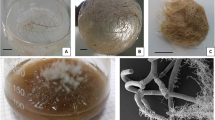
Abstract
Polygonum multiflorum Thunb. is an important medicinal plant that synthesizes an array of phenolic compounds. Its roots are used in a variety of pharmacological and cosmetic formulations, notably as hair dye. In the present study, the inoculum density (3–15 g/L) and culture period (1–7 weeks) were optimized in a 3 L bioreactor. High root biomass (14.18 g/L dry weight (DW)) was recorded with an inoculum of 7 g/L (p ≤ 0.05), which is consistent with the results for 5 and 10 g/L. However, significantly higher yield of bioactive compounds (53.87 mg/g DW total phenolics and 27.96 mg/g DW total flavonoids) with high free radical scavenging activity was obtained in root samples from 5 g/L inoculum density. A 4 week culture period was sufficient for optimum root growth and metabolite production. The optimized conditions were used for large-scale (5 and 20 L) and pilot-scale (500 L) studies. Considering that the continuous aeration of root cultures may lead to oxidative stress, antioxidant enzyme activity and lipid peroxidation also were studied. The results revealed high catalase (CAT) and guaiacol peroxidase (G-POD) activities, and low malondialdehyde (MDA) production, with increasing culture scale (20 and 500 L), which may indicate low-level oxidative damage to the cultures. An optimal yield of 4.01 kg dry root biomass with 287.12 mg/L of total phenolic productivity was achieved in a 500 L pilot-scale bioreactor. This work can pave the way for commercial production of biomass and secondary metabolites at the industrial level, and meet the rising demand for natural ingredients, especially in the pharmaceutical and cosmetic industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polygonum multiflorum Thunb. (family Polygonaceae) is an important medicinal herb of Southeast Asia. Various plant parts are used for specific therapeutic purposes such as antiaging, antidiabetic, anticancer, anti-inflammatory, antimicrobial, and antioxidant (Bounda and Feng 2015). In traditional Chinese medicine, P. multiflorum has been advocated as a tonic for growth and as a hair dye (Han et al. 2015). It is known to contain a range of bioactive compounds such as anthraquinones, stilbenes, flavonoids, tannins, and phospholipids (Li et al. 2015). However, the rising demand for these bioactive compounds led to overexploitation of natural habitat. Alternative production systems must be identified for these bioactive compounds. The production of biologically active metabolites using plant cell and organ culture is an attractive and reliable proposition. Scientific advances in plant biotechnology have led to a better understanding of in vitro conditions and scale-up processes. This is evident from the industrial production of paclitaxel, ginsenosides, and berberine using plant cell and tissue culture (Paek et al. 2009; Murthy et al. 2014d). Metabolic production and biosynthetic pathways are specific for plant species, tissue, or organ, for specific growth and developmental stages, and for specific macro–micro environmental conditions of culture (Paek et al. 2005; Murthy et al. 2014c). A number of in vitro investigations using plant cell cultures are available in the literature, which focus on optimizing factors and parameters to obtain high metabolic yield (Murthy et al. 2014d). However, low productivity, altered metabolic profile, and cell line instability are big threats to the industrial feasibility of such processes. In addition to advances in plant cell culture, recent successes in large-scale bioreactor production of several plant secondary metabolites through adventitious root culture seems to be a promising approach (Paek et al. 2009). Compared with cell cultures, adventitious roots displayed higher stability of secondary metabolite biosynthesis with high growth rates (Sivakumar 2006). In vitro factors like culture medium properties, hormonal balance, gaseous composition, growth kinetics, inoculum density, and culture period are important for scale-up and vary from species to species (Murthy et al. 2008; Baque et al. 2012). In the present study, different process parameters were optimized and successfully employed for pilot-scale production of P. multiflorum adventitious roots.
Materials and methods
Adventitious root culture
The adventitious root cultures of P. multiflorum were maintained in liquid MS (Murashige and skoog 1962) medium supplemented with 9.84 µM indole-3-butyric acid (IBA) and 50 g/L sucrose. Culturing was performed in a balloon-type bubble (air-lift) bioreactor (BTBB) (Lee et al. 2015). These root cultures were regularly subcultured with a 4 week interval and maintained at 24 ± 1 °C under dark conditions.
Optimization of inoculum density, culture period, and scale-up
The adventitious roots were cultivated for 4-weeks at different inoculum densities (3, 5, 7, 10, and 15 g/L FW) to identify the optimum inoculum density for producing high biomass levels and increased levels of bioactive compounds. The optimum period of culture was determined by cultivating the adventitious roots up to 7 weeks. The experiments were performed in a 3 L BTBB (3 L cap) at fixed temperature (24 ± 1 °C) and dissolved oxygen [0.1 vvm (air volume/culture volume min)] under dark conditions throughout the culture period. Each bioreactor contained 2 L full-strength MS medium with an initial pH of 5.7–5.8. The medium was supplemented with 9.84 µM IBA and 5% sucrose. The process was standardized by establishing the optimized reaction variables at different production scales [0.25 L (flask), 3 L, 5 L, 20 L (BTBB), and 500 L (pilot-scale tank bioreactor)].
Determination of growth kinetics
Roots were harvested and washed with distilled water, and excess surface water was removed with tissue paper before drying. The DW was recorded after drying the root to a constant weight at 60 °C. The growth index was determined as follows:
The relative growth rate (RGR) was calculated as follows: RGR = (lnW2 − lnW1)/CP, where ln is the natural log; W1 and W2 are the initial weight and final weight, respectively; and CP is the culture period. The overall productivity (P) of the process was calculated as follows:
Quantification of total phenolic and flavonoid contents
Preparation of root extract
The dried roots (0.5 g) were refluxed (LS-2050-S10, LS-TECH, Korea) with 15 mL of 80% methanol at 80 °C for 1 h, and then filtered through filter paper (Advantec 110 mm, Toyo Rosihi Kaisha Ltd., Japan).
Determination of total phenolic content
The total phenolic content (TPC) was analyzed using the Folin–Ciocalteu colorimetric method (Folin and Ciocalteu 1927). The methanolic extracts (0.05 mL) were mixed with 2.55 mL distilled water, followed by the addition of 0.1 mL (2 N) of Folin–Ciocalteu reagent. After 5 min, 20% Na2CO3 solution (2.5 mL) was added and thoroughly mixed, and then reactions were kept at room temperature under dark conditions. The resulting color change was recorded after 30 min by measuring the absorbance at 760 nm using a spectrophotometer (Optizen POP, Mecasys Co., Ltd, Korea). The data were compared with a standard curve obtained for gallic acid (Sigma Chemical Co., St. Louis, MO, USA) and expressed as mg gallic acid equivalent (GAE) per g DW adventitious roots.
Determination of total flavonoid content
The total flavonoid content (TFC) was determined colorimetrically using the method of Wu et al. (2006). The methanolic root extracts and (+)-catechin (Sigma Chemical Co.) standard (0.25 mL) were mixed with 1.25 mL of distilled water. The 0.075 mL of 5% NaNO2 solution was added later and the mixture was vigorously shaken. After 6 min, 0.15 mL of 10% AlCl3 solution was added. The solution was left for 5 min at room temperature, and the absorbance was measured at 510 nm using a spectrophotometer (Optizen POP, Mecasys Co., Ltd, Korea). The results were expressed as mg (+)-catechin equivalents per g DW adventitious roots.
Determination of free radical scavenging (DPPH) activity
The free radical scavenging activities of P. multiflorum adventitious roots were determined using 1,1-diphenyl-2-picrylhydrazyl (DPPH, Sigma Chemical Co.) according to a previously published method (Hatano et al. 1998). Briefly, 0.8 mL of 200 µM DPPH radical solution was added to 0.2 mL of the methanolic extract. The control contained 0.8 mL DPPH and 0.2 mL of 40% methanol. The solution was kept at room temperature for 5 min, and absorbance was measured at 517 nm using a spectrophotometer (Optizen POP, Mecasys Co., Ltd, Korea). Antioxidant activity was analyzed using following equation:
Determination of bioactive compounds by high performance liquid chromatography
The dry root (0.5 g) was ground with a mortar and pestle. The root powder was extracted with 80% methanol after 3 h sonication (Sonicator, Mujigae, Korea) to ensure the complete extraction of bioactive compound. The extract was filtered through filter paper (Advantic, 110 mm, Japan), and the methanol was evaporated under vacuum to dryness. The residue was dissolved in 10% methanol and fractionated twice with 10 mL of diethyl-ether:ethyl-acetate (1:1), and again evaporated under vacuum to dryness. The residues of both extracts were combined and dissolved in methanol, and filtered using a membrane filter (0.2 μm pore size; Whatman, England). An HPLC (2690 Separation Module, Waters Chromatography, Mildford, USA) system equipped with a photodiode array (PDA) detector was used to quantify phenolic compounds. Separation was primarily achieved using a Fortis C18 column (5 μL, 150 × 4.6 mm). The mobile phase consisted of acetonitrile [solvent (A)] and 0.1% aqueous acetic acid (v/v) [solvent (B)]. Separation was achieved using the following linear gradient program: 8–10% A at 0–2 min, 10–30% A at 2–27 min, 30–90% A at 27–50 min, 90–100% A at 50–51 min, 100% A at 51–60 min, and 100–8% A at 60–70 min. Re-equilibrium was allowed for 10 min between sample injections. The flow rate was 1.0 mL/min, and 20 μL aliquots were injected into the HPLC. Calibration plots were obtained by measuring the peak areas. Compound identification was made on the basis of UV absorption spectra and retention time.
Evaluation of enzyme activity
Enzyme extraction and protein estimation
For enzyme assays, roots were collected after harvest and washed immediately with distilled water. In each case, 1 g of fresh roots was weighed and stored at −70 °C until further analysis. The root tissues (1 g) were powdered in liquid nitrogen using a pre-chilled mortar and pestle. The powdered samples were homogenized in 2.0 mL of 50 mM potassium phosphate buffer (pH 7.0) containing 2% (w/v) insoluble polyvinylpolypyrrolidone (PVPP), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM EDTA. The homogenate was filtered through two layers of muslin cloth and centrifuged at 10,000 rpm (Smart R17 Micro Refrigerated centrifuge, Hanil Science Co., Ltd., Korea) at 4 °C for 10 min. The soluble protein content was measured using bovine serum albumin (BSA) as a standard (Bradford 1976).
Antioxidant enzyme activity assay
Catalase (CAT) activity was determined using a reaction mixture that contained 100 mM H2O2 in 100 mM potassium phosphate buffer (pH 7.0) and enzyme extract (modified after Bisht et al. 1989). After 10 min of reaction, the absorbance was measured at 240 nm using a spectrophotometer (Optizen POP, Mecasys Co., Ltd, Korea). For the blank control, 2 N H2SO4 (1 mL) was added to the reaction mixture before the addition of enzyme extract. Guaiacol-peroxidase (G-POD) activity was measured by monitoring the formation of tetraguaiacol (extinction coefficient of 6.39 m/M cm) at 436 nm according to the method described by Putter (1974).
Determination of lipid peroxidation
Lipid peroxidation was measured as the amount of malondialdehyde (MDA) reacting with thiobarbituric acid (TBA) (Sigma, USA) to form the TBA-MDA complex. Crude extract was prepared according to the method reported by Heath and Packer (1968), and the final absorbance was measured using a UV–VIS spectrophotometer (Optizen POP, Mecasys Co., Ltd, Korea) at 532 and 600 nm. MDA equivalents were calculated according to the following equation (Heath and Packer 1968):
In this equation, 532 nm represents the maximum absorbance of the TBA-MDA complex; 600 nm is the correction for non-specific turbidity, and 155 m/M cm is a molar extinction coefficient for MDA.
Statistical analysis
The experiment was conducted using a completely randomized design with three replicates, if not specified otherwise. Significant differences were determined by Duncan’s multiple range test (DMRT) using SAS software (Version 9.4; SAS Institute, USA).
Results and discussion
Effect of inoculum density on root growth and bioactive compound production
Adventitious root growth
The inoculum density is a key factor that affects the overall biomass yield and accumulation of bioactive compounds under in vitro plant culture conditions (Lee et al. 2011; Shohael et al. 2014; Wu et al. 2014; Murthy et al. 2014c, 2016). In the current study, a significantly high root biomass (fresh (FW) as well as dry weight (DW) basis) was recorded in all the inoculum densities as compared to 3 g/L treatment (Table 1). The highest percent root DW (14.16%) was obtained from 5 g/L inoculum density. Contrary to biomass yield, growth index was negatively affected by increasing inoculum density. Lower inoculum density (3 g/L) had significantly lower RGR (2.39) and root biomass yield (10.47 g/L DW) compared with those of other inoculum densities. This can be attributed to the slow growth and longer lag phase resulting from extended culture periods. The maximum root biomass (114.99 g/L FW) was observed at 10 g/L inoculum density, which is statistically (p ≤ 0.05) higher than 3 g/L inoculum size and par with rest of the treatments. It is evident from the data that increasing the inoculum densities greater than 5 g/L reduced the efficiency with respect to biomass accumulation. This can be ascribed to comparable growth rates and high growth index with 5 g/L inoculum, compared with those of higher initial inoculum densities. Higher growth rate, reduced nutrient and oxygen availability, and increased biomass may be responsible for the radical decline in growth index observed with higher inoculum densities. In Eleutherococcus koreanum, high initial inoculum density of adventitious root culture is reported to have a short lag phase followed by early stationary and senescence phases, compared with cultures from low inoculum densities (Lee et al. 2011). Lee et al. (2011) also reported the fivefold increase in CO2 concentration inside the bioreactor vessel compared with that of the initial stage, and suggested a possible reason for early cell senescence at high inoculum density. Echinacea angustifolia and Scoplia parviflora adventitious root cultures showed similar results, where high initial inoculum densities result in high root biomass and low growth index (Wu et al. 2006). The optimum inoculum density also varies with respect to plant species, cell line, nutrient composition, medium conditioning, and production scale (Baque et al. 2012). Withania somnifera and E. angustifolia adventitious root cultures had the highest biomass from 10 g/L inoculum density (Praveen and Murthy 2010; Wu et al. 2006), whereas the optimum yield of Hypericum perforatum was obtained from considerably lower (3 g/L) inoculum density (Cui et al. 2011). These combined results indicate that 5 g/L inoculum density is optimum for producing substantial P. multiflorum adventitious root biomass in 3 L BTBB bioreactors.
Polyphenol yield and free radical scavenging (DPPH) activity
The influence of inoculum density on polyphenol accumulation is quite evident from the results in P. multiflorum adventitious root cultures. The total phenolic and flavonoid contents decreased with increasing inoculum density from 3 to 15 g/L (Fig. 1a). As per trend observed, phenolic accumulation was higher at low inoculum densities (3 and 5 g/L) with a gradual decline thereafter. Significantly high bioactive compound productivity was observed in samples with 5 g/L inoculum density (Fig. 1b). Higher inoculum density (≥7 g/L) adversely affected the yield of bioactive compounds. This may be due to a significant decrease in the accumulation of flavonols and hydroxybenzoic acid derivatives at high initial inoculum density (Fig. 2). A total of 17 bioactive compounds were determined by HPLC analysis. Consistent with the phenolic and flavonoid contents, the highest yield of total polyphenols (13.22 mg/g DW root biomass) in P. multiflorum adventitious root culture was observed with 3 g/L inoculum density. The yield of anthraquinones was relatively higher with low inoculum density. It appears that the metabolic pathway or regulatory enzyme activity leading to phenolic compound synthesis may be attenuated with high inoculum density. These results are in agreement with a previous report on Morinda citrifolia adventitious root culture, where polyphenol synthesis (PAL) activity is reported to be significantly lower with high inoculum density (>10 g/L), thereby resulting in low yield of phenolics (Baque et al. 2013). The high CO2 concentration inside the bioreactor vessel at high inoculum density might have a role in the low yield of bioactive compounds. It was reported to adversely affect the accumulation of bioactive compounds in E. koreanum adventitious root culture (Lee et al. 2011). Earlier reports on adventitious root cultures of E. angustifolia and H. perforatum also suggest that high inoculum density negatively affects the accumulation of bioactive compounds (Wu et al. 2006, 2014). Low accumulation of scopolamine was reported in S. parviflora adventitious root culture with high initial inoculum density (Min et al. 2007). In the present study, no significant differences in total phenolic compound yield were observed with 3, 5, and 7 g/L inoculum densities (Fig. 2); however, the high productivity obtained with 5 g/L inoculum could be a decisive factor (Fig. 1).
Effect of inoculum density on phenolic compound profiles determined by HPLC analysis in Polygonum multiflorum adventitious roots. a Total of phenolic compounds, b content of phenolic groups, c content of specific compounds. Bars with different letters differ significantly from each other per DMRT (p ≤ 0.05)
Free radical scavenging activity (1,1-diphenyl-2-picrylhydrazyl, DPPH) was assessed and revealed good antioxidant potential of cultivated adventitious roots (Fig. 3a). A significantly high DPPH activity was recorded in root samples with 7 g/L inoculum density, which is at par with activity in 5 g/L inoculum density root cultures. The antioxidant activity in plant extracts was reported to be associated with phenolic and flavonoid accumulation (Zou et al. 2012; Jiang et al. 2015). The results of the present study corroborate the data for total phenolic and flavonoid yield (Fig. 1). These results provide evidence that the predominant source of antioxidant activity is derived from phenolic compounds, especially quercetin (Fig. 2c). The low inoculum density culture may possibly be exposed to high shear stress, and therefore accumulated high levels of phenolic compounds. The culture with low inoculum density had greater agitation due to the high liquid-to-biomass ratio reported to accumulate high levels of phenolic compounds and scavenge free radicals generated due to oxidative burst-mediated shear stress (Baque et al. 2013). The oxidative burst in Taxus cuspidata suspension culture is believed to change the membrane permeability and lead to the induction of secondary metabolism, resulting in the activation of PAL activity and accumulation of phenolic compounds (Han and Yuan 2004).
Effect of inoculum density on DPPH activity (a), malondialdehyde (MDA) content (b), and antioxidant enzyme activity [CAT (c), G-POD (d)] in adventitious root cultures of Polygonum multiflorum. Values represent mean ± SE (n = 3). z Bars with different letters differ significantly from each other per DMRT (p ≤ 0.05)
Antioxidant enzyme activity (CAT and G-POD) and lipid peroxidation (MDA) levels
Aerobic metabolism results in the production of reactive oxygen species (ROS), which can generate oxidative stress in organisms (Sharma et al. 2012; Salimi et al. 2016) and cause oxidative damage to tissues. These ROS also act as subcellular messengers in complex processes such as cell proliferation regulation (Michiels et al. 1994). It can also lead to the overproduction of MDA, which is the final product of lipid peroxidation. In the present study, adventitious roots of P. multiflorum were grown in the presence of continuous air flow of 0.1 vvm. Under these conditions, it was relevant to measure antioxidant enzyme activities (CAT and G-POD) and MDA production. The results showed a gradual increase in CAT activity with increasing inoculum density from 3 to 15 g/L at the end of a 4-week culture period in the BTBB bioreactor (Fig. 3c). Significantly high CAT activity (31.43 µmol H2O2/mg protein) was recorded at the end of the culture period with 15 g/L inoculum density, which is statistically (p ≤ 0.05) at par with all inoculum densities except for 3 g/L. G-POD activity also was significantly higher (p ≤ 0.05) in 15 g/L inoculum density (Fig. 3d). High antioxidant enzymes (CAT and G-POD) activity at higher inoculum density (15 g/L) indicates that P. multiflorum adventitious root cells were confronting oxidative stress environment might have built up due to reduced oxygen transfer. Such activities of antioxidant enzymes are generally correlated with increased stress tolerance (Ali et al. 2006). The variation in the activities of these enzymes might be attributed to their specific affinity. CAT help in removal of H2O2 generated in peroxisomes by oxidases involved in β-oxidation of fatty acids, photorespiration and purine catabolism, whereas G-POD decomposes indole-3-acetic acid (IAA) and play crucial role by scavenging H2O2 at plasma membrane interface (Mika and Lüthje 2003). The data showed that MDA gradually decreased with increasing inoculum density, with the exception of 3 g/L inoculum density (Fig. 3b). A significantly high accumulation of MDA was observed with 5 and 7 g/L inoculum densities (62.37 and 59.18 nmol MDA/g FW, respectively). High CAT and G-POD activity and low MDA accumulation at high inoculum densities suggests that root tissues were under oxidative stress from high H2O2 levels. Lipid peroxidation (MDA) possibly occurred in the adventitious roots to mitigate the stress conditions caused by free radicals (Alexieva et al. 2001). These results are in agreement with those of M. citrifolia adventitious root cultures, which showed high activities of antioxidant enzymes with 5 and 10 g/L inoculum, which further declined with increasing inoculum density (Baque et al. 2013). The high levels of MDA observed in these samples suggest that the antioxidant activities were not able to counter the adverse effect of high H2O2 levels, which led to lipid peroxidation in the root tissues. Cui et al. (2011) reported high CAT activity with low MDA content at high inoculum density in adventitious root culture of H. perforatum, which resulted from lipid peroxidation to counter the damage caused by free radicals.
These results clearly highlight the importance of inoculum density in maximizing the overall productivity in terms of root growth and accumulation of phenolic compounds in adventitious root cultures of P. multiflorum. It can be inferred from these results that 5 g/L is the optimal inoculum density to achieve maximum productivity.
Effect of culture period on root biomass and bioactive production
Adventitious root biomass
The adventitious roots of P. multiflorum were cultured at 5 g/L inoculum density for extended times to determine the exact growth and metabolite accumulation period. The results showed that adventitious roots followed a sigmoidal growth pattern (Fig. 4a). The first week of culture was the lag phase, and it was followed by accelerated growth in the second and third weeks (log phase). The growth appears to cease around the fourth week of culture and decline thereafter up to the seventh week. The experiments were discontinued after the seventh week because of the continuous decline in root biomass yield. A significantly high root biomass (13.46 g/L DW) and percent dry weight were recorded in samples obtained after a 5 week culture period (Fig. 4a). However, no significant differences (p ≤ 0.05) were recorded in root biomass yield and growth index of samples obtained after the fourth and fifth weeks of culture (Fig. 4b, c). The relative root growth rate of 5 week old cultures is high (2.62) and statistically (p ≤ 0.05) at par with that in 3, 4, and 6 week cultures (Fig. 4d). Therefore, 5 weeks appears to be optimum for maximum root biomass productivity. Nutrient depletion, especially depletion of the carbon source, attenuated the RGR and growth index after 5 weeks of culture. These results are consistent with those of an earlier study, which showed a gradual decline in sucrose content of spent media after 3 weeks of culture of E. angustifolia adventitious roots (Cui et al. 2013).
Numerous studies have reported similar growth behaviors of adventitious root suspension cultures in different plant species, although the precise time of growth decline varied (Wu et al. 2007a; Jeong et al. 2009a, b; Baque et al. 2012; Cui et al. 2014). In the scale-up studies of E. angustifolia adventitious roots, the maximum root biomass yield was achieved after 7 weeks of culture in 5 L BTBB (Cui et al. 2013). The exponential phase in that study lasted for 4 weeks (i.e., 2–6 weeks of culture). For W. somnifera, maximum root biomass yield was recorded after 4 weeks of culture (Praveen and Murthy 2010), whereas it took 7 weeks of adventitious root suspension culture in Panax ginseng to achieve the highest root biomass (Jeong et al. 2009a). For M. citrifolia, a 4 week culture period was optimum for maximum biomass production, but the authors did not show any lag phase (Baque et al. 2012). Some authors reported that sucrose concentrations (1 and 5%) affect biomass accumulation in M. citrifolia. These combined results suggest that the optimum culture period of a particular plant species depends on its growth characteristics and can be highly variable. The optimum culture period also is reported to be influenced by medium composition, especially the sucrose concentration and the culture environment (Cui et al. 2011; Lulu et al. 2015; Jiang et al. 2015).
Production of bioactive compounds and antioxidant activity
The polyphenol type(s), TPC and TFC, were measured at 1 week intervals during the 7 week cultivation period of P. multiflorum adventitious roots cultured at 5 g/L inoculum density (Figs. 5, 6). The polyphenol contents and productivity gradually increased from the first week of culture, and reached a maximum after 4 weeks of culture, thereafter declining to the lowest level at the end of cultivation (Fig. 5). Significantly high phenolic (53.87 mg/g DW) and flavonoid (27.96 mg/g DW) contents were recorded in 4-week-old cultures (Fig. 5a). Irrespective of the culture period, the TPC yield was approximately double that of TFC in P. multiflorum adventitious root cultures. The productivity of total phenolics and flavonoids was highest in root samples collected after 4 weeks of culture (Fig. 5b). HPLC analysis of phenolic compounds showed similar accumulation patterns, with a significantly high yield of bioactive compounds (12.50 mg/g DW) after 3 weeks of culture followed by 4 weeks of culture (11.42 mg/g DW) (Fig. 6). The highest yield was hydroxybenzoic acid phenolics (30.3 mg/g DW) followed by flavonols (21.9 mg/g DW). The anthraquinone yield also was also higher in roots after 3 and 4 weeks of culture. Most polyphenols were significantly higher after 3 and 4 weeks of culture, except for p-hydroxybenzoic acid accumulation, which peaked after 2 weeks in culture. The variation in total polyphenols and the specific metabolite yield could be attributed to the depletion of specific macro or micro nutrients, which affected the secondary metabolism biosynthetic processes (Wu et al. 2007a; Baque et al. 2013). The initial growth stage was challenged by a new environment, especially high air supply in the bioreactor and low root density. Low levels of polyphenols were observed during the first week of culture in E. angustifolia roots and peaked at approximately 37 mg/g root DW after 4 weeks of culture, which was similar to the results of our study (Cui et al. 2013). The Echinacea purpurea adventitious root culture also showed a linear increase in phenolics and flavonoids with maximum yields (92.8 mg/g root DW) after 5 weeks of culture (Jeong et al. 2009b). The variations in results of different studies can be attributed to plant species, cultivation conditions, and nutritional components in the medium. Bioactive compound productivity is one of the crucial parameters for determining the optimal culture period. Considering the results of our present study, we conclude that a 4 week culture period is sufficient for maximum productivity of both root biomass and bioactive compounds in P. multiflorum adventitious root culture.
Effect of culture period on bioactive compound profiles determined by HPLC analysis in Polygonum multiflorum adventitious roots. a Total of phenolic compounds, b content of phenolic groups, c content of specific compounds. Bars with different letters differ significantly from each other per DMRT (p ≤ 0.05)
DPPH free radical has been widely used to evaluate the antioxidant activity of specific plant extracts (Kim et al. 2007; Shekhar and Anju 2014). DPPH activity is generally associated with high levels of bioactive compounds during different stages of culture. However, DPPH levels also reflect the tissue stress levels. DPPH radical scavenging activity was significantly low during the first 2 weeks of adventitious root culture compared with root samples after 3 weeks in culture (Fig. 7a). In contrast to our results, relatively higher DPPH activity was observed in M. citrifolia adventitious root suspension cultures after 1 week of culture, and gradually declined thereafter up to 4 weeks of culture (Baque et al. 2012). However, Cui et al. (2011) reported a gradual increase in DPPH activity in adventitious root cultures of H. perforatum, which subsequently increased during the culture period.
Effect of culture period on DPPH activity (a), malondialdehyde (MDA) content (b), and antioxidant enzyme activity [CAT (c), G-POD (d)] in adventitious root cultures of Polygonum multiflorum. Values represent mean ± SE (n = 3). z Bars with different letters differ significantly from each other according to DMRT (p ≤ 0.05)
Antioxidant enzyme (CAT and G-POD) activity and malondialdehyde (MDA) production
The activities of antioxidant enzymes (CAT and G-POD) varied greatly during P. multiflorum adventitious root culture for 7 weeks. Significantly high CAT activity (25.06 µmol H2O2/mg protein) was recorded in samples after 5 weeks of culture, followed by samples after 4 and 6 weeks of culture (Fig. 7c). For G-POD, significantly high activity was observed in 4-week-old root cultures, followed by 5-week-old root cultures (Fig. 7d). Significantly high malondialdehyde (MDA) levels were recorded in 4-week-old root samples (Fig. 7b). Continuous oxygen exposure and diminishing nutritional components could explain the high antioxidant activity and MDA accumulation during the fourth and fifth weeks of culture (Low and Merida 1996; Cui et al. 2011). Considering the P. multiflorum adventitious root growth and overall productivity with respect to bioactive compound accumulation, a 4-week culture period appears to be optimum for bioreactor culture. Cui et al. (2011) reported that H2O2 levels were highest in H. perforatum adventitious root cultures after 4 weeks, followed by a gradual decline until the end of the culture period (7 weeks). However, the MDA levels slowly increased up to 6 weeks, before sharply declining thereafter. These results suggest that lipid peroxidation may counter the adverse effects of high H2O2 production, which leads to increased generation of free radical species.
Effect of scale-up culture on biomass and bioactive compounds in P. multiflorum
The adventitious root cultures of a few plant species have been successfully scaled up from 500 to 10,000 L in industrial bioreactors (Cui et al. 2014; Paek et al. 2009; Wu et al. 2007b). To scale-up adventitious root cultures, the BTBB is reported to be a suitable system for the accumulation of both biomass and bioactive compounds (Baque et al. 2012; Cui et al. 2013). BTBB provides optimum conditions for growth and bioactive compound accumulation by efficiently controlling the culture environment, foam generation, reducing shear stress, and supplying optimal oxygen (Murthy et al. 2014a, b, 2016). However, a pilot-scale study had to contend with root interweaving after attaining a certain growth stage, which may hamper productivity (Murthy et al. 2016). The current pilot-scale study (500 L BTBB) was conducted to assess the productivity of root biomass and bioactive compounds in BTBB cultures of P. multiflorum adventitious roots. We performed a comparative evaluation at different production scales (0.25 (flask), 3, 5, 20, and 500 L) with optimized parameters (i.e., 5 g/L inoculum density and 4 week culture period). Other parameters such as temperature (24 ± 1 °C), dissolved oxygen (0.1 vvm), pH (5.7–5.8), and culture under dark conditions were the same as those used for optimization experiments.
Adventitious root growth
The performance of optimized parameters was assessed at different production scales. The total adventitious root biomass was understandably higher (4.01 kg DW) for the 500 L BTBB at the end of the 4 week culture period compared with that of other production scales (Table 2). Significantly high root biomass yield was recorded in 3 L BTBB (14.09 g/L DW) followed by 5 L BTBB (12.62 g/L DW). The increase in production scale beyond 3 L capacity significantly reduced root biomass yield. Similar declines were observed in the culture growth indices; however, the RGRs ranged between 1.98 and 2.38 per day, with a maximum in 3 L BTBB and a gradual decline thereafter. The biomass reduction often occurs along with an increase in the scale of cell and tissue cultures, which might result from factors like changes in mixing, oxygen diffusion, head space O2/CO2 ratio, shear stress, and nutritional uptake (Zhong 2002; Murthy et al. 2008; Lee et al. 2011). A significant decrease in root biomass and growth ratio also was reported in adventitious root culture of E. angustifolia during scale-up from 5 to 500 L BTBB (Cui et al. 2013). E. purpurea adventitious root production in 20 L BTBB, which was later scaled up to 500 L BTBB and 1000 L drum type bioreactor also led to a decrease in root biomass (Wu et al. 2007b). These authors found that the yield of root biomass decreased with an increase in production scale (i.e., 11 g/L DW in 20 L, 7.2 g/L DW in 500 L, and 5.1 g/L DW in 1000 L bioreactor). The adventitious root biomass yield in the present study (approximately 35 kg FW) is significantly high compared with that of a previous report on E. purpurea (26.3 kg FW) and E. angustifolia (25.4 kg FW), but was lower than that of H. perforatum (80.4 kg FW). The variations in root biomass yield in these reports seem to be species-dependent. It is understood that dynamic factors such as nutritional uptake, gaseous composition, and agitation or mixing patterns change with process scale-up, which may lead to shifts in root growth and bioactive accumulation.
Bioactive production and antioxidant activity
The accumulation of TPC is higher than that of TFC, irrespective of the production scale. Significantly high levels of both TPC (52.97 mg/g DW) and TFC (27.03 mg/g DW) were obtained in 3 L bioreactor vessels compared with those of flasks and other production scales (Fig. 8a). Similarly, the overall productivity was significantly high (p ≤ 0.05) in 3 L BTBB followed by 5 and 20 L bioreactor vessels (Fig. 8b). This suggests that the increase in production scale led to a decrease in total yield of bioactive compounds. The decline in secondary metabolite yield during scale-up of plant tissue culture may be due to changes in the culture environment or shifts in growth and productivity kinetics. In a similar study, caffeic acid derivatives in E. purpurea adventitious root cultures were reported to decrease from 39 mg/g DW in 20 L BTBB to 27.4 mg/g in 500 L BTBB (Wu et al. 2007b). Contrary to the results of the present study, the scale-up of H. perforatum adventitious root culture from 100 to 500 L BTBB resulted in a slightly higher yield of both phenolic and flavonoid contents (Cui et al. 2014). The pilot-scale culturing of E. angustifolia adventitious roots also showed approximately two- to three-fold increases in polyphenol contents from 20 to 500 L BTBB. However, the increase in bioactive compounds was attributed to elicitation by methyl jasmonate (Cui et al. 2013). Although root biomass, growth index, and bioactive compounds declined with increasing scale beyond 3 L capacity, the concentration of phenolic compound derivatives in the 500 L BTBB (10.78 mg/g DW) was recorded as significant, on par with 3 L (11.87 mg/g DW) and 5 L (10.76 mg/g DW) bioreactor vessels (Fig. 9). It is therefore evident from the results that pilot-scale production resulted in a comparable yield of bioactive compounds. Among various groups of polyphenols, the highest contents were recorded for hydroxybenzoic acid phenolics followed by flavonols, irrespective of the production scale. The DPPH radical scavenging activity of P. multiflorum adventitious root extract showed significantly high activity in 3 L bioreactor vessel samples compared with those in 500 L BTBB, but at par (p ≤ 0.05) with 5 and 20 L bioreactor samples (Fig. 10a). This appears to be comparable with the yield of phenolic compounds.
Bioactive compound profiles determined by HPLC analysis in Polygonum multiflorum adventitious root at different production scales. a Total of phenolic compounds, b content of phenolic groups, c content of specific compounds. Bars with different letters differ significantly from each other per DMRT (p ≤ 0.05)
Effect of scale-up on DPPH activity (a), malondialdehyde (MDA) content (b), and antioxidant enzyme activity [CAT (c), G-POD (d)] in adventitious root cultures of Polygonum multiflorum. Values represent mean ± SE (n = 3). z Bars with different letters differ significantly from each other according to DMRT (p ≤ 0.05)
Activity of antioxidant enzymes and MDA production in adventitious roots of P. multiflorum
Significantly high activities of CAT (25.23 µmol H2O2/mg protein) and G-POD (0.36 units/mg protein) were obtained in the 500 L BTBB compared with other production scales. The G-POD activity in 500 L BTBB was twofold higher than that in the root samples of 3 L bioreactors (Fig. 10c, d). MDA levels were highest in 3 L BTBB (57.55 nmol/g FW), followed by 20 L bioreactor vessels, and significantly lower in 250 mL flask samples (Fig. 10b). Increased antioxidant enzyme activity was observed in 500 L BTBB root samples, which might be due to high ROS generation (Cui et al. 2013). These results indicate that adventitious roots are subjected to more stressful conditions in the pilot-scale studies than in the small-scale studies.
Scaling-up of adventitious root cultures has been achieved for several plant species with different bioreactor types (Murthy et al. 2016). After optimization of medium composition and culture conditions, the adventitious roots were successfully applied for large-scale (500 L) production of biomass and bioactive compounds in P. ginseng (Paek et al. 2009, Murthy et al. 2014a, b, d), E. purpurea (Wu et al. 2007b), and H. perforatum (Cui et al. 2014). Our study also successfully established a pilot-scale 500 L BTBB for adventitious root culture of P. multiflorum (Fig. 11). These studies will provide raw material to meet the demands of pharmaceutical and cosmetic industries, and will support the conservation of P. multiflorum in its natural habitat.
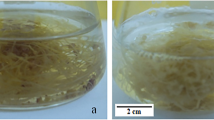
Culture of P. multiflorum adventitious roots at different bioreactor scales. a Adventitious root, b adventitious root in flask culture, c adventitious root in culture room, d 3 L BTBB, e adventitious root harvested from 3 L BTBB, f 500 L pilot-scale BTBB, g, i adventitious root harvested from 500 L BTBB, and h oven drying (60 °C) of adventitious roots
In conclusion
The present study established a pilot-scale culture of P. multiflorum adventitious roots in 500 L BTBB with 5 g/L inoculum density for 4 weeks. Although the biomass yield was comparatively reduced in the 500 L culture, the accumulation of bioactive compounds was not significantly different. The results revealed high antioxidant enzyme activity (CAT and G-POD) and low MDA production in the 500 L BTBB samples, which might mitigate the high oxidative stress generated at this production scale. Our results demonstrate the feasibility of using P. multiflorum adventitious root cultures for the production of bioactive compounds at the pilot scale (500 L) to meet the continuously expanding demands of the cosmetic, pharmaceutical, and food industries for natural ingredients. In addition, the production of anthraquinones like emodin and physcion at industrial scale could be a boon to ever rising herbal drug sector for management and treatment of human health ailments.
References
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultra violet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Ali MB, Singh N, Shohael AM, Hahn EJ, Paek KY (2006) Phenolics metabolism and lignin synthesis in root suspension cultures of Panax ginseng in response to copper stress. Plant Sci 171:147–154
Baque MA, Moh SH, Lee EJ, Zhong JJ, Paek KY (2012) Production of biomass and useful compounds from adventitious roots of high-value added medicinal plants using bioreactor. Biotechnol Adv 30:1255–1267
Baque MA, Shiragi MHK, Moh SH, Lee EJ, Paek KY (2013) Production of biomass and bioactive compounds by adventitious root suspension cultures of Morinda citrifolia (L.) in a liquid-phase airlift balloon-type bioreactor. In Vitro Cell Dev Biol Plant 49:737–749
Bisht SS, Sharma A, Chaturvedi K (1989) Certain metabolic lesions of chromium toxicity in radish. Indian J Agric Biochem 2:109–115
Bounda GA, Feng Y (2015) Review of clinical studies of Polygonum multiflorum Thunb. and its isolated bioactive compounds. Pharmacognosy Res 7:225–236
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cui XH, Murthy HN, Jin YX, Yim YH, Kim JY, Paek KY (2011) Production of adventitious root biomass and secondary metabolites of Hypericum perforatum L. in a balloon type airlift bioreactor. Bioresour Technol 102:10072–10079
Cui HY, Baque MA, Lee EJ, Paek KY (2013) Scale-up of adventitious root cultures of Echinacea angustifolia in a pilot-scale bioreactor for the production of biomass and caffeic acid derivatives. Plant Biotechnol Rep 7:297–308
Cui XH, Murthy HN, Paek KY (2014) Pilot-scale culture of Hypericum perforatum L. adventitious roots in airlift bioreactors for the production of bioactive compounds. Appl Biochem Biotechnol 174:784–792
Folin O, Ciocalteu V (1927) On trysonic and tryptophane determination in protein. J Biol Chem 27:627–650
Han RB, Yuan YJ (2004) Oxidative burst in suspension culture of Taxus cuspidate induced by a laminar shear stress in short-term. Biotechnol Prog 20:507–513
Han MN, Lu JM, Zhang GY, Yu J, Zhao RH (2015) Mechanistic studies on the use of Polygonum multiflorum for the treatment of hair graying. BioMed Res Int. doi:10.1155/2015/651048
Hatano T, Kagawa H, Yasuhara T, Okuda T (1998) Two new flavonoids and other constituents in licorice: their relative astringency and radical scavenging effects. Chem Pharm Bull 36:2090–2097
Heath RL, Packer L (1968) Photo-peroxidation in isolated chloroplasts I Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Jeong CS, Murthy HN, Hahn EJ, Lee HL, Paek KY (2009a) Inoculum size and auxin concentration influence the growth of adventitious roots and accumulation of ginsenosides in suspension cultures of ginseng (Panax ginseng C. A. Meyer). Acta Physiol Plant 31:219–222
Jeong JA, Wu CH, Murthy HN, Hahn EJ, Paek KY (2009b) Application of an airlift bioreactor system for the production of adventitious root biomass and caffeic acid derivatives of Echinacea purpurea. Biotechnol Bioprocess Eng 14:91–98
Jiang YJ, Piao XC, Liu JS, Jiang J, Lian ZX, Kim MJ, Lian ML (2015) Bioactive compound production by adventitious root culture of Oplopanax elatus in balloon-type airlift bioreactor systems and bioactivity property. Plant Cell Tiss Organ Cult 123:413–425
Kim HK, Bang CS, Choi YM, Lee JS (2007) Antioxidant and antiproliferative activities of methanol extracts from leafy vegetables consumed in Korea. Food Sci Biotechnol 16:802–806
Lee EJ, Moh SH, Paek KY (2011) Influence of inoculum density and aeration volume on biomass and bioactive compound production in bulb-type bioreactor cultures of Eleuterococcus koreanum Nakai. Bioresour Technol 102:7165–7170
Lee KJ, Park YK, Kim JY, Jeong TK, Yun KS, Paek KY, Park SY (2015) Production of biomass and bioactive compounds from adventitious root cultures of Polygonum multiflorum using air-lift bioreactors. Korean J Plant Biotechnol 42:34–42
Li LF, Ni BR, Lin HM, Zhang M (2015) Traditional usages, botany, phytochemistry and toxicology of Polygonum multiflorum Thunb.: a review. J Ethnopharmacol 159:158–183
Low PS, Merida JR (1996) The oxidative burst in plant defense: function and signal transduction. Physiol Plant 96:533–542
Lulu T, Park SY, Ibrahim R, Paek KY (2015) Production of biomass and bioactive compounds from adventitious roots by optimization of culturing conditions of Ericoma longifolia in balloon-type bubble bioreactor system. J Biosci Bioeng 119:712–717
Michiels C, Raes M, Toussaint O, Ramacle J (1994) Importance of Seglutathione peroxidase, catalase and Cu, Zn-SOD for cell survival against oxidative stress. Free Radical Biol Med 17:235–248
Mika A, Lüthje S (2003) Properties of guaiacol peroxidase activities isolated from corn root plasma membranes. Plant Physiol 132:1489–1498
Min JY, Jung HY, Kang SM, Kim YD, Kang YM, Park DJ, Prasad DT, Choi MS (2007) Production of tropane alkaloids by small-scale bubble column bioreactor cultures of Scopila parvi flora adventitious root. Bioresour Technol 98:1748–1753
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–479
Murthy HN, Hahn EJ, Paek KY (2008) Adventitious roots and secondary metabolism. Chin J Biotechnol 24:711–716
Murthy HN, Georgiev MI, Kim YS, Jeong CS, Kim SJ, Park SY, Paek KY (2014a) Ginsenosides: prospective for sustainable biotechnological production. Appl Microbiol Biotechnol 98:6243–6254
Murthy HN, Kim YS, Jeong CS, Kim SJ, Zhong JJ, Paek KY (2014b) Production of ginsenosides from adventitious root cultures of Panax ginseng. In: Paek KY, Murthy HN, Zhong JJ (eds) Production of biomass and bioactive compounds using bioreactor technology. Springer, Dordrecht, pp 625–654
Murthy HN, Lee EJ, Paek KY (2014c) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tiss Org Cult 118:1–16
Murthy HN, Dandin VS, Zhong JJ, Paek KY (2014d) Strategies for enhanced production of plant secondary metabolites from cell and organ cultures. In: Paek KY, Murthy HN, Zhong JJ (eds) Production of biomass and bioactive compounds using bioreactor technology. Springer, Dordrecht, pp 471–508
Murthy HN, Dandin VS, Paek KY (2016) Tools for biotechnological production of useful phytochemicals from adventitious root cultures. Phytochem Rev 15:129–145
Paek KY, Chakrabarty D, Hahn EJ, Zhong JJ (2005) Application of bioreactor systems for large scale production of horticultural and medicinal plants. Plant Cell Tiss Organ Cult 81:287–300
Paek KY, Murthy HN, Hahn EJ, Zhong JJ (2009) Large scale culture of ginseng adventitious roots for production of ginsenosides. Adv Biochem Eng Biotechnol 113:151–176
Praveen N, Murthy HN (2010) Production of withanolide-a from adventitious root cultures of Withania somnifera. Acta Physiol Plant 32:1017–1022
Putter J (1974) Peroxidases. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 685–690
Salimi F, Shekari F, Hamizei J (2016) Methyl jasmonate improves salinity resistance in German chamomile (Matricaria chamomilla L.) by increasing activity of antioxidant enzymes. Acta Physiol Plant. doi:10.1007/7s11738-015-2023-4
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. doi:10.1155/2012/217037
Shekhar TC, Anju G (2014) Antioxidant Activity by DPPH Radical Scavenging Method of Ageratum conyzoides Linn. leaves. Am J Ethnomed 4:244–249
Shohael AM, Murthy HN, Paek KY (2014) Pilot-scale culture of somatic embryos of Eleutherococcus senticosus in airlift bioreactors for the production of eleutheroside. Biotechnol Lett 36:1727–1733
Sivakumar G (2006) Bioreactor technology: a novel industrial tool for high-tech production of bioactive molecules and biopharmaceuticals from plant root. Biotechnol J 1:1419–1427
Wu CH, Dewir YH, Hahn EJ, Paek KY (2006) Optimization of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia. J Plant Biol 49:193–199
Wu CH, Murthy HN, Hahn EJ, Paek KY (2007a) Enhanced production of caftaric acid, chlorogenic acid and chichoric acid in suspension cultures of Echinacea purpurea by the manipulation of incubation temperature and photoperiod. Biochem Eng J 36:301–303
Wu CH, Murthy HN, Hahn EJ, Paek KY (2007b) Large-scale cultivation of adventitious root of Echinacea purpurea in air-lift bioreactors for the production of chichoric acid, chlorogenic acid and caftaric acid. Biotechnol Lett 29:1179–1182
Wu SQ, Yu XK, Lian ML, Park SY, Piao XC (2014) Several factors affecting hypericin production of Hypericum perforatum during adventitious root culture in airlift bioreactors. Acta Physiol Plant 36:975–981
Zhong JJ (2002) Plant cell culture for production of paclitaxel and other taxanes. J Biosci Bioeng 94:591–599
Zou Y, Liao S, Shen W, Liu F, Tang C, Chen C (2012) Phenolics and antioxidant activity of mulberry leaves depend on cultivar and harvest month in Southern China. Mol Sci 13:16544–16553
Acknowledgements
This study was funded by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Advanced Production Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (grant number 315013-4), and SB acknowledges the Council of Scientific and Industrial Research (CSIR), India, for awarding the Raman Research Fellowship.
Author contributions
T-TH, K-JL and J-DL contribute for acquisition data and writing of manuscript. SB and K-YP participated in interpreted data and revising for important intellectual content. S-YP made substantial contributions to conception and design this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ho, TT., Lee, KJ., Lee, JD. et al. Adventitious root culture of Polygonum multiflorum for phenolic compounds and its pilot-scale production in 500 L-tank. Plant Cell Tiss Organ Cult 130, 167–181 (2017). https://doi.org/10.1007/s11240-017-1212-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1212-9