Abstract
Chlorogenic acid and its derivatives (CADs) are the bioactive components in Gardenia jasminoides Ellis (GJE) fruits but occur at a low level. In the current paper, four types of calluses were induced from GJE leaf explants, and light yellow-green calluses with a fine appearance and texture were selected for the development of a cell suspension culture to produce CADs. Additionally, cultured cells were elicited by salicylic acid (SA) and methyl jasmonate (MeJA) to increase the CADs yield. Seven CADs compounds were identified via HPLC-TOF-MS/MS, namely, 3-caffeoylquinic acid, 4-caffeoylquinic acid, 5-caffeoylquinic acid, 3,5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid, 3,5-dicaffeoyl-4-o-(3-hydroxyl-3-methyl)-glutaroylquinic acid, and malonyl-4,5-o-dicaffeoylquinic acid. This is the first identification of malonyl-4,5-o-dicaffeoylquinic acid from GJE. Both SA and MeJA significantly promoted the CADs yield, but MeJA was more effective than SA. Cells elicited by 200 µM MeJA showed the maximum total CADs content and yield, up to 20.98 ± 1.1 mg g−1 and 232.32 ± 9.1 mg L−1, which were 19.51 times and 14.14 times those of the control, respectively. Furthermore, the total CADs extracted from cells elicited by 200 µM MeJA displayed a much higher antioxidant capacity than that of natural GJE fruits. Our research suggested that a GJE cell culture combined with MeJA elicitation has a promising application in natural CADs production.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gardenia jasminoides Ellis (GJE) is an evergreen shrub of the Gardenia genus in Rubiaceae, and its fruits are commonly used in traditional Chinese medicine and also production of gardenia yellow (a natural carotenoid pigment), which is widely used in the current food and textile industries in China (Carmona et al. 2006; Uekusa et al. 2007). In addition to the yellow pigment, many other bioactive components have also been identified in GJE, such as polyphenols (Bergonzi et al. 2012), iridoid glycosides (Chen et al. 2008), triterpenes (Qin et al. 2015), flavonoids (Zhu et al. 2014), and essential oils (Yan et al. 2006), etc. Among these components, chlorogenic acid and its derivatives (CADs), a variety of polyphenols consisting of hydroxycinnamic acid derivatives of quinic and shikimic acids, have recently attracted increasing attention from researchers (Bergonzi et al. 2012; He et al. 2010). He et al. (2010) identified six CADs from GJE fruits but found that the total CADs content was only 5.93 mg g−1. Although investigators demonstrated that CADs have various bioactivities, such as antioxidant, antibiosis, anti-inflammation, antivirus, and antitumor activities (Ali et al. 2017; Huang et al. 2017), the application of CADs in GJE fruits has been hindered by its low content.
Plant secondary metabolites such as CADs are usually extracted and purified from natural plants such as Lonicera japonica and Eucommia ulmoides (Wang et al. 2003; Zou et al. 2016). However, it normally takes a long time to grow the plants, and furthermore, plant growth is variably affected by the climate, environment, insect infestation, and microbial diseases during the entire growth cycle (Murthy and Praveen 2012), which makes it more difficult and expensive to extract secondary metabolites from natural plants. Therefore, researchers have attempted to find new alternative approaches to produce the bioactive secondary metabolites of plants. Among these new approaches, plant cell culture has been noted as one of the most promising methods because of its various prominent advantages. For example, this production method depends less on the climate and environment and makes it easier to control and enlarge the production scale, particularly because it has a shorter production cycle (Bonfill et al. 2011; Rao and Ravishankar 2002).
In our laboratory, Lin et al. (2017) optimized the GJE callus culture parameters and further identified three CADs compounds from this callus using HPLC, i.e., 3-caffeoylquinic acid, 3,5-dicaffeoylquinic acid, and 4,5-dicaffeoylquinic acid. However, the total CADs content of this GJE callus was only 2.54 mg g−1 (Lin et al. 2017), which was 42.8% of that in GJE fruit (He et al. 2010). It is difficult to produce CADs by culturing such calluses or cells with low CADs content. Certain novel techniques were required to increase the CADs yield, and therefore, we tested several technologies to improve CADs yield, such as callus and cell strain screening, elicitation, culture parameter optimization, and fed batch culture.
Elicitation is a potential strategy for increasing the metabolite yield of cultured plant cells with respect to high efficiency, low costs, and easy operation (Murthy et al. 2014; Xu et al. 2016). Methyl jasmonate (MeJA) and salicylic acid (SA) were two of the most recently studied elicitors for stimulating the synthesis of metabolites in cultured cells (Boroduske et al. 2016; Cai et al. 2017; Meier et al. 2015). It was reported that jasmonic acid (JA) and SA are critical endogenous signal molecules in the plant signal transduction network, which regulates many important physiological activities such as growth, differentiation, and metabolism (Wasternack and Hause 2013). Research indicated that exogenous MeJA and SA can lead to physiological activities similar to those of their endogenic counterparts, such as hypersensitivity and oxidative stress, and therefore could be used to promote metabolite synthesis (Wasternack and Hause 2013; Zhang et al. 2016). Nopo-Olazabal et al. (2014) reported that MeJA stimulated resveratrol synthesis in Vitis rotundifolia hairy root culture with a maximum content of 466 nmol g−1 (7.36 times that of the control). SA was used as an elicitor to enhance flavonoid yield in cultured Andrographis paniculata (Burm. f.) Nees cells, and the total content of flavonoids in treated cells reached 1.72 mg g−1 (1.39 times that of the control) (Mendhulkar and Vakil 2013).
However, few studies exist on GJE callus induction and culture, and no reports are available on CADs production by GJE cell culture. In the current paper, GJE cell suspension culture was established to produce CADs, and MeJA and SA were used as elicitors to increase the CADs yield. Seven CADs compounds were identified from the cultured GJE cells via HPLC-TOF-MS/MS. In addition, the antioxidant activities of the total CADs extracts from the elicited cells were assessed using the radical-scavenging assay and reducing capacity assay. To the best of our knowledge, this is the first report of identification of malonyl-4,5-o-dicaffeoylquinic acid in GJE.
Materials and methods
Materials and chemicals
The aseptic GJE seedlings were cultured in the Jiangxi Key Laboratory of Natural Products and Functional Food at Jiangxi Agricultural University, Nanchang, China. The main culture conditions are described as follows: Murashige and Skoog (MS) solid medium supplemented with 0.5 mg L−1 6-benzylaminopurine, 0.2 mg L−1 1-naphthylacetic acid, 0.7% agar powder, and 30 g L−1 sucrose under a photoperiod of 16/8 h (light/dark) at 28 ± 1 °C. Authentic compounds, including 3-caffeoylquinic acid (HPLC grade, ≥ 98%), 4-caffeoylquinic acid (HPLC grade, ≥ 98%), 5-caffeoylquinic acid (HPLC grade, ≥ 98%), 3,5-dicaffeoylquinic acid (HPLC grade, ≥ 97%), and 4,5-dicaffeoylquinic acid (HPLC grade, ≥ 97%), were purchased from Solarbio Co. (Beijing China). 3,5-dicaffeoyl-4-o-(3-hydroxyl-3-methyl)-glutaroylquinic acid (HPLC grade, ≥ 98%) was purchased from Lunan Pharmaceutical Group Co. (Linyi, China). Other reagent-grade chemicals obtained from Sigma-Aldrich Co. (Shanghai, China).
Callus induction
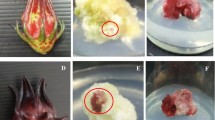
Young GJE leaves (Fig. 1a) were excised from the aseptic GJE seedlings, cut into 5 mm × 5 mm segments, and inoculated on MS solid medium supplemented with kinetin (KT) and 1-naphthylacetic acid (NAA) at different concentrations (concentrations of KT and NAA are shown in Table 1). Thirty leaf segments were involved in each hormone combination group, and each experiment was performed in triplicate. To obtain a callus suitable for establishing the cell suspension culture, different types of calluses were collected from the leaf explants, subcultured at day 40, and repeatedly subcultured and screened every 30 days.
Cell suspension culture
The cell suspension culture was established according to the reported method with selected modifications (Sang et al. 1991). The detailed procedures are described as follows: 18 g fresh light yellow-green calluses (fresh weight, FW) with fine appearance and texture were inoculated into a 250 mL culture flask containing 120 mL liquid MS medium supplemented with 0.3 mg L−1 KT, 0.5 mg L−1 NAA, and 30 g L−1 sucrose, cultivated in a gyratory shaker with 115 rpm at 28 ± 1 °C under continuous light, and sub-cultured every 10 days. Before the first sub-culture, cells were sieved through a 60-mesh stainless steel sieve to filter out the large cell masses. After the 10th subculture, the suspended cultured cells were homogeneous and stable and thus suitable for further experiments.
Growth characteristic assay
Amounts of 6 g of fresh cells were collected on the 10th day after inoculation, inoculated into a 100 mL culture flask containing 40 mL MS liquid medium supplemented with 0.3 mg L−1 KT, 0.5 mg L−1 NAA, and 30 g L−1 sucrose, and cultivated in a gyratory shaker with 115 rpm at 28 ± 1 °C under continuous light. Cells were harvested every 2 days during the entire 16-day growth cycle. The dry weight (DW) and CADs content of the collected cells were determined. The growth curve and CADs accumulation curve were generated according to the changes in cell biomass and CADs content.
Elicitation experiment
SA and MeJA were used as elicitors to increase the CADs yield of the cultured GJE cells. The elicitation procedures are described as follows: 6 g of fresh cells were transferred into a culture flask containing 40 mL fresh MS medium supplemented with 0.3 mg L−1 KT, 0.5 mg L−1 NAA, and 30 g L−1 sucrose and cultured in a gyratory shaker with 115 rpm at 28 ± 1 °C under continuous light. On the 5th day, elicitors at serial concentrations (SA: 10, 25, 50, and 100 µM; MeJA: 50, 100, 200, and 400 µM) were individually added into the cultures, and cells were collected on the 1st, 2nd, and 3rd day after elicitation. The dry weight and CADs content of the elicited cells were determined.
CADs extraction
CADs extraction were performed in accordance with the method described by Fu et al. (2015). An amount of 200 mg of fine-powdered cell sample (dried at 60 °C until a constant weight was observed) was extracted by 4 mL 70% methanol (v/v) in an ultrasonic water bath for 50 min and centrifuged at 4000 rpm for 5 min. The supernatant was collected and diluted to 10 mL before filtering through a 0.22µ m membrane for HPLC-TOF-MS/MS and HPLC determination.
Identification of CADs by HPLC-TOF-MS/MS
CADs from the cultured GJE cells were identified via HPLC-TOF-MS/MS (6430 HPLC-MS system, Aglient Technologies, USA). The determination was conducted according to the method reported by Zhang et al. (2013).
Quantification of CADs by HPLC
CADs from the cultured GJE cells were quantified via HPLC (1260 HPLC system, Aglient Technologies, USA) on a C18 column (4.6 mm × 250 mm, 5 µm; Waters, Milford, MA, USA). The HPLC parameters are described as follows: mobile phase: acetonitrile (A) and 0.2% acetic acid water (B); flow rate: 1 mL min−1; elution procedure: 0–35 min, 5–38.5% A; 35–40 min, 38.5–80% A; 40–45 min, 80–5% A; detection wavelength: 327 nm; column temperature: 40 °C; and volume of sample injection: 10 µL.
Authentic compounds (10.0 mg) were dissolved and diluted to 10 mL with 70% methanol (v/v), and the concentrations were adjusted to the appropriate ranges: 5, 10, 20, 40, 80, 120, 160, and 200 µg mL−1 (3-CQA); 5, 10, 20, 40, 50, 60, 80, and 100 µg mL−1 (4-CQA and 5-CQA); 20, 40, 60, 80, 120, 160, 180 and 200 µg mL−1 (3,5-diCQA); 20, 30, 40, 50, 60, 70, 80, and 100 µg mL−1 (4,5-diCQA); 20, 40, 80, 160, 200, 300, 360, and 400 µg mL−1 (3,5-diC(HMG)QA). The established linear regression equations based on the HPLC determination are shown in Table 1.
Antioxidant activity assay
The antioxidant activities of total CADs extracted from the cultured GJE cells and natural fruits were assessed using the radical-scavenging assay (DPPH and ABTS+) and reducing capacity assay (Ferricyanide/Prussian blue assay) according to the methods of Carocho et al. (2012) and Zhang et al. (2017).
Data analysis and statistics
All experiments were performed in triplicate, and the results were given as the mean value ± standard deviation (SD). The data were analysed using DPS 6.55 statistical software. Statistical analysis was performed with ANOVA followed by Duncan’s multiple range test (DMRT) (at P ≤ 0.05).
Results and discussion
GJE callus induction
Previous studies showed that to a great extent, callus induction was significantly affected by the type, concentration, and combination of plant hormones (Roy et al. 2008). The current paper investigated the effects of hormones at different concentrations and ratios on the induction of GJE callus, as shown in Table 2 and Fig. 1. The callus grew out in all of the treated groups. However, the occurrence time, colour, callus formation rate, and appearance were significant different among these groups. Generally, the callus appeared on the edge of the leaf explants between the 12th and 19th day, expanded gradually, and finally covered the entire explants.
In terms of GJE callus formation and growth rate, NAA played a more important role than KT. When the KT concentration remained unchanged, the callus grew quite well in the medium with a moderate concentration of NAA (0.25–0.5 mg L−1), but excessively high or low NAA concentration was unfavourable to callus formation and growth (Table 2). Overall, MS medium with 0.3 mg L−1 KT and 0.5 mg L−1 NAA was most suitable for GJE callus induction and produced a callus formation rate of 97.78 ± 1.54%.
Hormones exerted noticeable effects not only on the callus formation rate but also on the callus appearance and texture. In our GJE callus induction experiments, four types of calluses with different colours were obtained, namely, brown, green, light yellow–green, and gray–white (Fig. 1). In general, the induced callus usually appeared brown (Fig. 1b) or green (Fig. 1c) in colour when the KT concentration was greater than that of NAA. In contrast, the callus often appeared light yellow–green (Fig. 1d) or gray–white (Fig. 1e). The calluses with different colour normally differed in growth rate, texture, and surface morphology. The brown and green calluses generally showed a low growth rate, loose texture, and smooth surface, whereas the yellow–green and gray–white calluses exhibited a high growth rate, compact texture, and granular surface.
Establishment of GJE cell suspension culture
The light yellow–green GJE calluses were subcultured and screened repeatedly to find an excellent callus for establishment of the cell suspension culture. After ten repetitions of subculturing and screening, we obtained a callus strain with a high growth rate and fine appearance, which was suitable for the further suspension culture experiment. This callus strain was transferred to the liquid MS medium and subcultured repeatedly. After another ten subculture repetitions, a stable GJE cell suspension culture was achieved with a high growth rate and small granular texture (Fig. 1f). Similar to our results, Sang et al. (1991) also reported that repeated subculture contributed to establishment of the cell suspension culture.
Qualitative analysis of CADs from suspended cultured GJE cells
HPLC-TOF-MS/MS analysis was conducted to identify the main secondary metabolites in the suspended cultured GJE cells. The results displayed seven main peaks in the HPLC chromatogram of GJE cell extracts (Fig. 2), which produced quasi-molecular ion peaks at m/z 353, 353, 353, 515, 515, 659, and 601, respectively (Fig. 3). Furthermore, these compounds showed one or several fragment ions at m/z 515, 353, 191, 179, 173, or 135, which could be viewed as diagnostic fragment-ions (DFIs) for the identification of CADs. The accurate structures of the CADs could be further deduced according to the relative intensity of the DFIs compared with that of the base ion (Zhang et al. 2013).
HPLC chromatogram of CADs extracted from different suspended cultured GJE cells (327 nm). ① CADs extracted from the cells stimulated by 200 µM MeJA (collected on the 3rd day after elicitation); ② CADs extracted from the cells stimulated by 50 µM SA collected on the 2nd day after elicitation; ③ CADs extracted from the cells of the control (untreated). Peak 1 5-CQA, Peak 2 3-CQA, Peak 3 4-CQA, Peak 4 3,5-diCQA, Peak 5 4,5-diCQA, Peak 6 3,5-diC (HMG) QA, Peak 7 M-4,5-diCQA
Chromatograms of HPLC-TOF-MS/MS determinations of CADs from GJE cells. a Compound 1: 5-CQA (peak 1 in Fig. 2); b Compound 2: 3-CQA (peak 2 in Fig. 2); c Compound 3: 4-CQA (peak 3 in Fig. 2); d Compound 4: 3,5-diCQA (peak 4 in Fig. 2); e Compound 5: 4,5-diCQA (peak 5 in Fig. 2); f Compound 6: 3,5-diC (HMG) QA (peak 6 in Fig. 2); g Compound 7: M-4,5-diCQA (peak 7 in Fig. 2)
In our MS/MS determination, compounds 1 (peak 1, RT (retention time): 11.4 min), 2 (peak 2, RT: 13.6 min), and 3 (peak 3, RT: 15.4 min) all exhibited a quasi-molecular ion peak ([M–H]−) at m/z 353 (Fig. 3a–c) and DFIs at m/z 191, 179, 173, and 135, which might derive from mono-caffeoylquinic acids (mono-CQAs). Compounds 4 (peak 4, RT: 24.6 min) and 5 (peak 5, RT: 26.4 min) showed a [M–H]− at m/z 515 and the DFIs from di-caffeoylquinic acids (di-CQAs) at m/z 353, 191, 179, 173, and 135 (Fig. 3e, f). Therefore, these compounds might be preliminarily identified as di-CQAs. Compound 6 (peak 6, RT: 27.5 min) and 7 (peak 7, RT: 28.6 min) displayed [M–H]− at m/z 659 (Fig. 3f) and 601 (Fig. 3g), respectively, and the DFIs from di-CQAs at the same time, which suggested that a di-CQA group was present in these two compounds. The deduced fragmentation pathways of mono-CQA, di-CQA, and malonyl-CQA in MS/MS determination are shown in Fig. 4 (taking 5-CQA, 4,5-diCQA, and malonyl-4,5-o-dicaffeoylquinic acid as examples). Based on published data (Gouveia and Castilho 2009; Zhang et al. 2007, 2013) and HPLC-TOF-MS/MS and HPLC analyses of the authentic chemicals, compounds 1–7 were confirmed as 5-CQA, 3-CQA, 4-CQA, 3,5-diCQA, 4,5-diCQA, 3,5-dicaffeoyl-4-o-(3-hydroxyl-3-methyl)-glutaroyl-quinic acid (3,5-diC (HMG) QA), and malonyl-4,5-o-dicaffeoylquinic acid (M-4,5-diCQA), respectively. To the best of our knowledge, this is the first time that M-4,5-diCQA was found in GJE.
He et al. (2010) identified six CADs from GJE fruit, i.e., three mono-CQAs (5-CQA, 3-CQA,and 4-CQA) and three di-CQAs (3,4-diCQA, 3,5-diCQA, and 4,5-diCQA). Selected di-CQA derivatives such as 3,5-dicaffeoyl-4-o-(3-hydroxyl-3-methyl)-glutaroylquinic acid and 3,4-dicaffeoyl-5-o-(3-hydroxyl-3-methyl)-glutaroylquinic acid were also separated and identified from the GJE fruit (Nishizawa et al. 1987). However, it was noteworthy that there were certain caffeoylquinic acid derivatives with a sinapoyl group such as 3-caffeyol-4-sinapoylquinic acid, methyl 5-o-caffeoyl-4-o-sinapoylquinate, ethyl 3-caffeoyl-5-sinapoylquinate, and their isomers, which were previously found in the GJE fruits (Fujimoto and Nishizawa 1986; Yang et al. 2013), were not detected in the cultured cells.
Interestingly, no gardenia yellow pigment was detected in the suspended cultured GJE cells. The gardenia pigment, a type of water-soluble natural carotenoid colorant extracted from GJE fruits, has good resistance to light and temperature in neutral and weak alkaline media. Currently, this colorant is in wide use in foods and textiles, especially in foods made primarily from starches and proteins (Uekusa et al. 2007). Most of the plant secondary metabolites are usually synthesized in differentiated tissues such as leaf, flower, and fruit. In our experiments, this pigment did not appear in the GJE calluses and suspended cultured cells, perhaps because both of these represented dedifferentiated cells. Further research is still needed to understand the detailed mechanism, which could be helpful to increase the production of gardenia pigment by cultured GJE cells.
Growth characteristics and CADs accumulation of suspended cultured GJE cells
The growth curve of suspended cultured GJE cells displayed a typical S-shape (as shown in Fig. 5a). An entire growth cycle lasted for 16 days and could be roughly divided into the following four stages: (1) lag phase (0–4th day); (2) exponential growth phase (4th–10th day); (3) stationary phase (10th–12th day), and (4) declining phase (12th–16th day). The cells required 4 days to adapt to the new culture environment and grew slowly after inoculation. In the exponential growth phase, cells proliferated quickly, and as a result, the dry weight of cells increased 1.94-fold in 6 days. Peak biomass appeared on the 12th day (23 ± 0.25 g L−1, DW) and was 3.96 times that of the initial inoculation biomass. From the 12th day, the biomass began to gradually decrease, perhaps due to exhaustion of nutrients in the culture medium (Yin et al. 2013).
The content of six CADs in suspended cultured GJE cells was determined and calculated according to the determination regression equations (Table 1) every 2 days during a growth cycle. Compound 7 was not quantified because no authentic M-4,5-diCQA was obtained. The results (Fig. 5b) indicated that the contents of 5-CQA and 4-CQA were notably low and steady, and their maxima were only 0.009 ± 0.001 and 0.04 ± 0.01 mg g−1 (DW), respectively, during the whole growth cycle. Compounds 3-CQA, 3,5-diCQA, 4,5-diCQA, and 3,5-diC (HMG) QA were the main CADs components in the cells, with contents much higher than those of 5-CQA and 4-CQA. Among the quantified six CADs, 3,5-diC (HMG) QA was the most dominant component and reached 0.46 ± 0.02 mg g−1 (DW) on the 16th day.
The total CADs content and yield in suspended cultured GJE cells during the entire growth cycle are shown in Fig. 5c, d. In the first 4 days, the total content of CADs increased swiftly, and the rate of increase subsequently slowed. From the 6th to 10th day, the total content decreased quickly, and remained stable until the end. The maximal total CADs content reached 1.24 ± 0.07 mg g−1 (DW) on the 6th day, which was 4.13 times the initial value. However, the total CADs yield showed a different profile. The total yield continued to grow rapidly and linearly from the beginning to the 10th day, remained steadily as culture time progressed, and peaked at the 14th day (23.42 ± 0.9 mg L−1, 13.16 times the initial value). Consequently, from the point of view of production, the 14th day was the optimal harvest time.
It was reported that the total CADs content of GJE fruit was 5.93 mg g−1 (He et al. 2010), which was higher than that of our suspended cultured cells (1.24 ± 0.07 mg g−1). Therefore, further elicitation experiments were conducted to increase the CADs content in GJE cells.
Effects of MeJA and SA elicitation on GJE cell growth
According to Mendhulkar and Vakil (2013), elicitation improved the yield of target compounds but inhibited cell growth in Andrographis paniculata (Burm. f.) Nees cell culture. In our elicitation experiments, a similar inhibition of GJE cell growth was also observed under high MeJA and SA concentrations (Fig. 6a, b). When the cells were elicited by 100 µM SA or 400 µM MeJA, the dry weights of cells collected on the 3rd day after elicitation were 11.81 ± 0.21 and 10.05 ± 0.27 g L−1 (DW), respectively, which were 77.3 and 65.8% that of the control.
Effects of MeJA and SA elicitation on biomass (dry weight), total CADs content, and total CADs yield of suspended cultured GJE cells. a Effects of SA elicitation on biomass; b Effects of MeJA elicitation on biomass; c Effects of SA elicitation on total CADs content; d Effects of SA elicitation on total CADs yield; e Effects of MeJA elicitation on total CADs content; f Effects of MeJA elicitation on total CADs yield
Effects of MeJA and SA elicitation on the total CADs content and yield in GJE cells
Both SA and MeJA elicitation significantly improved the CADs content and yield of the cultured GJE cells (as shown in Figs. 2, 6c–f). Comparatively speaking, MeJA elicitation was more effective than SA. In the SA elicitation experiment, the peak total CADs content and yield (compound 7 was not included) were 3.34 times (4.61 ± 0.2 mg g−1, DW) and 2.86 times (49.15 ± 1.3 mg L−1) that of the control, respectively, and were recorded on the 2nd day under a 50 µM SA elicitation. However, much higher content and yield values were found in MeJA clicitation experiments. When the cells were stimulated with 200 µM MeJA, the highest total CADs content and yield were recorded on the 3rd day, reaching up to 19.51 (20.98 ± 1.13 mg g−1, DW) and 14.14 (232.32 ± 9.1 mg L−1) times that of the control, respectively.
It should be noted that another main component (Peak 7, M-4,5-diCQA) was not included in the total CADs content because it could not be quantified without an authentic compound. However, the content of M-4,5-diCQA could be estimated according to its peak area in the HPLC chromatogram. With SA and MeJA elicitation, the maximum peak areas of this component were 14.1 and 50.3 times that of the control, respectively. Including this compound (its putative content was calculated according to the regression equation for 4,5-diCQA determination), the total CADs content and yield of the cells elicited by 200 µM MeJA could reached approximately 26.27 mg g−1 (DW) and 290.91 mg L−1, respectively.
Several other plants have high CADs content, e.g., Lonicera japonica and Eucommia ulmoides. It was reported that the major CADs components in Lonicera japonica Thunb flowers were mono-CQAs, di-CQAs and that its total CADs content reached 28.58 mg g−1 (DW) (Zou et al. 2016). Similarly, mono-caffeoylquinic acids were the main CADs component in Eucommia ulmoides Oliver leaves, and the total CADs contents ranged from 23.4 to 25.6 mg g−1 (DW) (Wang et al. 2003). Compared with the above-mentioned two plants, the total CADs content of our suspended cultured cells was relatively low (1.24 ± 0.07 mg g−1, DW). However, under MeJA elicitation, the total CADs content increased to 20.98 ± 1.13 mg g−1 (DW), which was 19.51-fold that of the cells without the elicitor and 3.54-fold that of the GJE fruit (5.93 mg g−1, DW). If M-4,5-diCQA (compound 7) was counted in, the total CADs content reached approximately 26.27 mg g−1 (DW). Furthermore, it is much more convenient and easy to extract and purify CADs from cultured GJE cells than from plant leaves, flowers, or fruits because there are fewer impurities in the cultured plant cells. Moreover, in addition to the common mono-CQAs and di-CQAs, two unusual CADs compounds (3,5-diC (HMG) QA and M-4,5-diCQA) were found in the elicited GJE cells at high content. In summary, MeJA elicitation could dramatically enhance the total CADs content and yield in suspended cultured GJE cells and is therefore promising in CADs production.
Effects of MeJA and SA elicitation on the content of individual CADs in GJE cells
As shown in Fig. 7, the elicitors displayed different stimulation effects on synthesis of the six CADs in suspended cultured GJE cells. Among the quantified six CADs, the contents of 5-CQA and 4-CQA were minimally affected by the elicitation of SA and MeJA within our tested concentration during the entire elicitation experiment period. However, the synthesis of 3,5-diC (HMG) QA, 3,5-diCQA, and 3-CQA rose significantly under elicitation, especially 3,5-diC (HMG) QA, which led to a massive increase in the total CADs content of the cells. Overall, the contents of 3,5-diC (HMG) QA, 3,5-diCQA, and 3-CQA first increased and subsequently decreased with the increase in MeJA and SA concentration.
Effects of MeJA and SA elicitation on individual CADs compound content of suspended cultured GJE cells. a Effects of SA elicitation on individual CADs compound content (cells collected on the 1st day after elicitation); b Effects of MeJA elicitation on individual CADs compound content (cells collected on the 1st day after elicitation); c Effects of SA elicitation on individual CADs compound content (cells collected on the 2nd day after elicitation); d Effects of MeJA elicitation on individual CADs compound content (cells collected on the 2nd day after elicitation); e Effects of SA elicitation on individual CADs compound content (cells collected on the 3rd day after elicitation); f Effects of MeJA elicitation on individual CADs compound content (cells collected on the 3rd day after elicitation)
In the SA elicitation experiments, the peak content of 3,5-diCQA and 3-CQA appeared on the 2nd day under stimulation of 50 µM SA, whereas the peak content of 3, 5-diC (HMG) QA was observed on the 3rd day under 100 µM SA elicitation, and was 4.43 times that of the control. However, all of the six CADs contents reached their peaks on the 3rd day when the cells were elicited with 200 µM MeJA. Within the tested concentrations of this paper, MeJA showed a better stimulative effect on CADs synthesis than SA. Under stimulation by MeJA, the maximal content of 3,5-diC (HMG) QA was 20.83 times that of the control and reached 9.79 ± 0.13 mg g−1 (DW), whereas under the elicitation of SA, the peak content of 3,5-diC (HMG) QA was 4.43 times that of the control (2.08 ± 0.08 mg g−1, DW).
Antioxidant activities of the total CADs from the elicited GJE cells
In this study, the antioxidant activities of CADs extracted from the cultured GJE cells (elicited by 200 µM MeJA) and natural fruits were both evaluated via radical-scavenging activity assay (DPPH and ABTS+) and reducing capacity assay. Overall, the total CADs from both cells and fruits showed strong antioxidant activities, and an obvious dose-dependent relationship was observed between the CADs concentration and antioxidant activity (Fig. 8). Compared with total CADs from fruits, the total CADs from cells had a much stronger radical-scavenging activity, which was only slightly lower than that of Vc (vitamin C, positive control) at the same concentration in both DPPH and ABTS+ assay (Fig. 8a, b). The IC50 values of the DPPH radical-scavenging activity of total CADs from cells and fruits were 3.34 and 4.93 µg mL−1, respectively, and the IC50 values of ABTS+ radical-scavenging activity were 36.85 and 45.14 µg mL−1, respectively. In terms of the reducing capacity, the total CADs from cells and fruits and Vc all showed outstanding activity (Fig. 8c). Similar to the above-mentioned two radical-scavenging activities, the reducing capacity of the total CADs from cells was stronger than that from natural fruits but lower than that of Vc.
Antioxidant activities of total CADs extracted from GJE cells elicited by MeJA, total CADs extracted from natural GJE fruits, and vitamin C; a DPPH radical-scavenging activity of total CADs extracts and vitamin C, b ABTS+ radical-scavenging activity of total CADs extracts and vitamin C; c Reducing capacity of total CADs extracts and vitamin C
It was reported that CADs were the major contributors to the ABTS+ free-radical scavenging activity in GJE fruit extracts, which were responsible for 86.39% of this activity, and 3,5-diCQA was the major antioxidant compound among these CADs (He et al. 2010). The antioxidant capacity of CADs might depend on the hydroxy group number in their molecules, and consequently, di-CQAs normally exhibited a better antioxidant activity than mono-CQAs because one more caffeoyl moiety is present in di-CQAs. (Islam et al. 2003) In our cultured GJE cells elicited by 200 µM MeJA, the contents of 3,5-diCQA, 4,5-diCQA, and 3,5-diC (HMG) QA were 4.82 ± 0.14, 1.08 ± 0.07, and 9.79 ± 0.13 mg g−1 (DW), respectively, and the total contents of these components accounted for 76.8% of the total quantified content of the six CADs. Therefore, di-CQAs might be the principal contributors to the antioxidant activities of total CADs from cultured GJE cells. The difference in antioxidant activities between the total CADs from cultured cells and natural fruits might be caused by their difference in CADs compounds.
Conclusion
GJE cell suspension culture was developed to produce CADs. Seven CADs compounds from the cultured cells were identified via HPLC-TOF-MS/MS. Both SA and MeJA elicitation significantly increased the CADs yield, but MeJA was more effective than SA. The total CADs content and yield of cells elicited by 200 µM MeJA reached up to 20.98 ± 1.1 mg g−1 and 232.32 ± 9.1 mg L−1, respectively. Furthermore, the total CADs from elicited GJE cells showed outstanding antioxidant activities, and di-CQAs might be the principal contributors to these activities. Our research indicated that GJE cell suspension culture combined with MeJA elicitation has promising applications in natural CADs production.
Abbreviations
- GJE:
-
Gardenia jasminoides Ellis
- CADs:
-
Chlorogenic acid and its derivatives
- SA:
-
Salicylic acid
- MeJA:
-
Methyl jasmonate
- NAA:
-
1-Naphthylacetic acid
- KT:
-
Kinetin
- DFIs:
-
Diagnostic fragment-ions
- 3-CQA:
-
3-caffeoylquinic acid
- 4-CQA:
-
4-caffeoylquinic acid
- 5-CQA:
-
5-caffeoylquinic acid
- 3,5-diCQA:
-
3,5-dicaffeoylquinic acid
- 4,5-diCQA:
-
4,5-dicaffeoylquinic acid
- 3,5-diC(HMG)QA:
-
3,5-dicaffeoyl-4-o-(3-hydroxyl-3-methyl)-glutaroylquinic acid
- M-4,5-diCQA:
-
Malonyl-4,5-o-dicaffeoylquinic acid
References
Ali N, Rashid S, Nafees S, Hasan SK, Shahid A, Majed F, Sultana S (2017) Protective effect of chlorogenic acid against methotrexate induced oxidative stress, inflammation and apoptosis in rat liver: an experimental approach. Chem Biol Interact 272:80–91
Bergonzi MC, Righeschi C, Isacchi B, Bilia AR (2012) Identification and quantification of constituents of Gardenia jasminoides Ellis (Zhizi) by HPLC-DAD-ESI-MS. Food Chem 134:1199–1204
Bonfill M, Mangas S, Moyano E, Cusido RM, Palazón J (2011) Production of centellosides and phytosterols in cell suspension cultures of Centella asiatica. Plant Cell Tiss Organ Cult 104:61–67
Boroduske A, Nakurte I, Tomsone S, Lazdane M, Boroduskis M, Rostoks N (2016) In vitro culture type and elicitation affects secoiridoid and xanthone LC–ESI–TOF MS profile and production in Centaurium erythraea. Plant Cell Tiss Organ Cult 126:1–5
Cai J, Ma Y, Hu P, Zhang Y, Chen J, Li X (2017) Elicitation of furanocoumarins in Changium smyrnioides suspension cells. Plant Cell Tiss Organ Cult 130:1–12
Carmona M, Zalacain A, Sanchez AM, Novella JL, Alonso GL (2006) Crocetin esters, picrocrocin and its related compounds present in Crocus sativus stigmas and Gardenia jasminoides fruits. Tentative identification of seven new compounds by LC-ESI-MS. J Agric Food Chem 54:973–979
Carocho M, Antonio AL, Barros L, Bento A, Botelho ML, Kaluska I, Ferreira IC (2012) Comparative effects of gamma and electron beam irradiation on the antioxidant potential of Portuguese chestnuts (Castanea sativa Mill.). Food Chem Toxicol 50:3452–3455
Chen QC, Youn U, Min BS, Bae K (2008) Pyronane monoterpenoids from the fruit of Gardenia jasminoides. J Nat Prod 71:995–999
Fu X, Yin ZP, Chen JG, Shangguan XC, Wang X, Zhang QF, Peng DY (2015) Production of chlorogenic acid and its derivatives in hairy root cultures of Stevia rebaudiana. J Agric Food Chem 63:262–268
Fujimoto YB, Nishizawa M (1986) Isolation and structural elucidation of a new lipoxygenase inhibitor from gardeniae fructus. Chem Pharm Bull 34:1419–1421
Gouveia SC, Castilho PC (2009) Analysis of phenolic compounds from different morphological parts of Helichrysum devium by liquid chromatography with on-line UV and electrospray ionization mass spectrometric detection. Rapid Commun Mass Spectrom 23:3939–3953
He W, Liu X, Xu H, Gong Y, Yuan F, Gao Y (2010) On-line HPLC-ABTS screening and HPLC-DAD-MS/MS identification of free radical scavengers in Gardenia (Gardenia jasminoides Ellis) fruit extracts. Food Chem 123:521–528
Huang WY, Fu L, Li CY, Xu LP, Zhang LX, Zhang WM (2017) Quercetin, hyperin, and chlorogenic acid improve endothelial function by antioxidant, antiinflammatory, and ACE inhibitory effects. J Food Sci 82:1239–1246
Islam MS, Yoshimoto M, Yamakawa O (2003) Distribution and physiological functions of caffeoylquinic acid derivatives in leaves of sweetpotato genotypes. J Food Sci 68:111–116
Lin P, Yin ZP, Chen JG, Wu S (2017) Effects of different culture conditions on callus growth and chlorogenic acid accumulation in Gardenia jasminoides Ellis. Mod Food Sci Technol 33:181–188
Meier P, Hotti H, Rischer H (2015) Elicitation of furanocoumarins in poison hemlock (Conium maculatum L.) cell culture. Plant Cell Tiss Organ Cult 123:443–453
Mendhulkar VD, Vakil MMA (2013) Elicitation of flavonoids by Salicylic acid and Penicillium expansum in Andrographis paniculata (Burm. f.) Nees. cell culture. Res Biotechnol 4:01–09
Murthy HN, Praveen N (2012) Influence of macro elements and nitrogen source on adventitious root growth and with an olide-A production in Withania somnifera (L.) Dunal. Nat Prod Res 26:466–473
Murthy HN, Lee E-J, Paek K-Y (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tiss Organ Cult 118:1–16
Nishizawa M, Izuhara R, Kaneko K, Fujimoto Y (1987) 3-Caffeoyl-4-sinapoylquinic acid, a novel lipoxygenase inhibitor from Gardeniae fructus. Chem Pharm Bull 35:2133
Nopo-Olazabal C, Condori J, Nopo-Olazabal L, Medina-Bolivar F (2014) Differential induction of antioxidant stilbenoids in hairy roots of Vitis rotundifolia treated with methyl jasmonate and hydrogen peroxide. Plant Physiol Biochem 74:50–69
Qin FM, Liu BL, Zhang Y, Zhou GX (2015) A new triterpenoid from the fruits of Gardenia jasminoides var. radicans Makino. Nat Prod Res 29:633–637
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Roy A, Ghosh S, Chaudhuri M, Saha P (2008) Effect of different plant hormones on callus induction in Gymnema sylvestris R. Br.(Asclepiadaceae). Afr J Biotechnol 7:2209–2211
Sang H, Park YG, LEE AYH (1991) Suspension culture of Gardenia jasminoides Ellis cell for production of yellow pigment. J Microbiol Biotechnol 1:142–149
Uekusa Y, Sugimoto N, Sato K, Yun YS, Kunugi A, Yamazaki T, Tanamoto K (2007) Neocrocin A: a novel crocetin glycoside with a unique system for binding sugars isolated from gardenia yellow. Chem Pharm Bull 55:1643–1646
Wang J, Liao X, Zhang H, Du J, Chen P (2003) Accumulation of chlorogenic acid in cell suspension cultures of Eucommia ulmoides. Plant Cell Tiss Organ Cult 74:193–195
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111:1021–1058
Xu A, Zhan JC, Huang WD (2016) Combined elicitation of chitosan and ultraviolet C enhanced stilbene production and expression of chitinase and β-1,3-glucanase in Vitis vinifera cell suspension cultures. Plant Cell Tiss Organ Cult 124:105–117
Yan S, Xin W, Luo G, Wang Y, Cheng Y (2006) Chemical fingerprinting of Gardenia jasminoides fruit using direct sample introduction and gas chromatography with mass spectrometry detection. J AOAC Int 89:40–45
Yang L, Peng K, Zhao S, Chen L, Qiu F (2013) Monoterpenoids from the fruit of Gardenia jasminoides Ellis (Rubiaceae). Biochem Syst Ecol 50:435–437
Yin Z, Shangguan X, Chen J, Zhao Q, Li D (2013) Growth and triterpenic acid accumulation of Cyclocarya paliurus cell suspension cultures. Biotechnol Bioproc Eng 18:606–614
Zhang Y, Shi P, Qu H, Cheng Y (2007) Characterization of phenolic compounds in Erigeron breviscapus by liquid chromatography coupled to electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 21:2971–2984
Zhang JY, Zhang Q, Li N, Wang ZJ, Lu JQ, Qiao YJ (2013) Diagnostic fragment-ion-based and extension strategy coupled to DFIs intensity analysis for identification of chlorogenic acids isomers in Flos Lonicerae Japonicae by HPLC-ESI-MS(n). Talanta 104:1–9
Zhang X, Dong J, Liu H, Wang J, Qi Y, Liang Z (2016) Transcriptome sequencing in response to salicylic acid in salvia miltiorrhiza. PLoS ONE 11:e0147849
Zhang XX, Shi QQ, Ji D, Niu LX, Zhang YL (2017) Determination of the phenolic content, profile, and antioxidant activity of seeds from nine tree peony (Paeonia section Moutan DC.) species native to China. Food Res Int 97:141–148
Zhu H et al (2014) Simultaneous determination of two iridoid glycosides, two anthraquinones and four flavonoid glycosides of Zhi-Zi-Da-Huang decoction in rat plasma by UFLC-MS/MS: application to a comparative pharmacokinetic study in normal and cholestatic liver injury rats. J Chromatogr B 960:116–125
Zou R, You Y, Chen Z, Kai HU, Ran L (2016) Effects of drying methods on polyphenol compounds and antioxidant activities of Lonicera japonica flower. Food Sci 37:78–83
Acknowledgements
The authors are grateful for the financial support of research projects from the Natural Science Foundation of PR China (No. 31260368; No. 31460436) and Food and Drug Administration of Jiangxi Province of China (No. 2015yp17). The authors also acknowledge the HPLC-TOF-MS/MS technical assistance of Analysis and Determination Center, University of Nanchang, Jiangxi, China.
Author information
Authors and Affiliations
Contributions
ZPY and XCS conceived the idea and designed the experiments. ZBL and JGC performed the experiments. ZBL, TL, and PL analyzed the data. All the authors contributed to writing and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Sergio J. Ochatt.
Rights and permissions
About this article
Cite this article
Liu, ZB., Chen, JG., Yin, ZP. et al. Methyl jasmonate and salicylic acid elicitation increase content and yield of chlorogenic acid and its derivatives in Gardenia jasminoides cell suspension cultures. Plant Cell Tiss Organ Cult 134, 79–93 (2018). https://doi.org/10.1007/s11240-018-1401-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-018-1401-1