Abstract
The induction and establishment of hairy root cultures of Gentiana dinarica using two strains of Agrobacterium rhizogenes (A4M70GUS and 15834/PI) is reported for the first time. Strain 15834/PI had higher induction rate of hairy roots (32.15 %) than strain A4M70GUS (6.12 %). Transgenic nature of the roots was confirmed by GUS assay and PCR analysis. Two clones per strain (A4M70GUS-D and -I, and 15834/PI-2 and -3) with marked differences in general morphology and growth rate were further studied. The methanol extracts of hairy root clones were analyzed for xanthones content using HPLC method. The effects of the type of carbohydrate source (sucrose, fructose or glucose) at different concentrations on the growth parameters (growth index, dry weight, fresh/dry weight ratio), phenolic and xanthone production, and free radical scavenging activity of the transgenic clones were evaluated. Statistical two level factorial design was used to define optimal conditions for growth and successful secondary metabolite production in G. dinarica hairy root clones. The results showed that clones A4M70GUS-D and 15834/PI-3 were the superior ones. These two clones had the highest dry weight on 116.8 mM sucrose, producing up to threefold higher amounts of total phenolics and norswertianin-1-O-primeveroside than other clones, untransformed roots and roots of wild-grown plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gentiana dinarica Beck. is a sub-alpine, perennial species found in open plains on carbonate soil of Dinaric Alps Mountains of the Balkan Peninsula (Jovanović-Dunjić 1973). Gentians (Gentiana spp.) have been used in the traditional medicines in many countries mostly for the preparation of bitter tonic which is usually used as a remedy for digestive problems (Pontus et al. 2006). Secoiridoids, xanthones and flavone-C-glucosides are the main secondary metabolites isolated from Gentiana species (Hostettmann-Kaldas et al. 1981). The aerial parts of G. dinarica contain secoiridoids and flavone-C-glucosides, while the roots are rich in xanthones and secoiridoids, along with flavone-C-glucosides (Krstić et al. 2004). Xanthones are yellow pigments occurring in a few higher plant families and are almost exclusively found in Gentianaceae and Guttiferae (Jensen and Schripsema 2002). Xanthones with 1,3,7,8-oxygenation pattern such as norswertianin and its O-glycosides are characteristic for G. dinarica, but are not very common in gentians. Apart from G. dinarica, these compounds have been found only in two other gentians, G. bavarica and G. verna (Peres et al. 2000). Interestingly, this type of xanthones has been isolated from many Swertia species, and is more characteristic for Swertia than to Gentiana species. Naturally occurring xanthones are widely reported for their significant biological and pharmacological properties. Numerous studies demonstrated that xanthones and their derivatives exhibit antioxidant (Luo et al. 2013), anti-inflammatory (Mahendran et al. 2013), antimicrobial (Larcher et al. 2004; Yimdjo et al. 2004), antiviral (Cao et al. 2013), vasodilator (Chericoni et al. 2003) and anti-diabetic activities (Zheng et al. 2014). It has been shown that norswertianin exhibited antibacterial, antifungal, antioxidant and hypoglycemic activity (Fotie and Bohle 2006), with potential to act as a chemopreventive agent (Hirakawa et al. 2005).
In vitro culture techniques have been well developed for the major European gentians, and micropropagation of G. dinarica has been described recently (Vinterhalter et al. 2012). The effects of different carbohydrates on root growth and xanthone production in root cultures of G. dinarica have been reported (Krstić-Milošević et al. 2013). It has been shown that sucrose was the preferred carbohydrate source for the excised root growth and xanthone production. However, fructose and glucose were also suitable carbohydrates supporting growth of root cultures and high accumulation of xanthones.
Transformed root cultures have received increasing attention in recent years as they offer many advantages, such as fast growth in hormone-free media, genetic and biochemical stability, and capacity for secondary metabolite production with the possibility of increasing their levels by line selection, elicitation or manipulation of culture conditions (Georgiev et al. 2007; Hu and Du 2006). Transformation of Gentiana species in most studies was done using Agrobacterium rhizogenes with two main goals in mind. One was to improve transformation protocols in producing hairy roots with increased secondary metabolite production, while the second goal was to transfer other traits that may be of horticultural interest. In this latter sense, Hosokawa et al. (1997) and Nishihara et al. (2006) produced dwarf gentian clones (Gentiana triflora × G. scabra) with different flower colors, and Mishiba et al. (2006) studied a flower color stability among transformants. However, majority of the numerous gentian studies were dedicated to the production of superior hairy root cultures with increased secondary metabolite content suitable for production in bioreactors. Secoiridoid compounds were analyzed in hairy roots of G. cruciata (Hayta et al. 2011), G. punctata (Menković et al. 2000a), and G. macrophylla (Tiwari et al. 2007; Zhang et al. 2010), whereas secoiridoids and xanthones were reported in transformed G. lutea (Menković et al. 2000b). Secondary metabolite production in root cultures was usually studied in relation to the type of transformation vectors, presence of rol genes, morphological differences between genotypes/clones, initial inoculum size, medium type, culture conditions, mineral medium composition and concentration of carbohydrates (Keil et al. 2000; Jeong et al. 2002; Weathers et al. 1997). Most of the studies analyzed the influence of a single factor while the interactions between the factors have not been examined thoroughly. Therefore, in order to optimize the culture conditions it would be necessary to investigate the influence of different factors on growth and secondary metabolism using experimental design. This strategy could provide identification and prediction of experimental conditions for the best culture performance (Wu and Hamada 2009).
In the present study, we report for the first time successful genetic transformation of G. dinarica and establishment of hairy root cultures. For transformation we used A. rhizogenes strains A4M70GUS and 15834/PI. Moreover, for each bacterial strain we have investigated the contribution and interactive effects of clone, carbohydrate type and concentration on the dry weight, total phenolic content and xanthone production in hairy root cultures using a factorial 23 experimental design.
Materials and methods
Bacterial strains
Two agropine-producing A. rhizogenes strains A4M70GUS (Tepfer and Casse-Delbart 1987) and 15834/PI, carrying two independently expressed proteinase inhibitor (PI) genes (Smigocki et al. 2009), were used. Bacteria were maintained on YEB media (Van Larebake et al. 1977) supplemented with 50 mg/l neomycin for A. rhizogenes A4M70GUS or 50 mg/l kanamycin for A. rhizogenes 15834/PI. For infection of plants, bacteria were cultured overnight at 28 °C in liquid YEB media without antibiotics. The transgenic status of hairy roots was confirmed by PCR reactions for rolA, rolB, rolC, rolD and virD1 genes as previously described by Ninković et al. (2010). β-Glucuronidase enzyme activity was determined histochemically (GUS assay) after an overnight incubation at 37 °C using X-Gluc as a substrate, at pH 7.0 (Jefferson et al. 1987).
Plant material and hairy root induction
Micropropagated shoots of G. dinarica (Vinterhalter et al. 2012), about 15 mm long, were inoculated by wounding with a needle dipped in the bacterial suspensions. Inoculated shoots were cultured for 15 days on MS (Murashige and Skoog 1962) medium without plant growth regulators and then transferred to the same medium supplemented with 250 mg/l cefotaxime (Tolycar®, Cefotaxim-Na, Jugoremedia, Zrenjanin, Serbia).
Formed roots, about 20 mm in length, were excised from the shoot explants and transferred on ½ MS medium plus cefotaxime without plant growth regulators in Petri dishes. Concentration of cefotaxime was gradually decreased over the next two subcultures (2 × 5 weeks) down to the antibiotic-free medium.
Hairy root culture
Hairy roots were propagated in 40 ml of liquid ½ MS medium without plant growth regulators supplemented with 58.4 mM sucrose in 100 ml wide neck Erlenmeyer flask with cotton wool stoppers mounted on an orbital shaker at 85 rpm in a growth chamber at 25 ± 2 °C and 2 µmol m2 s−1 irradiance under 16 h (long day) photoperiod.
To assess the effect of carbohydrate type and concentration on growth, total phenolic content and xanthone production, hairy root cultures were grown on media containing 29.2, 58.4, 116.8 or 175.2 mM sucrose, glucose or fructose. Erlenmeyer flasks containing 40 ml media and approximately 400 mg of fresh root explants were cultured on an orbital shaker under the same conditions as the propagated hairy roots. After 5 weeks, root growth was measured in terms of fresh weight (data not shown) and dry weight. Growth index [(final fresh weight − initial fresh weight)/initial fresh weight], dry weight, fresh/dry weight ratio, accumulation of total phenolics and xanthones, and DPPH radical scavenging activity were determined for the harvested roots.
Factorial design
A 23 full factorial design was used to evaluate the statistical significance of independent variables, i.e. hairy root clones (1), type of carbohydrate (2) and concentration of carbohydrate (3) on the root dry weight, total phenolic content and xanthone production (dependent variables). Each factor was tested at two significantly different levels using the following upper and lower limits chosen on the basis of growth pattern—clones (clones D and I for strain A4M70GUS; clones 2 and 3 for strain 15834/PI), two types of carbohydrate (sucrose and fructose), and two carbohydrate concentrations (58.4 and 116.8 mM). An overview of experimental conditions and studied variables is shown in Table 1. For statistical analysis of factorial design multivariate ANOVA has been performed using STATISTICA 7.0 software, where factor influence on the dry weight, total phenolic content and xanthone production, for each bacterial strain, were observed through absolute values of standardized estimated effects and presented on Pareto charts with level of significance set at P ≤ 0.05.
Preparation of extracts
Hairy roots were air-dried at room temperature and ground to fine powder using a mortar and a pestle. The obtained powder (500 mg) was extracted with 10 ml of methanol in ultrasonic bath for 20 min. After sonication, extraction was continued by maceration for 48 h in the dark at room temperature. The extracts were filtered through Whatman filter paper No. 1 into 10 ml volumetric flasks, adjusted to the volume with methanol, and were used for further chemical analysis.
HPLC analysis
Prior to HPLC analysis, hairy root extracts were filtered through a 0.45 μm membrane filters. Chromatographic analysis was carried out on Agilent series 1100 HPLC instrument, with a DAD detector, on a reverse phase Zorbax SB-C18 (Agilent) analytical column (150 mm × 4.6 mm i.d., 5 µm particle size). The mobile phase consisted of solvent A (1 %, v/v solution of orthophosphoric acid in water) and solvent B (acetonitrile) using the gradient elution as follows: 98–90 % A 0–5 min, 90 % A 5–10 min, 90–85 % A 10–13 min, 85 % A 13–15 min, 85–70 % A 15–20 min, 70–40 % A 20–24 min, 40–0 % A 24–28 min. The injection volume was 5 µl. Detection wavelengths were set at 260 and 320 nm, and the flow rate was 1 ml min−1. Standards of gentioside, norswertianin, norswertianin-1-O-glucoside and norswertianin-1-O-primeveroside were previously isolated in our laboratory (Krstić et al. 2004). Quantification was performed using calibration curve with external standards. All experiments were repeated at least two times. The results are presented as milligrams per gram of dry weight.
Total phenolic content
The total phenolic content of hairy root extracts was estimated by Folin–Ciocalteu method as described by Singleton and Rossi (1965), with slight modifications. Briefly, a 100 µl aliquot of methanol extract (concentration 12.5 mg/ml) was mixed with 500 µl of 1:10 diluted Folin–Ciocalteu reagent (Sigma-Aldrich). After 5 min, 400 µl of sodium carbonate (7.5 g/ml) was added, and mixture was incubated at room temperature in the dark for 2 h. The absorbance of samples was measured at 765 nm using an Agilent 8453 UV–Visible spectrophotometer. Gallic acid (0–100 mg/l) was used for calibration curve. Results were expressed as milligrams of gallic acid equivalent per gram of dry weight (mg GAE/g DW). Triplicate measurements were taken and mean values were calculated.
DPPH radical scavenging activity
The free radical scavenging activity of hairy root extracts against stable DPPH radical was determined according to the procedure described previously (Brand-Williams et al. 1995), with some modifications. The antiradical activity was evaluated using various concentration of each extract ranging from 0.06 to 1 mg/ml. The reaction mixture contained 500 µl of extract and 500 µl of daily prepared 150 μM DPPH solution in methanol. After thorough mixing, the solutions were incubated for 20 min at room temperature in the dark. Thereafter, absorbance of the samples was measured spectrophotometrically at 517 nm. All tests were performed in triplicate, using Trolox [(±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid, Sigma Aldrich, USA] as a positive control.
The percentage of inhibition was calculated using the equation:
where \(A_{0}\) is the absorbance of the control solution (containing only DPPH) and \(A_{1}\) is the absorbance of the samples. The IC50 values were calculated by linear regression of plots where the abscissa represented the concentration of tested samples and ordinate the average percentage of inhibition from three separate tests.
Statistical analysis
Each treatment lasting 5 weeks was repeated 3–5 times with three samples per treatment. Significant differences between means of each treatment within the same clone were determined using Fisher’s least significant difference (LSD) test at P ≤ 0.05 after performing ANOVA multifactorial analysis. All calculations of the data from DPPH assay and statistical analysis were performed by Prism 4.0 for Windows (GraphPad Soft. Inc., USA).
Results
Establishment of hairy root cultures
Hairy roots appeared on wounded shoots after 25 days of inoculation with each A. rhizogenes strain. Transformation frequency for the A4M70GUS strain was 6.12 %, i.e. 149 of 924 explants produced hairy roots at the wound site (Fig. 1a). Transformation frequency for strain 15834/PI was 32.15 % (118 out of 367 explants). Most of the regenerated roots did not survive. Thus in the second subculture, only 27 clones of A4M70GUS and 25 clones of 15834/PI survived. Twenty four out of 27 A4M70GUS clones (88.9 %) were GUS positive, with a variable degree of GUS staining. GUS activity was highest in clones E, D and B, while clones H and G had barely visible blue stains in the epidermis (Fig. 1b). The number of transformed clones continued to decline with each consecutive subculture, and only five A4M70GUS and nine 15834/PI clones survived after 10 subcultures. PCR analysis confirmed the presence of rolA, rolB and rolC genes in all of the A4M70GUS and 15834/PI derived clones (Fig. 2).
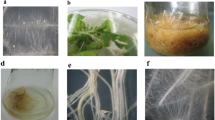
a Appearance of hairy roots 5 weeks after the inoculation of shoots with A. rhizogenes A4M70GUS bacterial suspension. b Differences in GUS gene expression following root treatments with X-Gluc solution. c Fast growing hairy root clones D (A4M70GUS) and 3 (15834/PI) after 5 weeks on hormone-free liquid medium. d, e Hairy root cultures of clone D on ½ MS liquid medium with 29.2, 58.4, 116.8 and 175.2 mM sucrose (d) or fructose (e), from left to right, after 5 weeks
PCR analysis of rolA, rolB and rolC genes in G. dinarica Beck. hairy root cultures transformed by a A. rhizogenes A4M70GUS. Lane 1 control, nontransformed roots; lanes 2–6 hairy root clones B, D, E, I and J, respectively; lane Ar A4M70GUS DNA; lane L 1 kb DNA ladder; b A. rhizogenes 15834/PI. Lane 1 control, nontransformed roots; lanes 2–10 hairy root clones 2, 3, 5, 11, 14, 16, 17, 18 and 19, respectively; lane Ar 15834/PI DNA; lane L 1 kb DNA ladder. PCR analysis of all transformants for Agrobacterium contamination using Agrobacterium virD1-gene specific primers (bottom panels; 441 bp)
Root clones were characterized on the basis of growth pattern and type of branching, and two A4M70GUS (D and I) and two 15834/PI (2 and 3) clones were selected for further experiments. They all had typical hairy root phenotype with pronounced plagiotropic growth response, but exhibited marked differences in general morphology and growth rate. Clone D roots were thick, elongated and produced less lateral roots than clone I roots. Clone 2 roots were similar to roots of clone I (less elongated and well branched), while clone 3 roots were thin, elongated and branched.
The effects of sucrose and its constitutive hexoses on the growth and phenotype characteristics of the transgenic clones are shown in Fig. 3. On the medium supplemented with sucrose, maximum growth index for all transgenic clones, except for clone D, was observed at concentration of 58.5 mM (Fig. 3a). Clone D had the highest growth index at higher concentration of sucrose (116.8 mM). At 175.2 mM sucrose, necrosis was observed in all clones. Similar pattern was noticed for fructose-supplemented medium where the highest growth index for clones I, 3 and 2 was observed at 116.8 mM fructose, while clone D required higher fructose concentration (175.2 mM). On glucose- supplemented medium all clones had the maximum growth index at 175.2 mM. Analysis of variance revealed that carbohydrate concentration was significant for the growth index (F = 46.777, P < 0.000001), while carbohydrate type had no significant effect (F = 0.004, P < 0.995821).
Effect of sucrose (suc), glucose (glc) and fructose (fru) on the growth parameters of G. dinarica hairy root clones: A growth index, B dry weight, C fresh/dry weight ratio. To calculate the final root dry weight in mg per liter of growth medium, values along ordinate should be multiplied with factor 25. Data represent mean values (±SE) of five measurements. Values denoted by the same letter are not significantly different according to the Fisher’s (LSD) test at P ≤ 0.05 following ANOVA multifactorial analysis
The dry weight of hairy roots was affected by carbohydrate concentration in all clones (Fig. 3b). The maximum dry weight on sucrose-supplemented medium was at the concentration of 116.8 mM, while on glucose-supplemented medium it was at 175.2 mM concentration. On the fructose-supplemented medium, clones D and 3 had the highest dry weight at 175.2 mM, while the dry weight of the slow growing clones I and 2 was slightly higher at 116.8 mM than at 175.2 mM.
Carbohydrate concentration had a profound effect on root morphology, with roots becoming dark green, thick and brittle at higher concentrations (Fig. 1d, e). The root dry weight increased with carbohydrate concentration but the fresh/dry weight ratio decreased at higher carbohydrate concentrations (116.8 and 175.2 mM), as the roots became more compact (Fig. 3C). The decrease in total water content of the roots, indicated by decrease in fresh/dry weight ratio at higher carbohydrate concentrations in the medium, was greater on sucrose- and fructose- than on glucose-supplemented medium.
Total phenolic content
After 5 weeks of cultivation, hairy root clones D, I, 2 and 3 grown in media supplemented with 29.2, 58.4, 116.8 and 175.2 mM of sucrose fructose or glucose, were analyzed for their total phenolic content. Generally, the increase in the carbohydrate concentration up to 116.8 mM led to an increase in the phenolic content (Fig. 4). Higher sugar concentration (175.2 mM) decreased the production of phenolics (except in clone I cultured on fructose and glucose, and clone 3 cultured on glucose). Phenolic levels of the roots transformed with 15834/PI ranged from 107.0 to 411.7 mg GAE/g DW. In comparison, A4M70GUS hairy roots clones had lower phenolic levels (60.5–355.5 mg GAE/g DW).
Effects of carbohydrates (sucrose—suc, glucose—glc and fructose—fru) on the total phenolic content of G. dinarica hairy root clones. Treatments within clones denoted by the same letter are not significantly different according to the Fisher’s (LSD) test at P ≤ 0.05 following ANOVA multifactorial analysis
Effect of carbohydrates on xanthone production in hairy roots
The HPLC analysis of methanol extracts of G. dinarica hairy roots revealed the presence of characteristic xanthones, namely norswertianin-1-O-primeveroside, norswertianin-1-O-glucoside, gentioside and norswertianin, in all analyzed clones (Fig. 5). Their contents differed among hairy root clones depending on the type and the concentration of carbohydrate in the growth medium. Norswertianin-1-O-primeveroside was the most abundant xanthone in all hairy root clones. Xanthone levels increased with increasing concentration of sucrose up to 116.8 mM. When glucose or fructose was supplied to the medium, the highest xanthone accumulation was determined at 175.2 mM (Fig. 5A). Among the analyzed hairy root clones, the highest amount of norswertianin-1-O-primeveroside (32.4 mg/g) was observed in clone 3 cultured on medium with 116.8 mM sucrose. Norswertianin-1-O-glucoside content ranged from 1.6 (clone I at 29.2 mM sucrose) to 6.0 mg/g (clone 3 at 116.8 mM sucrose). Clones D and 3 produced higher content of this xanthone glucoside than clones I and 2 (Fig. 5B). The content of the second xanthone primeveroside namely gentioside was significantly higher (0.07–2.32 mg/g) in clones grown on sucrose compared to the fructose and glucose (Fig. 5C); and the highest accumulation was observed in clone 3 at 116.8 mM sucrose. On the other hand, accumulation of xanthone aglycone norswertianin decreased with increasing concentration of tested carbohydrates. Lower concentrations of sugars stimulated norswertianin production, especially in thin elongated roots such as clone 3. Clones I and 2 grew more slowly and accumulated norswertianin in traces (Fig. 5D). Since norswertianin-1-O-primeveroside was the most abundant xanthone in roots of the transformed G. dinarica, this compound was chosen as a marker for metabolite production in factorial design experiment.
Xanthone content in G. dinarica hairy root clones grown on medium supplemented with different concentrations of sucrose (suc), glucose (glc) and fructose (fru). A norswertianin-1-O-primeveroside; B norswertianin-1-O-glucoside; C gentioside; D norswertianin. Data represent mean values (±SE) of three measurements. Values denoted by the same letter are not significantly different according to the Fisher’s (LSD) test at P ≤ 0.05 following ANOVA multifactorial analysis
Free radical scavenging activity
DPPH assay was performed as a routine assay for estimating free radical scavenging activity of methanol extracts of hairy root clones. Figure 6 presents free radical scavenging activity of methanol extracts of hairy roots expressed as IC50 values. The highest activity (IC50 = 0.195 mg/g DW) was observed in extract of clone D cultured on 175.2 mM fructose. At the highest sucrose and glucose concentration, clone D also exhibited high scavenging activity with an IC50 value of 0.268 and 0.271 mg/g DW, respectively. The lowest activity was found in extract of clone I.
Effect of sucrose (suc), glucose (glc) and fructose (fru) on the DPPH radical-scavenging activity of G. dinarica hairy root clones. Data represent mean values (±SE) of three measurements. Values denoted by the same letter are not significantly different according to the Fisher’s (LSD) test at P ≤ 0.05 following ANOVA multifactorial analysis
Full factorial design
Since 58.5 and 116.8 mM sucrose exhibited the highest promoting effect on root growth, these concentrations were selected for the factorial design experiment. Root growth on glucose and fructose supplemented medium was similar, however, as xanthone production in fast growing clones D and 3 (Fig. 1c) was higher on fructose than glucose, the former was selected as the second carbohydrate source to compare with sucrose.
Statistical 23 full factorial design was performed to evaluate the influence of carbohydrate type, carbohydrate concentration and type of clone on the dry weight, total phenolic content and norswertianin-1-O-primeveroside production in hairy root cultures of G. dinarica, and to identify the most significant factor in this system. This multivariate optimization method also allows the evaluation of the interactions between the factors. Figure 7a–f presents the Pareto charts of the factors that have a significant effect on the response obtained at P ≤ 0.05 significance level. The lengths of the bars are proportional to the absolute value of the estimated effects and the values which are located on the right of the dashed line are significant. Factor 2 (carbohydrate type) had the most significant effect on the dry weight of roots derived from A4M70GUS, while factor 3 (carbohydrate concentration) was most significant for roots derived from 15834/PI (Fig. 7a, b). A higher intensity interaction was the interaction between 1 (type of clone) and 3 in roots derived from A4M70GUS transformation, while the other interactions were not significant since they presented probability higher than 0.05. For the roots derived from the 15834/PI transformation, all interactions between factors presented significant effect. Factor 3 caused significant effect on the total phenolic content in roots of A4M70GUS strain, whereas factor 1 showed significant effect in roots of strain 15834/PI (Fig. 7c, d). Interaction of 1 × 3 was the most statistically significant interaction in A4M70GUS strain since its absolute value was higher than other interactions, whereas in strain 15834/PI it was interaction between 2 and 3. Factor 3 was the most significant independent variable for the production of norswertianin-1-O-primeveroside in roots of both strains, and all interactions between factors presented significant effect (Fig. 7e, f).
Discussion
Susceptibility of various Gentiana species to A. rhizogenes strains has been previously reported by a number of authors (Suginuma and Akihama 1995; Momčilović et al. 1997; Vinterhalter et al. 1999, 2000; Tiwari et al. 2007; Zhang et al. 2010; Hayta et al. 2011). In this study, we report for the first time successful Agrobacterium transformation and establishment of hairy root clones of G. dinarica, endemic and endangered medicinal plants from Balkan Peninsula.
It was already shown that transformation frequency in Gentiana species besides explants type and method of inoculation depends on Agrobacterium strain used for plant infection (Momčilović et al. 1997; Tiwari et al. 2007; Hayta et al. 2011). In our study, we also report significant differences in transformation frequencies between two A. rhizogenes strains—A4GUS and 15834/Pi. Lower transformation frequency of G. dinarica obtained with A. rhizogenes A4GUS (6 %) in comparison with A. rhizogenes 15834/Pi (32 %) was expected since A4 wild-type strain has already shown less efficiency of hairy root formation in various plant species comparing to 15834 strain (Porter 1991). Survival rate of the G. dinarica clones (7.5 % for 15834/Pi vs. 3.3 % for A4GUS) confirms high efficiency of A. rhizogenes 15834/Pi strain as a transformation vector. The observed differences in general morphology and the growth rate of G. dinarica individual hairy root clones could be explained by positional effects, variable copy numbers and differential expression of T-DNA inserts into genome similarly as in many other reports on A. rhizogenes-transformed species (Cho et al. 1998; Batra et al. 2004; Hayta et al. 2011).
In most studies, the biomass production of transgenic root cultures was higher than in excised root cultures of adventitious roots (Verma et al. 2007; Chaudhuri et al. 2005). In relation to the adventitious root cultures of G. dinarica (Krstić-Milošević et al. 2013), A4GUS clone D and 15834/Pi clone 3 had higher while the other clones had lower growth indexes. The two fast growing clones (D and 3) could be recommended for the further bioreactor studies since they had constantly high growth rate and secondary metabolite production over 5 years of continuous cultivation. On the contrary, the growth rate of adventitious root cultures was satisfactory only for a couple of subcultures after which it declined and cultures had to be replaced every year (Krstić-Milošević et al. 2013). The root dry weight of clones D and 3 was higher than that of clones 2 and I, the latter being similar to the dry weight of the untransformed roots (Krstić-Milošević et al. 2013). A lower fresh/dry weight ratio of most of the analyzed hairy root clones indicates their more compact structures which actually becomes an advantage in the case of production in bioreactors. Under conditions of increased concentration of carbohydrates in the medium, growth index of hairy root cultures followed a similar pattern as in cultures of adventitious roots (Krstić-Milošević et al. 2013). Namely, only at lower concentrations (29.2 and 58.4 mM) sucrose was superior in comparison to its constitutive hexoses at the same concentrations. In previous studies of hairy root cultures in different plant species, sucrose was reported to be superior in comparison to fructose and glucose, applied individually or in combination (Jeong et al. 2002; Praveen and Murthy 2012). In hairy root cultures of G. punctata, the highest biomass production was obtained on 58.4 mM (2 %) sucrose (Vinterhalter et al. 1999, 2000), while in Swertia chirata (Keil et al. 2000) the highest biomass production was registered at very high sucrose concentration (6–9 %). In general, to maintain the high biomass production, sucrose can be replaced with its constitutive hexoses but their content needs to be 2–3 times higher than that of sucrose. It should be noted that at equimolar concentration sucrose has twice the number of C-atoms present in fructose or glucose.
Regarding secondary metabolite composition, secoiridoids were not detected in either adventitious or hairy roots of G. dinarica, but they accumulated the same xanthone compounds as plants from the nature. Hairy roots of clones D, 2 and 3 contained higher amount of norswertianin-1-O-primeveroside than adventitious root cultures, regardless of the type and concentration of the carbohydrate source. Moreover, all analyzed hairy roots had higher norswertianin-1-O-primeveroside levels than roots of the plants collected in nature (Vinterhalter et al. 2013). In G. dinarica hairy root cultures, high concentration of sugars stimulated production of polyphenolics. The total phenolic content is considerably lower in roots of the wild-grown plants (99.2 mg GAE/g) as well as in untransformed roots (93.0–132.4 mg GAE/g) than in hairy roots (Krstić-Milošević et al. 2013).
Secondary metabolite production in Gentiana species transformed with A. rhizogenes has been well documented. It is noteworthy that the main secoiridoid compound gentiopicroside was present in much lower concentration in hairy roots of G. lutea, G. cruciata, G. punctata and G. macrophylla, than in roots of the wild-grown plants (Menković et al. 2000a; Hayta et al. 2011; Menković et al. 2000b; Zhang et al. 2010). Gentiopicroside and other secoiridoids were absent from both hairy root and adventitious root cultures of G. dinarica, they produced only xanthones. Contrary to secoiridoids, xanthone production in some hairy root clones of G. lutea and other members of Gentianaceae family such as Centaurium erythraea and C. pulchellum was higher than in roots of the plants grown in the nature (Menković et al. 2000b; Janković et al. 2002). The results obtained in our study are in accordance with these reports. Although decreased production or the absence of secoiridoids in hairy roots was observed, they still retain the capacity for producing high amounts of xanthones.
Factorial design is a good and simple statistical tool that can be used to simultaneously study the effects that several factors may have on a particular process. The factorial design determines which factor has a significant effect on a response and allows measuring the interactions between different factors. For A4M70GUS and 15834/PI bacterial strains, the carbohydrate source and concentration, respectively, are the most important factors for the biomass production. Analysis of optimal conditions for xanthone production revealed carbohydrate concentration as the most significant factor. The interaction of clone × carbohydrate concentration was also statistically significant with high absolute values.
Conclusion
This is the first report on the establishment of hairy root cultures of G. dinarica Beck. A. rhizogenes-mediated transformation of G. dinarica was feasible, enabling the production and maintenance of a number of hairy root clones induced by bacterial strains 15834/PI and A4M70GUS. Hairy root cultures displayed high growth rates, and biomass production over 5 years was accompanied by equally high and constant production of secondary metabolites. They exhibited high-level production of xanthone that exceeded those found in roots grown in nature and adventitious roots. Full factorial design was used to determine the effects of clone and carbohydrate source and concentration on root biomass and phenolic production. Clones D and 3 cultured on 116.8 mM sucrose can be recommended for large scale production in bioreactors.
Abbreviations
- MS:
-
Murashige and Skoog (1962)
- DW:
-
Dry weight
- FW:
-
Fresh weight
- BA:
-
6-Benzyladenine
- NAA:
-
α-Naphthaleneacetic acid
- PCR:
-
Polymerase chain reaction
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
References
Batra J, Dutta A, Singh D, Kumar S, Sen J (2004) Growth and terpenoid indole alkaloid production in Catharanthus roseus hairy root clones in relation to left- and right-termini-linked Ri T-DNA gene integration. Plant Cell Rep 23:148–154
Brand-Williams W, Cuvelier ME, Berset C (1995) Use a free radical method to evaluate antioxidative activity. LWT Food Sci Technol 28:25–30
Cao TW, Geng CA, Ma YB, He K, Wang HL, Zhou NJ, Zhang XM, Tao YD, Chen JJ (2013) Xanthones with anti-hepatitis B virus activity from Swertia mussotii. Planta Med 79:697–700
Chaudhuri KN, Ghosh B, Tepfer D, Jha S (2005) Genetic transformation of Tylophora indica with Agrobacterium rhizogenes A4: growth and tylophorine productivity in different transformed root clones. Plant Cell Rep 24:25–35
Chericoni S, Testai L, Calderone V, Flamini G, Nieri P, Morelli I, Martinotti E (2003) The xanthones gentiacaulein and gentiakochianin are responsible for the vasodilator action of the roots of Gentiana kochiana. Planta Med 69:767–770
Cho HJ, Widholm JM, Tanaka NY, Nakanishi Y, Merooka Y (1998) Agrobacterium rhizogenes-mediated transformation and regeneration of the legume Astragalus sinicus (Chinese milk vetch). Plant Sci 138:53–65
Fotie J, Bohle S (2006) Pharmacological and biological activities of xanthones. AntiInfect Agents Med Chem 5:15–31
Georgiev M, Pavlov A, Bley T (2007) Hairy root type plant in vitro systems as sources of bioactive substances. Appl Microbiol Biotechnol 74:1175–1185
Hayta S, Gurel A, Aagun IH, Altan F, Ganzera M, Tanyolac B, Bedir E (2011) Induction of Gentiana cruciata, hairy roots and their secondary metabolites. Biologia 66:618–625
Hirakawa K, Yoshida M, Nagatsu A, Mizukami H, Rana V, Rawat MSM, Oikawa S, Kawanishi S (2005) Chemopreventive action of xanthone derivatives on photosensitized DNA demage. Photochem Photobiol 81:314–319
Hosokawa K, Matsuki RM, Oikawa Y, Yamamura S (1997) Genetic transformation of gentian using wild-type Agrobacterium rhizogenes. Plant Cell Tissue Organ Cult 51:137–140
Hostettmann-Kaldas M, Hostettmann K, Sticher O (1981) Xanthones, flavones and secoiridoids of American Gentiana species. Phytochemistry 20:443–446
Hu ZB, Du M (2006) Hairy root and its application in plant genetic engineering. J Integr Plant Biol 48:121–127
Janković T, Krstić D, Šavikin-Fodulović K, Menković N, Grubišić D (2002) Xanthones and secoiridoids from hairy root cultures of Centaurium erythraea and C. pulchellum. Planta Med 68:944–946
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: b-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jensen SR, Schripsema J (2002) Chemotaxonomy and pharmacology of Gentianaceae. In: Struwe L, Albert VA (eds) Gentianaceae: systematics and natural history. Cambridge University Press, Cambridge, pp 573–631
Jeong G-T, Park D-H, Ryu H-W, Lee W-T, Park K, Kang C-H, Hwang B, Woo J-C (2002) Optimum conditions for transformed Panax ginseng hairy roots in flask culture. Appl Biochem Biotech 98–100:1129–1139
Jovanović-Dunjić R. (1973) Gentiana L. In: Josifović M (ed) Flore de la Republique Socialiste de Serbie V. Academie Serbe des Sciences et des Artes, Belgrade, pp 412–425
Keil M, Härtle B, Guillaume A, Psiorz M (2000) Production of amarogentin in root cultures of Swertia chirata. Planta Med 66:452–457
Krstić D, Janković T, Aljančić I, Šavikin-Fodulović K, Menković N, Milosavljević S (2004) Phytochemical investigation of Gentiana dinarica. Biochem Syst Ecol 32:937–941
Krstić-Milošević D, Janković T, Vinterhalter B, Menković N, Aljančić I, Vinterhalter D (2013) Influence of carbohydrate source on xanthone content in root cultures of Gentiana dinarica Beck. Plant Growth Regul 71:147–155
Larcher G, Morel C, Tronchin G, Landreau A, Seraphin D, Richomme P, Bouchara JP (2004) Investigation of the antifungal activity of caledonixanthone E and other xanthones against Aspergillus fumigatus. Planta Med 70:569–571
Luo C-T, Mao S-S, Liu F-L, M-x Yang, Chen H, Kurihara H, Li Y (2013) Antioxidant xanthones from Swertia mussoti, a high altitude plant. Fitoterapia 91:140–147
Mahendran G, Manoj M, Rajendra Prasad KJ, Narmatha Bai V (2013) Evaluation of anti-inflammatory and antinoceceptive activity of xanthones from Swertia corymbosa (Griseb.) Wight ex C.B. Clarke. Int J Pharm Pharm Sci 5:523–529
Menković N, Šavikin Fodulović K, Vinterhalter B, Vinterhalter D, Janković T, Krstić D (2000a) Secoiridoid content in hairy roots of Gentiana punctata. Pharm Pharmacol Lett 2:73–75
Menković N, Šavikin-Fodulović K, Momčilović I, Grubišić D (2000b) Quantitative determination of secoiridoid and γ-pyrone compounds in Gentiana lutea cultured in vitro. Planta Med 66:96–98
Mishiba K, Nishihara M, Abe Y, Nakatsu T, Kawamura H, Kodama K, Takesawa T, Abe J (2006) Production of dwarf potted gentian using wild-type Agrobacterium rhizogenes. Plant Biotechnol NAR 23:33–38
Momčilović I, Grubišić D, Kojić M, Nešković M (1997) Agrobacterium rhizogenes-mediated transformation and plant regeneration of four Gentiana species. Plant Cell Tissue Organ Cult 50:1–6
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ninković S, Djordjević T, Vinterhalter B, Uzelac B, Cingel A, Savić J, Radović S (2010) Embryogenic responses of Beta vulgaris L. callus induced from transgenic hairy roots. Plant Cell Tissue Organ Cult 103:81–91
Nishihara M, Nakatsuka T, Hosokawa K, Yokoi T, Abe Y, Mishiba K, Yamamura S (2006) Dominant inheritance of white-flowered and herbicide-resistant traits in transgenic gentian plants. Plant Biotechnol 23:25–31
Peres V, Nagem TJ, de Oliviera FF (2000) Tetraoxygenated naturally occurring xanthones. Phytochemistry 55:683–710
Pontus S, Michael AP, Chaim I (2006) Use of Gentiana lutea extracts as an antimicrobial agent. European Patent EP1663271
Porter J (1991) Host range and implications of plant infection by Agrobacterium rhizogenes. Crit Rev Plant Sci 10:387–421
Praveen N, Murthy HN (2012) Synthesis of withanolide A depends on carbon source and medium pH in hairy roots of Withania somnifera. Ind Crops Prod 35:241–243
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–153
Smigocki AC, Puthoff DP, Zuzga S, Ivic-Haymes SD (2009) Low efficiency processing of an insecticidal Nicotiana proteinase inhibitor precursor in Beta vulgaris hairy roots. Plant Cell Tissue Organ Cult 97:167–174
Suginuma C, Akihama T (1995) Transformation of Gentiana with Agrobacterium rhizogenes. Acta Hortic 392:153–160
Tepfer M, Casse-Delbart F (1987) Agrobacterium rhizogenes as a vector for transforming higher plants. Microbiol Sci 4:24–28
Tiwari RK, Trivedi M, Guang ZC, Guo GQ, Zheng G-C (2007) Genetic transformation of Gentiana macrophylla with Agrobacterium rhizogenes: growth and production of secoiridoid glucoside gentiopicroside in transformed hairy root cultures. Plant Cell Rep 26:199–210
Van Larebake N, Genetello CH, Hernalsteens JP, De Picker A, Zaenen I, Messens E, Van Montagu M, Schell J (1977) Transfer of Ti plasmids between Agrobacterium strains by mobilization with the conjugative plasmid RP4. Mol Gen Genet 152:1119–1124
Verma PC, Rahman LU, Negi AS, Jain DC, Khanuja SPS, Banerjee S (2007) Agrobacterium rhizogenes-mediated transformation of Picrorhiza kurroa Royle ex Benth.: establishment and selection of superior hairy. Plant Biotechnol Rep 1:169–174
Vinterhalter B, Orbović V, Vinterhalter D (1999) Transgenic root cultures of Gentiana punctata L. Acta Soc Bot Pol 68:275–280
Vinterhalter B, Momčilović I, Vinterhalter D (2000) High biomass producing root cultures of Gentiana punctata L. transformed with Agrobacterium tumefaciens C58C1 (pArA4b). Arch Biol Sci 52:85–90
Vinterhalter B, Krstić-Milošević D, Janković T, Milojević J, Vinterhalter D (2012) In vitro propagation of Gentiana dinarica Beck. Cent Eur J Biol 7:690–697
Vinterhalter B, Krstić-Milošević D, Janković T, Zdravković-Korać S, Vinterhalter D (2013) Quantitative determination of secoiridoid and xanthone glycosides of Gentiana dinarica Beck cultured in vitro. Acta Physiol Plant 35:567–574
Weathers PJ, Hemmavanh DD, Walcerz DB, Cheetham RD, Smith TC (1997) Interactive effects of nitrate and phosphate salts, sucrose, and inoculum culture age on growth and sesquiterpene production in Artemisia annua hairy root cultures. In Vitro Cell Dev Biol Plant 33:306–312
Wu CFJ, Hamada M (2009) Experiments: planning, analysis and parameter design optimization, 2nd edn. John Wiley, New York
Yimdjo MC, Azebaze AG, Nikengfack AE, Meyer AM, Bodo B, Fomum ZT (2004) Antimicrobial and cytotoxic agents from Calophyllum inophyllum. Phytochemistry 65:2789–2795
Zhang HL, Xue SH, Pu F, Tiwari RK, Wang XY (2010) Establishment of hairy root lines and analysis of gentiopicroside in the medicinal plant Gentiana macrophylla. Russ J Plant Physiol 57:110–117
Zheng H-H, Luo C-T, Chen H, Lin J-N, Ye C-L, Mao S-S, Li Y-L (2014) Xanthones from Swertia mussoti as multitarget-directed antidiabetic agents. Chem Med Chem 9:1374–1377
Acknowledgments
Bacterial strain A4M70GUS was obtained by courtesy of Dr. Landre, Univ. Pierre and Marie Curie, Paris, France. The present work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, Grant No. 173015.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vinterhalter, B., Krstić-Milošević, D., Janković, T. et al. Gentiana dinarica Beck. hairy root cultures and evaluation of factors affecting growth and xanthone production. Plant Cell Tiss Organ Cult 121, 667–679 (2015). https://doi.org/10.1007/s11240-015-0737-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0737-z