintaining dark all throughout the protocol, except the final regeneration stage and onwards; (ix) rapid change of media, every 7–8 days; and lastly, but most importantly, (x) use of optimum concentration of selection antibiotic, for both selection and regeneration media, that should be standardized for each cultivar to be transformed.
A good quality seed lot showing synchronous immediate and complete germination as a starting material is of ultimate importance as it dramatically improves callus quality, their doubling time and also promotes better recovery of cells during the stressful event of co-cultivation. Removal of unwanted tissues and sub-culturing the 7–8 day old calli on to fresh medium for 5–6 days before co-cultivation dramatically enhanced the growth rate of peripheral cells which were observed to be highly amenable for transformation and regeneration. Similar observations were made by Azria and Bhalla (2011). Hiei et al. (1997), showed that actively dividing cells are a pre-requisite for successful transformation as process related to DNA synthesis and cell division are required for integration of foreign DNA into the plant genome. The importance of high quality calli as a starting material for successful and efficient transformation have also been emphasized by several earlier reports (Chakraborty et al. 2016; Kumar et al. 2005; Karthikeyan et al. 2011). Hiei et al. (1997) and Toki et al. (2006), pointed out the importance of keeping the callusing phase as short as possible due to concerns of introduction of somaclonal variations during prolonged callusing. In compliance to it, the callus phase of our proposed protocol was kept short, for not more than 38 days, before transferring to the regeneration medium.
Not all rice varieties, especially indica cultivars, respond well on a single callus induction medium and some are highly recalcitrant to tissue culture. Hence, in order to obtain compact calli with robust growth across the different cultivars, standardization of cultivar specific medium and growth conditions becomes important. A good callus induction protocol should be able to produce a reasonably sized compact callus within 7–10 days from majority of the seeds. In our study, we found 2 mg/L 2,4-D to be optimal for callus induction across all varieties. Several groups have reported similar findings (Azria et al. 2000; also see Supplementary File 1), although, extensive modifications of other media components have been proposed to suit different rice cultivars. We found that addition of proline and glutamine, in addition to multiple sources of carbon, such as combination of sucrose and maltose had stimulatory effects on callus induction and growth. Maltose has been reported earlier to enhance callusing and regeneration in some rice cultivars (Kumar et al. 2005; Rachmawati et al. 2004; Zhang et al. 1997). Addition of proline in callus induction, co-cultivation, selection and regeneration media has been reported by several groups and is thought to improve recovery from stress during tissue culture as well as co-cultivation (Chakraborty et al. 2016; James et al. 1993; Toki et al. 2006; Wakasa et al. 2007). A robust regeneration medium is of paramount importance as it can significantly improve the speed and efficiency of regeneration, adding to the overall transformation efficiency. We found that combination of lower strength of auxin (NAA) and higher strength of cytokinins (BAP and kinetin) improved the regeneration potential across distant cultivars, tremendously, compared to when only cytokinins were added. Regeneration was further enhanced when desiccation stress was applied to the calli by adding sorbitol to the medium. Several groups emphasized on combinatorial use of auxin (mostly NAA) and cytokinins (kinetin or BAP or both) to increase regeneration frequency across distant rice cultivars (Azria et al. 2000, 2006; Chakraborty et al. 2016; Ishizaki and Komashiro 2008; Kumar et al. 2005; Rachmavati et al. 2004). Stimulatory effects of osmotic/desiccation stress during regeneration have been reported by several groups (Azria and Bhalla 2006; Ishizaki and Komashiro 2008; Nishimura and Matsuoka 2006; Sahoo et al. 2011; Sahoo and Tuteja 2012; Wakasa et al. 2007).
We found that longer duration of infection with Agrobacterium improved transformation efficiency. Keeping the O.D. of the Agrobacterium suspension in the liquid co-cultivation medium low (0.1) and by lowering the co-cultivation temperature to 22 °C, the duration of infection can be increased. In the present study, three days of co-cultivation was found to be optimal at lower temperature (22 °C), which promoted higher transformation efficiency and checked excess growth of Agrobacterium. Lower temperatures have been known to facilitate pilus formation between Agrobacterium and plant cells and hence the process of T-DNA transfer (Gelvin 2003). Dillen et al. (1997), reported highest transformation efficiency at 22 °C in Phaseolus acutifolius calli and Nicotiana tabacum leaves which dropped significantly at 25 °C and was completely inhibited above 25 °C. In the present study, addition of a surfactant, 0.02% Silwet L-77, in the co-cultivation medium worked as a game changer and miraculously improved the transformation efficiency for all varieties of rice. A surfactant may work by bringing bacterial cells closer to the callus cells as it lowers the water surface tension, thereby facilitating Agrobacterium penetration into the callus tissue and increase in cell infectivity. Use of Silwet L-77 to improve transformation efficiency has been reported earlier in a few plant species, involving various explants; such as immature embryos and embryogenic calli in wheat (Cheng et al. 1997); floral buds in Arabidopsis (Clough and Bent 1998); embryogenic calli in maize (Yang et al. 2006); embryogenic calli in Cyclamen persicum (Ratjens et al. 2018). Cheng et al. (1997), reported 19-fold increase in transformation efficiency when 0.05% Silwet was used during Agrobacterium infection in wheat embryogenic calli. High infection efficiency (> 90%) was obtained across all cultivars when 150–100 µM acetosyringone was added to the liquid and solid co-cultivation media, respectively.
Choice of Agrobacterium strains along with binary vector combinations, for infection, have been reported to make marginal to significant difference to the transformation efficiencies. EHA101, LBA4404 and EHA105 have been the most popular strains of Agrobacterium used worldwide (see Supplementary File 1). LBA4404 produced higher frequency of transformation, compared to the super-virulent strains EHA101 and EHA105, in both indica and japonica rice varieties (Hiei et al. 1994, 2008; Toki et al. 2006). Rachmavati et al. (2004), reported EHA101 to be more efficient, while Sahoo et al. (2011) reported both LBA4404 and EHA105 producing similar transformation efficiencies in some indica rice cultivars. In the present study, the EHA105 strain transformed with pB4NU binary vector was used, which produced more than 90% infection efficiency across all rice cultivars.
A short recovery period of 6–7 days was considered vital in the present study, before subjecting the calli to selection pressure. Prolonged and intense co-cultivation step was found to be stressful, especially in case of the recalcitrant cultivars such as IR64, Swarna and BPT5204, that often lead to tissue death and loss of calli. Recovery phase after co-cultivation have been found helpful, and often essential, by other groups as well (Duan et al. 2012; Ratjens et al. 2018; Risco et al. 2021).
Maintenance of higher temperature, i.e. 30–32 °C, throughout the recovery, callusing and regeneration stages significantly improved growth of rice calli and checked Agrobacterium growth. Higher temperature immediately after co-cultivation was found helpful in eliminating Agrobacterium as it enhanced resistance of calli to the bacteria (Toki et al. 2006). Dark incubation after co-cultivation, till the second phase of regeneration was also considered important in the present protocol. Co-cultivation was observed to be stressful to calli, more so in case of recalcitrant varieties, causing cessation of growth, browning and finally necrosis. Moreover, addition of selection pressure made their condition worse. Presence of light, soon after co-cultivation caused faster necrosis of the calli, possibly due to hypersensitive responses caused by oxidative burst, which is naturally enhanced in light. Dark phase helps in minimizing ROS production and also contributes to its quenching. Similar findings were reported by several previous reports (Karthikeyan et al. 2011; Sahoo et al. 2011; Risco et al. 2021).
Hygromycin is the most efficient and common selection agent used in rice transformation (see Supplementary File 1), and was also used in the present study. We observed that some rice cultivars, i.e. Swarna, IR64 and BPT5204 were more sensitive to hygromycin than the others. MTU1010 and Basmati-370 were moderately sensitive, whereas PB1 and Taipei-309 were the least sensitive. Choosing a fixed concentration of 50 mg/L hygromycin for all cultivars proved detrimental for the sensitive cultivars, leading to poor or no regeneration at all. Hence, it is sensible to select the antibiotic concentration least stressful for the transformed tissue, but at the same time, most effective in eliminating the non-transformed cells. Moreover, hygromycin was found to be extremely detrimental during the regeneration stages and completely inhibited regeneration from non-transformed calli, at concentrations as low as 20 mg/L. Lowering the hygromycin concentration to 20–25 mg/L during regeneration was seen as essential in our study. Similar observations were made in some previous reports where 20–30 mg/L concentration was considered essential for selection and 0–20 mg/L concentration was necessary at the regeneration stage, in Australian and African rice cultivars (Azria and Bhalla 2011; Ishizaki and Komashiro 2008).
In the present protocol, stable integration was confirmed in 100% of the regenerated plants by PCR amplification of the HPT-II gene, while complete T-DNA transfer was confirmed by GUS histochemical staining, where nearly 90% of the plants obtained were GUS-positive indicating complete integration of the T-DNA, as the uidA gene is located near the right border of the T-DNA. In our opinion, T-DNA mobilization and integration is dependent on Agrobacterium strain and the binary vector itself. All published protocols have shown T-DNA insertion to be random where the copy number varied from 1–6, irrespective of cultivar, Agrobacterium strain, vector and methodology. Moreover, the desirable single inserts have been reported in the range of only 30–40% (Hiei et al. 1997, 2008; Ishizaki and Komashiro 2008; Karthikeyan et al. 2011; Rachmavati 2004; Saika and Toki 2010; Sahoo et al. 2011, 2012; Toki et al. 2006).
Conclusions
Functional genomics studies in rice or other complex monocots often require raising rice transgenics. Agrobacterium-mediated transformation is the preferred method of choice because of higher transformation rates and stable and complete T-DNA transfer to the plant genome. The protocol put forward in this study used mature seeds as explant and emphasized on several factors leading to a successful transformation attempt in rice. High quality seeds, optimized callus induction and regeneration media, use of freshly transformed Agrobacterium and Silwet L-77 during co-cultivation, prolonged co-cultivation period, optimization of antibiotic strength for selection and regeneration media, maintenance of dark till the second regeneration stage, and a higher temperature during the entire process were found to be the most important factors governing successful transformation in rice. By strictly implementing these factors in the protocol with cultivar-specific negotiations, higher efficiency of transformation was achieved across all cultivars tested.
Abbreviations
- PEG:
-
Polyethylene glycol
- T-DNA:
-
Transfer-deoxyribonucleic acid
- MIC:
-
Minimum inhibitory concentration
- OD:
-
Optical density
- GUS:
-
Beta-glucuronidase
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- NAA:
-
1-Naphtheleneacetic acid
- BAP:
-
6-benzylaminopurine
- NB:
-
Modified Chu/Gamborg (N6/B5 medium)
- MS:
-
Murashige and Skoog’s medium
References
Azria D, Bhalla PL (2000) Plant regeneration from mature embryo-derived callus of Australian rice (Oryza sativa L.) varieties. Aust J Agric Res 51:305–312. https://doi.org/10.1071/AR98189
Azria D, Bhalla PL (2011) Agrobacterium-mediated transformation of Australian rice varieties and promoter analysis of major pollen allergen gene, Ory s 1. Plant Cell Rep 30:1673–1681. https://doi.org/10.1007/s00299-011-1076-0
Chakraborty M, Sairam Reddy P, Laxmi Narasu M et al (2016) Agrobacterium-mediated genetic transformation of commercially elite rice restorer line using nptII gene as a plant selection marker. Physiol Mol Biol Plants 22:51–60. https://doi.org/10.1007/s12298-015-0334-y
Cheng M, Fry JE, Pang S, Zhou H, Hironaka CM, Duncan DR, Conner TW, Wan Y (1997) Genetic transformation of wheat mediated by Agrobacterium. Plant Physiol 115:971–980
Christou P, Ford TL, Kofron M (1991) Production of transgenic rice (Oryza sativa L.) plants from agronomically important indica and japonica varieties via electric discharge particle acceleration of exogenous DNA into immature zygotic embryos. Bio/technology 9:957–962
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743. https://doi.org/10.1046/j.1365-313X.1998.00343.x
Datta SK, Peterhans A, Datta K, Potrykus I (1990) Genetically engineered fertile Indica-rice recovered from protoplasts. Bio/technology 8:736–740
Dillen W, Clercq JD, Kapila J, Zambre M, Montagu MV, Angenon G (1997) The effect of temperature on Agrobacterium tumefaciens-mediated gene transfer to plants. Plant J 12:1459–1463
Duan Y, Zhai C, Li H et al (2012) An efficient and high-throughput protocol for Agrobacterium-mediated transformation based on phosphomannose isomerase positive selection in Japonica rice (Oryza sativa L.). Plant Cell Rep 31:1611–1624. https://doi.org/10.1007/s00299-012-1275-3
Gelvin SB (2003) Agrobacterium-mediated plant transformation: the biology behind the “Gene-Jockeying” tool. Microbiol Mol Biol Rev 67:16–37. https://doi.org/10.1128/MMBR.67.1.16-37.2003
Hiei Y, Komari T (2008) Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nat Protoc 3:824–834. https://doi.org/10.1038/nprot.2008.46
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282. https://doi.org/10.1046/j.1365-313X.1994.6020271.x
Hiei Y, Komari T, Kubo T (1997) Transformation of rice mediated by Agrobacterium tumefaciens. Plant Mol Biol 35:205–218
Huang JQ, Wei ZM, An HL, Zhu YX (2001) Agrobacterium tumefaciens-mediated transformation of rice with the spider insecticidal gene conferring resistance to leaffolder and striped stem borer. Cell Res 11:149–155. https://doi.org/10.1038/sj.cr.7290080
Ishizaki T, Kumashiro T (2008) Genetic transformation of NERICA, interspecific hybrid rice between Oryza glaberrima and O. sativa, mediated by Agrobacterium tumefaciens. Plant Cell Rep 27:319–327. https://doi.org/10.1007/s00299-007-0465-x
James DJ, Uratsu S, Cheng J et al (1993) Acetosyringone and osmoprotectants like betaine or proline synergistically enhance Agrobacterium-mediated transformation of apple. Plant Cell Rep 12:559–563. https://doi.org/10.1007/BF00233060
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907. https://doi.org/10.1002/j.1460-2075.1987.tb02730.x
Karthikeyan A, Pandian SK, Ramesh M (2011) Agrobacterium-mediated transformation of leaf base derived callus tissues of popular indica rice (Oryza sativa L. sub sp. indica cv. ADT 43). Plant Sci 181:258–268. https://doi.org/10.1016/j.plantsci.2011.05.011
Kumar KK, Maruthasalam S, Loganathan M et al (2005) An improved Agrobacterium-mediated transformation protocol for recalcitrant elite Indica rice cultivars. Plant Mol Biol Rep 23:67–73. https://doi.org/10.1007/BF02772648
Lin J, Zhou B, Yang Y et al (2009) Piercing and vacuum infiltration of the mature embryo: a simplified method for Agrobacterium-mediated transformation of Indica rice. Plant Cell Rep 28:1065–1074. https://doi.org/10.1007/s00299-009-0706-2
Luu V, Stiebner M, Maldonado P et al (2020) Efficient Agrobacterium-mediated transformation of the elite-Indica rice variety komboka. Bio-Protocol 10:1–30. https://doi.org/10.21769/bioprotoc.3739
Molina-Risco M, Ibarra O, Faion-Molina M et al (2021) Optimizing Agrobacterium-mediated transformation and crispr-cas9 gene editing in the tropical japonica rice variety presidio. Int J Mol Sci. https://doi.org/10.3390/ijms222010909
Nishimura A, Aichi I, Matsuoka M (2007) A protocol for Agrobacterium-mediated transformation in rice. Nat Protoc 1:2796–2802. https://doi.org/10.1038/nprot.2006.469
Pathak H, Tripathi R, Jambhulkar NN, Bisen JP, Panda BB (2020) Eco-regional rice farming for enhancing productivity, profitability and sustainability. NRRI Research Bulletin, ICAR-National Rice Research Institute, Cuttack, p 28
Rachmawati D, Hosaka T, Inoue E, Anzai H (2004) Agrobacterium-mediated transformation of Javanica rice cv. Rojolele Biosci Biotechnol Biochem 68:1193–1200. https://doi.org/10.1271/bbb.68.1193
Ratjens S, Mortensen S, Kumpf A et al (2018) Embryogenic callus as target for efficient transformation of cyclamen persicum enabling gene function studies. Front Plant Sci 9:1–15. https://doi.org/10.3389/fpls.2018.01035
Sahoo RK, Tuteja N (2012) Development of Agrobacterium-mediated transformation technology for mature seed-derived callus tissues of indica rice cultivar IR64. GM Crops Food 3:123–128. https://doi.org/10.4161/gmcr.20032
Sahoo KK, Tripathi AK, Pareek A, Sopory SK, Pareek SLS (2011) An improved protocol for efficient transformation and regeneration of diverse indica rice cultivars. Plant Methods 7:49
Saika H, Toki S (2010) Mature seed-derived callus of the model indica rice variety Kasalath is highly competent in Agrobacterium-mediated transformation. Plant Cell Rep 29:1351–1364. https://doi.org/10.1007/s00299-010-0921-x
Shimamoto K, Terada R, Izawa T, Fujimoto H (1989) Fertile transgenic rice plants regenerated from transformed protoplasts. Nature 338:274–276
Shri M, Rai A, Verma PK et al (2013) An improved Agrobacterium-mediated transformation of recalcitrant indica rice (Oryza sativa L.) cultivars. Protoplasma 250:631–636. https://doi.org/10.1007/s00709-012-0439-x
Supartana P, Shimizu T, Shioiri H et al (2005) Development of simple and efficient in planta transformation method for rice (Oryza sativa L.) using Agrobacterium tumefaciens. J Biosci Bioeng 100:391–397. https://doi.org/10.1263/jbb.100.391
Terada R, Asao H, Iida S (2004) A large-scale Agrobacterium-mediated transformation procedure with a strong positive-negative selection for gene targeting in rice (Oryza sativa L.). Plant Cell Rep 22:653–659. https://doi.org/10.1007/s00299-003-0752-0
Toki S, Hara N, Ono K et al (2006) Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J 47:969–976. https://doi.org/10.1111/j.1365-313X.2006.02836.x
Wakasa Y, Ozawa K, Takaiwa F (2007) Agrobacterium-mediated transformation of a low glutelin mutant of “Koshihikari” rice variety using the mutated-acetolactate synthase gene derived from rice genome as a selectable marker. Plant Cell Rep 26:1567–1573. https://doi.org/10.1007/s00299-007-0373-0
Yang A, He C, Zhang K, Zhang J (2006) Improvement of Agrobacterium-mediated transformation of embryogenic calluses from maize elite inbred lines. Vitr Cell Dev Biol 42:215–219. https://doi.org/10.1079/IVP2006768
Yoshida S, Forno DA, Cock JA, Gomez KA (1976) Laboratory manual for plant physiological studies of rice. International Rice Research Institute, Philippines
Zhang J, Xu RJ, Elliott MC, Chen DF (1997) Agrobacterium-mediated transformation of Élite Indica and Japonica rice cultivars. Appl Biochem Biotechnol Part B 8:223–231. https://doi.org/10.1007/BF02760776
Acknowledgements
This research was funded by the Department of Science and Technology (DST), Government of India and J.C. Bose National Fellowship to JPK (SB/SR/JCB-13/2013) by the Science and Engineering Research Board (SERB), Government of India. Authors also acknowledge infrastructural support by the Department of Science and Technology (FIST and PURSE programmes), Government of India, and the University Grants Commission (UGC-SAP), New Delhi. NJ thanks the Council of Scientific and Industrial Research (CSIR), New Delhi, for providing financial assistance in the form of RA Fellowship. The authors are grateful to Dr. A.K. Singh, Department of Genetics, Indian Agricultural Research Institute, for kindly providing healthy seed lots of the seven rice cultivars, used in this study.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
NJ and JPK designed the research plan, NJ performed the experiments, analysed the data and wrote the manuscript. JPK and PK critically supervised the progress of research, gave vital inputs in improvement of the research work and edited the manuscript. JPK funded the cost of the entire research through his grants. All authors have read and approve the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no relevant financial or non-financial conflict of interest.
Additional information
Communicated by Manoj Prasad.
The research article is dedicated to Late Prof. Jitendra P. Khurana.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jain, N., Khurana, P. & Khurana, J.P. A rapid and efficient protocol for genotype-independent, Agrobacterium-mediated transformation of indica and japonica rice using mature seed-derived embryogenic calli. Plant Cell Tiss Organ Cult 151, 59–73 (2022). https://doi.org/10.1007/s11240-022-02331-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02331-3