Abstract
Water deficit limits the growth and productivity of plants worldwide. Improved water use efficiency (WUE) and drought tolerance are important adaptations to address these limitations. In this study, an epidermal patterning factor (EPF), PdEPF2, from a fast-growing poplar clone NE-19 (Populus nigra × (Populus deltoids × Populus nigra)) was isolated. Quantitative reverse transcription polymerase chain reaction showed that transcription of this gene was induced by drought and abscisic acid (ABA). To study the biological functions of PdEPF2, transgenic Arabidopsis plants harboring (35S:PdEPF2) in which PdEPF2 was constitutively expressed were generated. Compared with the wild type and epf2-3 mutant, the transgenic plants ectopically expressing PdEPF2 showed favorable osmotic parameters, such as seed germination rate, primary root length, proline and chlorophyll content, Fv/Fm, photosynthetic rate, and instantaneous leaf WUE, under drought stress. In addition, the transgenic Arabidopsis plants displayed enhanced drought tolerance as a result of decreased stomatal density, which would limit transpiration and reduce water loss. Compared with the wild-type, plants that overexpressed PdEPF2 had decreased sensitivity to exogenous ABA during germination and seedling development, whereas the epf2-3 mutant showed increased sensitivity to ABA. Furthermore, PdEPF2 positively regulated expression of two ABA signaling-related genes, ABI1 and ABI2. These findings indicate that PdEPF2 may enhance drought tolerance by regulating stomatal density and the response to the ABA signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants have developed adaptations to cope with adverse environmental conditions, such as drought, high salinity, and cold, as these abiotic stresses can limit growth and development (Zhu et al. 2007; Nakashima et al. 2009; Xing et al. 2011). Moreover, drought is a primary environmental constraint on plant growth and crop production, especially in arid and semi-arid regions. Water use efficiency (WUE) (defined as yield per unit of water) represents the relationship between biomass production and water consumption. Specifically, leaf WUE is the ratio of photosynthesis to transpiration and is also referred to as transpiration efficiency (Karaba et al. 2007). Under water-limited conditions, high WUE is necessary to maintain high biomass production (Han et al. 2013) and thus high WUE is imperative to improve and stabilize crop productivity under drought conditions (Bhatnagar-Mathur et al. 2007).
Increased WUE is achieved when plants enhance photosynthetic assimilation and reduce transpirational water loss. Stomata are major regulators of carbon dioxide uptake and water loss in plants (Hetherington and Woodward 2003). In addition, stomatal conductance regulates gas exchange through stomatal movements (opening and closing) and density (Chaerle et al. 2005; Yoo et al. 2009). Alteration of the stomatal aperture in response to drought is a short-term means through which plants cope with fluctuating water availability, although this strategy affects photosynthesis and WUE (Chaves et al. 2003; Kim et al. 2010). To adapt to long-term water deficits, plants adjust stomatal density during development. This modification of stomatal density varies between species and with drought severity (Quarrie and Jones 1977; Xu and Zhou 2008; Silva et al. 2009) and is closely associated with WUE and stomatal development (Yoo et al. 2010).
Stomatal development has been well studied in Arabidopsis. Each developmental transition is regulated by three basic helix–loop–helix (bHLH) transcription factors, namely SPEECHLESS (MacAlister et al. 2007), MUTE (Pillitteri et al. 2007) and FAMA (Ohashi-Ito and Bergmann 2006). In addition, different epidermal patterning factor (EPF) family members play different roles in stomatal development. For example, EPF2 modulates protodermal cell differentiation into meristemoid mother cells (Hara et al. 2009). EPFf peptides interact with three leucine-rich repeat receptor-like kinases (ERECTA, ERECTA-LIKE1, and ERECTA-LIKE2) and a leucine-rich repeat receptor-like protein called TOO MANY MOUTHS (TMM). These receptors contain extracellular leucine-rich repeat domains that often promote protein–protein interactions (Shpak et al. 2005; Bhave et al. 2008). Biochemical studies using biosensor chips have shown that ER-EPF2 was the predominant ligand-receptor pairs (Lee et al. 2012). In addition, stomatal development is regulated by environmental factors such as light, CO2, temperature, humidity, drought, and abscisic acid (ABA) (Royer 2001; Casson et al. 2009; Casson and Hetherington 2010; Tricker et al. 2012; Chater et al. 2014).
Stomatal development, including the density and size of stomata, is the primary defense mechanism of plants. Regulation of stomatal development may lead to increased WUE and drought tolerance (Boccalandro et al. 2009; Wang et al. 2014). Most previous research has focused on herbaceous plants, such as Arabidopsis thaliana, and few studies have examined mechanisms in woody plants. Poplars (Populus spp.) are among the fastest-growing trees in temperate climates, and their high productivity strongly depends on water availability (Tschaplinski and Blake 1989; Zsuffa et al. 1996; Monclus et al. 2006). In addition, poplar is generally easy to regenerate in vitro and is susceptible to Agrobacterium-mediated transformation (Han et al. 2000; Confalonieri et al. 2003).
We predicted 15 EPIDERMAL PATTERNING FACTOR-LIKE (EPFL) members in Populus trichocarpa (data unpublished). In a previous study, we isolated PdEPF1 from poplar and demonstrated that PdEPF1 improved WUE and conferred drought tolerance in Populus (Wang et al. 2015). In the present study, an additional member of the EPF family, PdEPF2, was isolated for further analysis. Morphological and physiological experiments revealed that overexpression of PdEPF2 reduced stomatal density and water loss, thus increasing drought tolerance. Moreover, our data showed that PdEPF2 is likely to be involved in response to the ABA signaling pathway.
Materials and methods
Plant materials and growth conditions
Cuttings of the poplar genotype NE-19 (Populus nigra × (Populus deltoids × Populus nigra)) with 15-cm-long stems were planted in April, 2013, in an open field at the experimental nursery of Beijing Forestry University, Beijing, China for genetic analysis. For expression analysis, uniformly developed plants (95–110 cm high, with 25–35 leaves and 15–35 cm root length) were subjected to drought and ABA treatments. For drought treatment, water was withheld from plants for 0, 3, 6, 9, or 12 days. Treatment with ABA treatment was performed by spraying the leaves once with a 250 µM ABA solution, following which young leaves were collected at 0, 3, 6, 9, or 12 h after the treatment. For both treatments, young leaves (the first one to three leaves from the shoot apex) were harvested, frozen in liquid nitrogen, and stored at −80 °C for subsequent RNA isolation.
Arabidopsis mutant and transgenic lines used were in the Columbia-0 (Col-0) background. A. thaliana ecotype Col-0 was selected as the wild-type (WT) control. Seeds of Col-0 and the mutant epf2-3 (SALK_047918) were obtained from the Arabidopsis Biological Resource Center at Ohio State University (Columbus, OH, USA). The mutant homozygous for T-DNA insertion within AtEPF2 (At1g34245) was verified by polymerase chain reaction (PCR). Transgenic plants harboring and expressing 35S:PdEPF2 and epf2-3/PdEPF2 were generated using the agrobacterium tumefaciens-mediated floral dip method (Zhang et al. 2006). Transformed lines were screened on hygromycin-supplemented media (containing 50 mg/L hygromycin) to generate independent transgenic lines. The homozygous T3 lines were used for further analyses. All seeds were surface sterilized for 1 min in 75 % ethanol followed by 10 min in 1 % NaClO and five washes in sterilized distilled water. Seeds were sown on half-strength Murashige and Skoog (1/2MS; Murashige and Skoog 1962) plates supplemented with 3 % (w/v) sucrose and 0.6 % (w/v) agar. The seeds were stratified for 2 days at 4 °C before transference to a growth room maintained at 22 °C under a 16 h/8 h (white light/dark) photoperiod, 80 % relative humidity, and 150 μmol m−2 s−1 irradiation. Ten days after germination, seedlings were transplanted and grown at a density of four plants per 7 × 7 × 6.5 cm pot containing a mixture of soil and vermiculite (2:1) under a 16/8 light/dark photoperiod at 22 °C, 70 % relative humidity, and 150 µmol m−2 s−1 irradiation.
Poplar gene cloning and plasmid construction
Total RNA isolation was performed using the CTAB reagent method (Chang et al. 1993). First-strand cDNA synthesis was performed using M-MLV Reverse Transcriptase and an oligo (dT) primer (Promega, Madison, WI, USA) in accordance with manufacturer’s instructions. The full-length cDNA of PdEPF2 was retrieved by homologous cloning-using the gene-specific primers FW 5′-ATGAAGTTCTTAGTTGGAGCCC-3′ and RV 5′-TCATGCTGAAGGCACATGG-3′. The PCR products were cloned into the pMD18-T cloning vector (Promega, Madison, WI, USA) and were subsequently subjected to sequencing analysis. The amplified PdEPF2 open reading frame was inserted into the XbaI and BamHI sites in the pBI121 vector under the control of the cauliflower mosaic virus (CaMV) 35S promoter to generate the fusion construct 35S:PdEPF2.
Phylogenetic tree construction
To understand the relationship between PdEPF2 and other EPFL family members from Arabidopsis, a phylogenetic analysis of PdEPF2 was performed using full-length amino acid sequences from Arabidopsis and Populus trichocarpa using phylogeny (http://www.phylogeny.fr/). The sequences used were retrieved from PopGenIE (http://popgenie.org/) and TAIR (https://www.arabidopsis.org/).
RT-PCR and qRT-PCR analysis
Total RNA from each sample was extracted by the CTAB method, and 1 µg RNA was used for reverse transcription. Subsequently, 100 ng cDNA was used as the template for reverse-transcription (RT)-PCR amplification. The PCR products were electrophoresed in a 2 % agarose gel stained with ethidium bromide. Quantitative (R) RT-PCRs were performed in 96-well plates containing 100 ng template (1 µL), 0.6 µL (10 µM) forward primer, 0.6 µL (10 µM) reverse primer, 10 µL SYBR® Green Master Mix, 2 µL ROX™ Reference Dye and 5.8 µL RNase-free ddH2O, comprising a total volume of 20 µL. The reaction was amplified for 40 cycles at 95 °C for 10 s, 55 °C for 30 s, and 72 °C for 32 s. Transcript levels of all candidate genes was determined using the 2−ΔΔCT method and relative transcript levels were calculated and normalized as described previously (Willems et al. 2008). The reactions were performed in biological triplicates using RNA samples extracted from three independent plant materials and all experiments were repeated three times. UBQ was used as an internal control to quantify the relative transcript level of PdEPF2 in each sample (Wang et al. 2014). Gene-specific primers were designed using the Primer6 (PRIMER-E, Ivybridge, UK) software listed in Online Resource 1.
Southern blot analysis
Southern blot analysis was performed to demonstrate transgene integration and gene copy number. Following digestion with XbaI overnight, DNA samples (30 µg) were separated by electrophoresis on a 1 % agarose gel and then transferred to a nylon membrane. DNA probes specific for PdEPF2 coding sequence were labelled with digoxigenin (DIG). Southern blot hybridization was performed according to the protocol of the DIG High Primer DNA Labeling and Detection Starter Kit I (Roche Diagnostics, Mannheim, Germany). After hybridization, the DNA filter was washed sequentially as follows: twice with 2× SSC and 0.1 % SDS for 5 min at room temperature and twice with 0.5× SSC and 0.1 % SDS for 15 min each at 65 °C.
ABA and drought tolerance assays
To determine the germination efficiency of transgenic seeds under stress conditions, seeds were germinated and seedlings grow on 1/2MS agar plates (supplemented with 300 mM mannitol, and 0.6 µM ABA) (Luo et al. 2013). Germination was scored daily, and seedlings were photographed after 10 days. For the root length assay, 5-day-old seedlings grown on 1/2MS agar plates were transferred to vertically oriented 1/2MS agar plates supplemented with or without 300 mM mannitol for 12 days before root length was measured and photographed. The solute potential of 300 mM mannitol was −0.74 MPa. Seventy seeds from each line were used to determine germination rates and six seeds from each line were used to compare root length. All experiments were repeated three times.
To test drought tolerance at subsequent developmental stages, the seedlings transplanted into the soil and watered for 3 weeks before water was withheld. After 2 weeks of water deficit, the pots were re-watered for 1 week. The control comprised plants that were well-watered continuously. Soil was collected at three stages (well-watered, drought, and re-watered), weighed immediately (fresh weigh), then oven-dried to a constant weight for 16 h at 105 °C and weighed for determination of soil water content (dry weigh). The percentage soil water contents was calculated as (fresh weight − dry weight)/fresh weight × 100.
To investigate ABA-regulated gene expression, total RNA was extracted from 2-week-old seedlings floated in 100 µM ABA solution for 6 h under continuous white light (150 µmol m−2 s−1). The synthetic ABA used was a racemic mixture.
Physiological measurements
The following physiological measurements were conducted on seedlings after water was withheld for 10 days. Photosynthetic and transpiration rates were measured using the Li-6400 Portable Photosynthesis System (Li-Cor, Lincoln, NE, USA). The leaves were held at an ambient CO2 concentration of 400 µmol mol−1, a photosynthetic photon flux density of 600 µmol m−2 s−1, and a chamber temperature of 24 °C. The maximum quantum yield of PSII (Fv/Fm) was measured using a Dual-PAM-100 measuring system (Walz Heinz GmbH, Effeltrich, Germany). Six fully expand leaves were detached from different plants of each line, then for each line 1.70 cm2 leaf discs were obtained with a 6-mm- diameter single-hole punch. Leaf chlorophyll was extracted with 80 % (v/v) acetone (Shu et al. 2010). A UV/visible spectrophotometer (YHB-061; GE Healthcare, Little Chalfont, Buckinghamshire, UK) was used to measure the absorbance at 663 nm for chlorophyll a and 645 nm for chlorophyll b. Chlorophyll a concentration was calculated using the equation C a = 12.72D 663 − 2.59D 645, where D is absorbance at 663 or 645 nm. Chlorophyll b was calculated using the equation Cb = 22.88D 645 − 4.67D 663. Total chlorophyll concentration was equal to Ca + Cb. The concentration of proline was estimated using the acid-ninhydrin method by measuring the absorbance at 520 nm. After water was withheld for 14 days, six plants from each line (oxPdEPF2#1, #2, #3, WT, epf2-3, and epf2-3/PdEPF2) were sampled and oven-dried for 72 h at 70 °C to measure total biomass.
Water loss measurements
Rosette leaves detached from transgenic plants, WT, epf2-3, and epf2-3/PdEPF2 grown under non-stress conditions for 3 weeks were used to measure the rate of water loss. Leaves weighing approximately 0.5 g were harvested, weighted and used immediately for experiments. The leaves were placed on a laboratory bench and weighed every 30 min (Ma et al. 2010). The percentage loss of fresh weight was calculated on the basis of the initial weight of the leaves. The experiments were replicated three times.
Stomatal density and stomatal aperture
The stomatal density and aperture of fully expanded leaves were recorded and photographed using a scanning electron microscope (Hitachi S-3400 N; Chiyoda-ku, Tokyo, Japan). Leaves of rosette stage plants were sampled from the oxEPF2 lines, WT and the epf2-3 mutant. The stomatal aperture assays were supplemented with 10 µM ABA for 2.5 h. The samples were fixed as described by Cao et al. (2007). The samples were first fixed in 25 % glutaraldehyde for 24 h. The leaves were then dehydrated in 30, 50, 70, 85, and 95 % ethanol (15 min each) and then twice in 100 % ethanol (15 min each). The dehydrated samples were then treated with isoamyl acetate: ethanol (1:1) and 100 % isoamyl acetate (15 min each).
Statistical analysis
For qPT-PCR, RNA samples were extracted from three independent plant materials and all experiments were repeated three times. Six root lengths were measured per strain, and three independent experiments were repeated. For physiological measurements, six leaves were measured per plant, and three individual plants were included in each group. For stomatal density, three leaves were measured per plant, and three individual plants were included in each group. All statistical analyses and plots were performed using SPSS 19.0 (IBM Corporation, Armonk, NY, USA). Statistical comparisons used one-way analysis of variance (ANOVA) in SPSS. Different letters indicate significant differences at α = 0.05 (one-way ANOVA). Data were presented as the mean ± standard error (SE).
Results
Identification and molecular characterization of PdEPF2
The PdEPF2 gene (GenBank accession number KR611990), comprising a complete open reading frame of 351 bp and encoding 116 amino acid residues with a predicted molecular mass of 12.88 kDa and an isoelectric point of 8.63 (http://web.expasy.org/compute_pi/), was cloned from the high WUE poplar genotype NE-19 (Populus nigra × (Populus deltoids × Populus nigra)) (Hao et al. 2011; Xing et al. 2011).
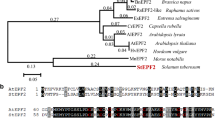
To identify homologous proteins of PdEPF2, a phylogenetic tree was constructed between the poplar and Arabidopsis EPF family members through their amino acid sequence alignment. PdEPF2 was clustered with AtEPF2 (Fig. 1a). The multiple protein sequence alignment revealed that PdEPF2 contained eight conserved cysteine residues at the C-terminal end (Fig. 1b), consistent with AtEPF1, AtEPF2, and AtEPFL7 (Hara et al. 2009; Shimada et al. 2011).
PdEPF2 gene of Populus nigra × (Populus deltoides × Populus nigra). a Phylogenetic relationships among poplar and Arabidopsis EPF family members. The phylogenetic tree was constructed with the neighbor-joining method using MEGA 6. b Multiple sequence alignment of the C-terminal region of PdEPF2 and homologous EPFs from Arabidopsis. The number of preceding amino acid residues is indicated in parentheses. Conserved cysteine residues are marked in yellow. (Color figure online)
Expression profile of PdEPF2
Transcription of PdEPF2 was tissue-specific. Transcripts were detected in roots, stems, young leaves, mature leaves, and senescent leaves of NE-19 under non-stress growth conditions. The results exhibited that PdEPF2 was expressed more highly in young leaves and mature leaves than in senescent leaves, but was the lowest in root (Fig. 2a).
Expression patterns of PdEPF2. a Tissue-specific expression pattern of PdEPF2 in poplar. “Young leaf”, the first one to three leaves from the shoot apex; “mature leaf”, a fully expanded leaf; “senescent leaf”, basal most two to three leaves above the root system. The level of the PdEPF2 transcript in the stem was set at 1. b, c Expression of PdEPF2 in response to drought and ABA in poplar. Data represent mean ± SE (n = 3)
Under drought stress, PdEPF2 transcription initially increased but subsequently decreased during days 3–12 of drought treatment (Fig. 2b). ABA is an important secondary signaling molecule, and its exogenous application can cause effects similar to those of osmotic stress and may modulate some drought-responsive genes (Zhu 2002). To investigate the involvement of PdEPF2 in responses to ABA stress, the transcript level of PdEPF2 under 250 µM ABA was quantified by qRT-PCR. Transcripts of PdEPF2 accumulated rapidly to about 5.2-fold 3 h after ABA application before slightly declining (Fig. 2c). These results indicate that the expression of PdEPF2 was induced by both drought and ABA treatments.
Analysis of transgenic Arabidopsis overexpressing PdEPF2
To elucidate the in vivo functions of PdEPF2, the 35S:PdEPF2 construct was transformed into WT Arabidopsis and the epf2-3 mutant to generate overexpression and complementation lines, respectively. We raised 11 independent transgenic lines, of which three were selected on the basis of qRT-PCR results. As expected, PdEPF2 transcripts were undetectable in the WT plants. Three overexpression lines (lines 1, 3, and 5) showed higher transcript levels than the other overexpression lines (Online Resource 2), and were designated oxPdEPF2#1, #2, and #3. One complementary line was selected for further analysis (Online Resource 3). DNA hybridization showed that PdEPF2 was integrated into the genomes of the transgenic Arabidopsis as one (oxPdEPF2#1, #2, #3 and epf2-3/PdEPF2), whereas no hybridization was observed in wild type (Online Resource 4).
Phenotypes of oxPdEPF2 plants under drought conditions
Mannitol was used to simulate drought stress. Seeds of transgenic plants, WT, epf2-3, and the complementary line epf2-3/PdEPF2 were sown on 1/2MS agar medium supplemented with or without 300 mM mannitol. In the presence of mannitol, seedlings from the oxPdEPF2 plants were greener (Fig. 3a) and showed more rapid germination than WT and epf2-3 seeds (Fig. 3b).The transgenic plants showed more green cotyledon than the WT and the mutant epf2-3 (Fig. 3c). When 5-day-old seedlings grown on 1/2MS medium were transferred to vertical agar plates containing 1/2MS medium supplemented with 300 mM mannitol, the primary root was 1.34 times as long in oxPdEPF2 plants as in WT plants; however, when compared with the epf2-3 mutant, the difference was significant (2.17 times). The mutant complementation restored the phenotype to that of the WT. Furthermore, compared with seedlings grown on standard 1/2MS medium, the primary root length of oxPdEPF2, WT, and epf2-3 seedlings subjected to osmotic stress decreased by approximately 50, 63.05, and 76.65 %, respectively (Fig. 3d).
Overexpression of PdEPF2 in Arabidopsis under drought stress. a–c Phenotype, germination time, and cotyledon greening at the seed germination stage of seedlings grown on 1/2MS medium supplemented with or without 300 mM mannitol. A seeds was considered to have germinated when the radicle had completely penetrated the seed coat. Photographs were taken 10 days after stratification. Data shown represent the means (±SE) of three independent experiments (70 seeds for each test). d Root length of oxPdEPF2 s, WT, epf2-3, and epf2-3/PdEPF2 seedlings grown on 1/2MS medium supplemented with or without 300 mannitol. Photographs were taken after 12 days of growth on the media. Data are mean ± SE (n = 6 × 3) of three independent experiments. Different letters indicate a significant difference at α = 0.05 (one-way ANOVA). e Morphological differences in drought experiments. The seedlings were grown in soil for 3 weeks under well-watered conditions; thereafter, water was withheld for 2 weeks, then plants were re-watered for 1 week
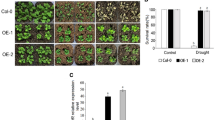
The capacity of oxPdEPF2 plants to respond to severe drought stress was investigated further. Watering of 3-week-old transgenic plants, WT, epf2-3, and the mutant complementation line epf2-3/PdEPF2 was withheld for 2 weeks, after which the plants were re-watered for 1 week. The soil water contents in the three phases of the experiment were 58.65 ± 1.99 g/g % (well-watered), 8.66 ± 1.83 g/g % (drought), and 46.64 ± 1.97 g/g % (re-watered), respectively. During water deprivation, plants of the WT and epf2-3 mutant wilted more severely than the transgenic plants, and the mutant complementation line showed a similar response to that of the WT (Fig. 3e). These results indicated that the transgenic plants showed enhanced drought tolerance.
Physiological analysis of oxPdEPF2 plants under drought stress
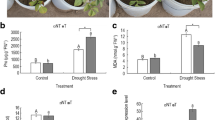
To investigate the physiological basis of the transgenic plants’ drought tolerance, we monitored changes in proline and chlorophyll content and F v/F m after water was withheld for 10 days. Although there were no differences under well-watered conditions, proline content in the transgenic lines was significantly higher than that of the WT (31 %) and epf2-3 plants (54 %) under drought stress (Fig. 4a). Likewise, at all tested drought levels, chlorophyll content in the transgenic plants was significantly higher than that of WT and epf2-3 (1.35 and 2.12 times, respectively) (Fig. 4b). F v/F m was significantly higher in the oxPdEPF2 plants under drought stress. When water was withheld for 10 days, F v/F m of the oxPdEPF2, WT, and epf2-3 plants decreased from 0.83 to 0.79, 0.82 to 0.70, and 0.82 to 0.63, respectively (Fig. 4c). During water deprivation, growth of the oxPdEPF2 lines continued, resulting in higher biomass, despite all lines suffering wilting, whereas no difference in biomass was observed among the lines under well-watered conditions (Fig. 4d). In addition, the oxPdEPF2 plants maintained a significantly higher photosynthetic rate and lower transpiration rate than those of the WT and epf2-3 plants under drought stress (Fig. 4e, f). Based on the higher photosynthetic capability and lower transpiration level, the oxPdEPF2 plants showed increased leaf WUE compared with the WT and epf2-3 mutant (Fig. 4g). These observations indicated that a positive relationship existed between PdEPF2 expression and drought stress tolerance.
Physiological analysis of oxPdEPF2 lines under drought stress. a–c Changes in proline content, chlorophyll content, and F v/F m of transgenic plants, WT, epf2-3 mutant, and epf2-3/PdEPF2 complementation line after water was withheld for 10 days. d Total plant biomass over the 14-day experimental period. Data are mean ± SE (n = 6). e–g Net photosynthetic rate, transpiration rate, and instantaneous WUE of leaves at three different stages. Data represent mean ± SE (n = 6). Different letters indicate a significant difference at α = 0.05 (one-way ANOVA)
Overexpression of PdEPF2 decreases stomatal density
Stomatal density is an important index of tolerance to drought stress in plants, as it affects water loss. Compared with the WT and epf2-3 plants, the transgenic plants showed a decrease in water loss (Fig. 5a) consistent with their superior drought tolerance. Given the close relationship between water loss and stomatal density, we recorded the number of stomata per unit leaf area (Fig. 5b). The number of stomata per mm2 in the oxPdEPF2 plants was <50 % that of the epf2-3 mutant plants and approximately 30 % less than that of WT plants. The epf2-3 mutant showed a significantly higher stomatal density than the WT, whereas the epf2-3/PdEPF2 complementation line showed a decreased stomatal density, as observed for the WT (Fig. 5c). These results indicated that overexpression of PdEPF2 may reduce water loss by influencing stomatal density.
Effect of PdEPF2 on stomatal density and leaf water loss. a Water loss from detached leaves; the water loss of 0.5 g detached leaves per plant was measured at the indicated time points in triplicate. Three measurements were averaged at each time point. Data are mean ± SE. b Scanning electron micrograph of the abaxial leaf epidermis. Scale bars 100 µm. c Abaxial stomatal densities in WT, transgenic plants, epf2-3 mutant, and the epf2-3/PdEPF2 complementation line. Different letters indicate a significant difference at α = 0.05 (one-way ANOVA)
Overexpression of the PdEPF2 gene decreases ABA sensitivity in seeding stage
Substantial evidence suggests that ABA plays a crucial role in responses to abiotic stresses, including drought and salinity (Shinozaki and Yamaguchi-Shinozaki 2007; Jin et al. 2011). Hence, the ABA-response phenotypes of the oxPdEPF2 plants were examined further. To observe germination and early seedling development, seeds were incubated on 1/2MS medium supplemented with or without 0.6 µM ABA. In the absence of ABA, no significant difference was observed among the different lines. In the presence of exogenous ABA, the oxPdEPF2 plants showed more green cotyledon (Fig. 6a) and seeds showed faster germination compared with the WT and mutant epf2-3 plants (Fig. 6b). These results indicated that oxPdEPF2 plants showed reduced sensitivity to ABA. To investigate molecular changes in response to ABA stress, we evaluated the transcription of two genes, ABI1 and ABI2, which are both associated with type 2C protein serine/threonine phosphatases and act as key regulators in the response to ABA (Merlot et al. 2001). The overexpression lines showed higher transcript levels of ABI1 and ABI2 compared with those of WT plants after ABA treatment. The mutant epf2-3 showed lower transcript levels than those of WT plants, whereas the complementation line (epf2-3/PdEPF2) showed similar transcript levels to those of WT plants (Fig. 6c). These results indicated that PdEPF2 may be involved in response to the ABA signaling pathway.
Overexpression of PdEPF2 in Arabidopsis decreased sensitivity to ABA. a, b Phenotype and time course of seed germination on 1/2MS medium supplemented with or without 0.6 µM ABA. Data are mean ± SE from three independent experiments (70 seedlings per experiment). c Expression of ABI1 and ABI2 in transgenic plants. Two-week-old seedlings were grown for 6 h on 1/2MS agar medium supplemented with or without 100 µM ABA. Quantitative RT-PCR was used to analyze ABA-responsive gene expression. The Arabidopsis actin gene was used as the internal control. Data are mean ± SE of three independent measurements
It is well known that ABA induces stomatal closure. We therefore compared stomatal apertures in the different lines under ABA treatment. The aperture length:width ratio was used as a measure of stomatal closure (Ren et al. 2010). As shown in Online Resource 5, all lines showed a reduced stomatal aperture in response to ABA treatment. However, no obvious difference in the length:width ratio of stomata was observed in the different lines.
Discussion
Drought is one of the most prevalent environmental stressors to plants. Therefore, identifying genes that confer drought tolerance, and thus enable plants to better survive under water deficit, is important for crop production and plant breeding. The present study identified novel roles for PdEPF2 in the response to drought stress, and transcription analyses indicated that PdEPF2 was differentially expressed and induced in response to drought and ABA (Fig. 2b, c). Furthermore, PdEPF2 transgenic plants differed phenotypically from WT and epf2-3 mutant plants under drought stress. First, transgenic plants showed superior germination capacity and increased primary root length under osmotic stress conditions (Fig. 3a–d). Second, drought-induced proline content was significantly higher in transgenic plants than that of WT and epf2-3 mutant plants (Fig. 4a). Proline is positively associated with abiotic stress tolerance (Liu and Zhu 1997; Xiang et al. 2007). Third, although net CO2 assimilation under well-watered conditions did not vary and photosynthetic rates decreased in all plants under water deprivation, the reduction in photosynthetic rate was least severe in transgenic plants (Fig. 4e), which was consistent with the observed changes in chlorophyll content and F v/F m (Fig. 4b, c). The transgenic lines maintained higher WUE (Fig. 4g) through reduction in the transpiration rate (Fig. 4f).
Transpiration and CO2 uptake occur primarily through stomatal pores, and conductance depends on stomatal density and pore aperture size (Hetherington and Woodward 2003; Nilson and Assmann 2007). In addition, reduced transpirational water loss is a key determinant of drought tolerance (Xiong et al. 2002). We observed that overexpression of PdEPF2 decreased the rate of water loss (Fig. 5a) and stomatal density (Fig. 5b, c), which resulted in enhanced tolerance of water deficiency. This likely occurred because the reduced stomatal density resulted in decreased transpiration during drought conditions, although we cannot rule out that other changes caused increased efficiency in water uptake in the oxPdEPF2 plants. Interestingly, the decreased stomatal density did not affect photosynthesis capacity, although gas exchange might be limited by the reduced number of stomata.
Generally, in C3 plants, photosynthetic rates become saturated as stomatal conductance increases because of limitations unrelated to stomata, such as the regeneration of ribulose 1,5-bisphosphate (Zeiger and Field 1982). Consequently, lower stomatal density does not necessarily affect carbon assimilation or biomass accumulation and therefore oxPdEPF2 plants showed continued growth and development, resulting in higher biomass compared with the other lines under drought conditions (Fig. 4d). This finding is supported by previous work that showed that overexpression of both HARDY and HDG11, and mutations of GTL1 and GPA1, reduced stomatal density and improved drought tolerance while not affecting carbon assimilation or biomass accumulation (Karaba et al. 2007; Yu et al. 2008; Nilson et al. 2010; Yoo et al. 2010). However, transpiration rate increases linearly over a certain range of stomatal conductance and therefore decrease in stomatal density could reduce transpiration rate, thereby increasing WUE without affecting carbon assimilation (Yoo et al. 2009).
Quantitative RT-PCR confirmed that exogenous ABA application induced PdEPF2 expression (Fig. 2c), indicating that PdEPF2 may respond to the ABA signaling pathway. HDG11, a transcription factor involved in the interplay between ABA biosynthesis, ABA signaling and stomatal development (Chater et al. 2014), has been shown to negatively regulate stomatal development via transactivation of the ERECTA promoter (Yu et al. 2008). Biochemical studies using a biosensor chip have shown that EPF2–ERECTA, as the ligand–receptor pair, regulates initiation of stomatal development (Lee et al. 2012). These studies indicate that the EPF2–ERECTA–MPK signal transduction pathways involved in stomatal development may share signaling pathways involved in ABA response. In addition, appraisal of ABA sensitivity in the current study showed that epf2-3 was more sensitive to ABA before germination, whereas oxPdEPF2 was less sensitive than WT plants to ABA (Fig. 6a, b). Analysis of two ABA-regulated genes suggested that the reduced sensitivity of PdEPF2 to ABA may be due to activation of ABI1 and ABI2 (Fig. 6c). Previous studies have shown that ABI1 and ABI2 are important for ABA signal transduction and act by negatively regulating ABA responses (Merlot et al. 2001). The significant up-regulation of ABI1 and ABI2 is consistent with the decreased ABA sensitivity of oxPdEPF2 plants during germination and seedling growth. Some evidence indicates that ABI1 and ABI2 negatively affect a plant’s stress tolerance (Jung et al. 2008; Seo et al. 2010). However, this negative relationship is not consistently supported. For example, transgenic Arabidopsis plants overexpressing GsWRKY20 showed reduced ABA sensitivity but were more tolerant of drought stress, possibly through up-regulation of ABI1 and ABI2 (Luo et al. 2013). In addition, overexpression of OZF2 in Arabidopsis improved salt stress tolerance and reduced ABA sensitivity, and the level of ABI2 transcripts was increased (Huang et al. 2012). Furthermore, transgenic plants overexpressing ZmPP2C showed reduced expression of ABI1 and ABI2 but were less sensitive to ABA and displayed decreased salt and drought tolerance (Liu et al. 2009). Consequently, stress tolerance depends on the expression of stress response genes as well as plant physiology and drought tolerance and thus is the result of their integrated effects. In the present study no obvious difference in stomatal aperture was observed in the different lines after treatment with ABA (Online Resource 5). ABA promotes stomatal closure mainly through import of Ca2+ and export of K+, Cl−, and malate in guard cells (Chen et al. 2010; Kim et al. 2010). These results demonstrate that the molecular mechanisms of PdEPF2 response to ABA are complex. Furthermore, different mechanisms might be involved at different stages of growth or due to other unknown factors.
In conclusion, in this study we identified and isolated PdEPF2 and showed that its expression in Arabidopsis plants improved drought tolerance. Our findings suggest that the gene may be useful in the breeding of drought-tolerant plants and in improving crop production under drought conditions. Further experiments are required to generate additional transgenic poplars and to elucidate the regulatory mechanisms of drought tolerance in woody plants.
References
Bhatnagar-Mathur P, Devi MJ, Reddy DS, Lavanya M, Vadez V, Serraj R, Yamaguchi-Shinozaki K, Sharma KK (2007) Stress-inducible expression of At DREB1A in transgenic peanut (Arachis hypogaea L.) increases transpiration efficiency under water-limiting conditions. Plant Cell Rep 26(12):2071–2082. doi:10.1007/s00299-007-0406-8
Bhave NS, Veley KM, Nadeau JA, Lucas JR, Bhave SL, Sack FD (2008) TOO MANY MOUTHS promotes cell fate progression in stomatal development of Arabidopsis stems. Planta 229(2):357–367. doi:10.1007/s00425-008-0835-9
Boccalandro HE, Rugnone ML, Moreno JE, Ploschuk EL, Serna L, Yanovsky MJ, Casal JJ (2009) Phytochrome B enhances photosynthesis at the expense of water-use efficiency in Arabidopsis. Plant Physiol 150(2):1083–1092. doi:10.1104/pp.109.135509
Cao WH, Liu J, He XJ, Mu RL, Zhou HL, Chen SY, Zhang JS (2007) Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol 143(2):707–719. doi:10.1104/pp.106.094292
Casson SA, Hetherington AM (2010) Environmental regulation of stomatal development. Curr Opin Plant Biol 13(1):90–95. doi:10.1016/j.pbi.2009.08.005
Casson SA, Franklin KA, Gray JE, Grierson CS, Whitelam GC, Hetherington AM (2009) phytochrome B and PIF4 regulate stomatal development in response to light quantity. Curr Biol: CB 19(3):229–234. doi:10.1016/j.cub.2008.12.046
Chaerle L, Saibo N, Van Der Straeten D (2005) Tuning the pores: towards engineering plants for improved water use efficiency. Trends Biotechnol 23(6):308–315
Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11(2):113–116
Chater CC, Oliver J, Casson S, Gray JE (2014) Putting the brakes on: abscisic acid as a central environmental regulator of stomatal development. New phytol 202(2):376–391. doi:10.1111/nph.12713
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30(3):239–264
Chen ZH, Hills A, Lim CK, Blatt MR (2010) Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant J Cell Mol Biol 61(5):816–825. doi:10.1111/j.1365-313X.2009.04108.x
Confalonieri M, Balestrazzi A, Bisoffi S, Carbonera D (2003) In vitro culture and genetic engineering of Populus spp. synergy for forest tree improvement. Plant Cell Tissue Organ Cult 72:109–138
Han KH, Meilan R, Ma C, Strauss SH (2000) An Agrobacterium tumefaciens transformation protocol effective on a variety of cottonwood hybrids. Plant Cell Rep 19:315–320
Han X, Tang S, An Y, Zheng DC, Xia XL, Yin WL (2013) Overexpression of the poplar NF-YB7 transcription factor confers drought tolerance and improves water-use efficiency in Arabidopsis. J Exp Bot 64(14):4589–4601. doi:10.1093/jxb/ert262
Hao S, Zhao T, Xia X, Yin W (2011) Genome-wide comparison of two poplar genotypes with different growth rates. Plant Mol Biol 76(6):575–591. doi:10.1007/s11103-011-9790-0
Hara K, Yokoo T, Kajita R, Onishi T, Yahata S, Peterson KM, Torii KU, Kakimoto T (2009) Epidermal cell density is autoregulated via a secretory peptide, EPIDERMAL PATTERNING FACTOR 2 in Arabidopsis leaves. Plant Cell Physiol 50(6):1019–1031. doi:10.1093/pcp/pcp068
Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424(6951):901–908
Huang P, Ju HW, Min JH, Zhang X, Chung JS, Cheong HS, Kim CS (2012) Molecular and physiological characterization of the Arabidopsis thaliana oxidation-related zinc finger 2, a plasma membrane protein involved in ABA and salt stress response through the ABI2-mediated signaling pathway. Plant Cell Physiol 53(1):193–203. doi:10.1093/pcp/pcr162
Jin YM, Jung J, Jeon H, Won SY, Feng Y, Kang JS, Lee SY, Cheong JJ, Koiwa H, Kim M (2011) AtCPL5, a novel Ser-2-specific RNA polymerase II C-terminal domain phosphatase, positively regulates ABA and drought responses in Arabidopsis. New Phytol 190(1):57–74. doi:10.1111/j.1469-8137.2010.03601.x
Jung C, Seo JS, Han SW, Koo YJ, Kim CH, Song SI, Nahm BH, Choi YD, Cheong JJ (2008) Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol 146(2):623–635. doi:10.1104/pp.107.110981
Karaba A, Dixit S, Greco R, Aharoni A, Trijatmiko KR, Marsch-Martinez N, Krishnan A, Nataraja KN, Udayakumar M, Pereira A (2007) Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc Natl Acad Sci USA 104(39):15270–15275. doi:10.1073/pnas.0707294104
Kim TH, Bohmer M, Hu H, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61:561–591. doi:10.1146/annurev-arplant-042809-112226
Lee JS, Kuroha T, Hnilova M, Khatayevich D, Kanaoka MM, McAbee JM, Sarikaya M, Tamerler C, Torii KU (2012) Direct interaction of ligand-receptor pairs specifying stomatal patterning. Genes Dev 26(2):126–136. doi:10.1101/gad.179895.111
Liu J, Zhu J-K (1997) Proline accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis. Plant Physiol 114(2):591–596
Liu L, Hu X, Song J, Zong X, Li D, Li D (2009) Over-expression of a Zea mays L. protein phosphatase 2C gene (ZmPP2C) in Arabidopsis thaliana decreases tolerance to salt and drought. J Plant Physiol 166(5):531–542
Luo X, Bai X, Sun X, Zhu D, Liu B, Ji W, Cai H, Cao L, Wu J, Hu M (2013) Expression of wild soybean WRKY20 in Arabidopsis enhances drought tolerance and regulates ABA signalling. J Exp Bot 64:2155–2169
Ma HS, Liang D, Shuai P, Xia XL, Yin WL (2010) The salt- and drought-inducible poplar GRAS protein SCL7 confers salt and drought tolerance in Arabidopsis thaliana. J Exp Bot 61(14):4011–4019. doi:10.1093/jxb/erq217
MacAlister CA, Ohashi-Ito K, Bergmann DC (2007) Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature 445(7127):537–540. doi:10.1038/nature05491
Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J (2001) The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J 25(3):295–303
Monclus R, Dreyer E, Villar M, Delmotte FM, Delay D, Petit JM, Barbaroux C, Le Thiec D, Brechet C, Brignolas F (2006) Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoides × Populus nigra. New Phytol 169(4):765–777. doi:10.1111/j.1469-8137.2005.01630.x
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue culture. Physiol Plant 15:473–497
Nakashima K, Ito Y, Yamaguchi-Shinozaki K (2009) Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol 149(1):88–95. doi:10.1104/pp.108.129791
Nilson SE, Assmann SM (2007) The control of transpiration. Insights from Arabidopsis. Plant Physiol 143(1):19–27. doi:10.1104/pp.106.093161
Nilson SE, Assmann SM (2010) The alpha-subunit of the Arabidopsis heterotrimeric G protein, GPA1, is a regulator of transpiration efficiency. Plant Physiol 152(4):2067–2077. doi:10.1104/pp.109.148262
Ohashi-Ito K, Bergmann DC (2006) Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. Plant Cell 18(10):2493–2505. doi:10.1105/tpc.106.046136
Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU (2007) Termination of asymmetric cell division and differentiation of stomata. Nature 445(7127):501–505. doi:10.1038/nature05467
Quarrie S, Jones H (1977) Effects of abscisic acid and water stress on development and morphology of wheat. J Exp Bot 28(1):192–203
Ren X, Chen Z, Liu Y, Zhang H, Zhang M, Liu Q, Hong X, Zhu JK, Gong Z (2010) ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis. Plant J Cell Mol Biol 63(3):417–429. doi:10.1111/j.1365-313X.2010.04248.x
Royer D (2001) Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Rev Palaeobot Palynol 114(1):1–28
Seo YJ, Park JB, Cho YJ, Jung C, Seo HS, Park SK, Nahm BH, Song JT (2010) Overexpression of the ethylene-responsive factor gene BrERF4 from Brassica rapa increases tolerance to salt and drought in Arabidopsis plants. Mol Cells 30(3):271–277. doi:10.1007/s10059-010-0114-z
Shimada T, Sugano SS, Hara-Nishimura I (2011) Positive and negative peptide signals control stomatal density. Cell Mol Life Sci CMLS 68(12):2081–2088. doi:10.1007/s00018-011-0685-7
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58(2):221–227. doi:10.1093/jxb/erl164
Shpak ED, McAbee JM, Pillitteri LJ, Torii KU (2005) Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science 309(5732):290–293
Shu Z, Zhang XS, Chen J, Chen GY, Xu DQ (2010) The simplification of chlorophyll content measurement. Plant Physiol Commun 46(4):399–402. doi:10.13592/j.cnki.ppj.2010.04.001
Silva EC, Nogueira RJ, Vale FH, Araújo FPd, Pimenta MA (2009) Stomatal changes induced by intermittent drought in four umbu tree genotypes. Braz J Plant Physiol 21(1):33–42
Tricker PJ, Gibbings JG, López CMR, Hadley P, Wilkinson MJ (2012) Low relative humidity triggers RNA-directed de novo DNA methylation and suppression of genes controlling stomatal development. J Exp Bot 63(10):3799–3813
Tschaplinski T, Blake T (1989) Water relations, photosynthetic capacity, and root/shoot partitioning of photosynthate as determinants of productivity in hybrid poplar. Can J Bot 67(6):1689–1697
Wang WH, Chen J, Liu TW, Chen J, Han AD, Simon M, Dong XJ, He JX, Zheng HL (2014a) Regulation of the calcium-sensing receptor in both stomatal movement and photosynthetic electron transport is crucial for water use efficiency and drought tolerance in Arabidopsis. J Exp Bot 65(1):223–234. doi:10.1093/jxb/ert362
Wang HL, Chen J, Tian Q, Wang S, Xia X, Yin W (2014b) Identification and validation of reference genes for Populus euphratica gene expression analysis during abiotic stresses by quantitative real-time PCR. Physiol Plant 152(3):529–545. doi:10.1111/ppl.12206
Wang C, Liu S, Dong Y, Zhao Y, Geng A, Xia X, Yin W (2015) PdEPF1 regulates water-use efficiency and drought tolerance by modulating stomatal density in poplar. Plant Biotechnol J. doi:10.1111/pbi.12434
Willems E, Leyns L, Vandesompele J (2008) Standardization of real-time PCR gene expression data from independent biological replicates. Anal Biochem 379(1):127–129. doi:10.1016/j.ab.2008.04.036
Xiang Y, Huang Y, Xiong L (2007) Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement. Plant Physiol 144(3):1416–1428. doi:10.1104/pp.107.101295
Xing HT, Guo P, Xia XL, Yin WL (2011) PdERECTA, a leucine-rich repeat receptor-like kinase of poplar, confers enhanced water use efficiency in Arabidopsis. Planta 234(2):229–241. doi:10.1007/s00425-011-1389-9
Xiong L, Schumaker KS, Zhu J-K (2002) Cell signaling during cold, drought, and salt stress. Plant Cell Online 14(suppl 1):S165–S183
Xu Z, Zhou G (2008) Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J Exp Bot 59(12):3317–3325. doi:10.1093/jxb/ern185
Yoo CY, Pence HE, Hasegawa PM, Mickelbart MV (2009) Regulation of transpiration to improve crop water use. Crit Rev Plant Sci 28(6):410–431. doi:10.1080/07352680903173175
Yoo CY, Pence HE, Jin JB, Miura K, Gosney MJ, Hasegawa PM, Mickelbart MV (2010) The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell 22(12):4128–4141. doi:10.1105/tpc.110.078691
Yu H, Chen X, Hong YY, Wang Y, Xu P, Ke SD, Liu HY, Zhu JK, Oliver DJ, Xiang CB (2008) Activated expression of an Arabidopsis HD-START protein confers drought tolerance with improved root system and reduced stomatal density. Plant Cell 20(4):1134–1151. doi:10.1105/tpc.108.058263
Zeiger E, Field C (1982) Photocontrol of the functional coupling between photosynthesis and stomatal conductance in the intact leaf blue light and par-dependent photosystems in guard cells. Plant Physiol 70(2):370–375
Zhang X, Henriques R, Lin S-S, Niu Q-W, Chua N-H (2006) Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 1(2):641–646
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273. doi:10.1146/annurev.arplant.53.091401.143329
Zhu J, Dong C-H, Zhu J-K (2007) Interplay between cold-responsive gene regulation, metabolism and RNA processing during plant cold acclimation. Curr Opin Plant Biol 10(3):290–295
Zsuffa L, Giordano E, Pryor L, Stettler R (1996) Trends in poplar culture: some global and regional perspectives. In: Stettler RF, Bradshaw HD Jr, Heilman PE, Hinckley TM (eds) Biology of Populus and its implications for management and conservation. NRC Research Press, Ottawa, pp 515–539
Acknowledgments
This research was supported by the Special Fund for forestry scientific Research in the Public Interests (201304301), the Hi-Tech Research and Development Program of China (2013AA102701), the National Natural Science Foundation of China (31270656), Program for Changjiang Scholars and Innovative Research Team in University (IRT13047) and the 111 Project (B13007). We thank Dun Zhang for his helpful comments on the manuscript and technical assistance. We also thank Junna Shi for her technical assistance with scanning electron microscopy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, S., Wang, C., Jia, F. et al. Secretory peptide PdEPF2 enhances drought tolerance by modulating stomatal density and regulates ABA response in transgenic Arabidopsis thaliana . Plant Cell Tiss Organ Cult 125, 419–431 (2016). https://doi.org/10.1007/s11240-016-0957-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-0957-x