Abstract
A highly efficient and reproducible regeneration system based on somatic embryogenesis in Gossypium hirsutum cv. Narasimha (NM), which has superior fiber qualities and is also used as a female parent in several hybrid cottons, has been developed. Embryogenic callus was obtained form both hypocotyls and cotyledonary leaves on Murashige and Skoog (MS) medium containing kinetin and 2,4-dichlorophenoxyacetic acid. Somatic embryogenesis was observed on hormone-free MS medium, but embryos did not grow well beyond globular stage. However, somatic embryos germinated well on MS medium containing B5 vitamins; addition of zeatin was found to be beneficial for their normal development. Most importantly, the media and culture conditions developed for NM were also found to be suitable for high-frequency somatic embryogenesis in Coker 310. In addition, the newly developed regeneration protocol has been successfully tested for genetic transformation through co-cultivation with Agrobacterium using embryogenic calli as explants. Molecular analysis confirmed the stable integration and expression of marker gene, green fluorescent protein (GFP). These results show that it is now possible to introduce foreign gene(s) directly into elite cultivar Narasimha with similar efficiency to in traditionally used Coker lines in a relatively short period of time. Development of efficient regeneration and transformation systems as demonstrated here should augment the introduction of new traits directly into cultivated varieties/hybrids, reducing the time required for back-crossing and the costs for seed production, besides aiding genomic research in cotton.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cotton is one of the most important crop species in the world, valued globally for textile and oilseed production, with about 32.4 million hectares planted worldwide. Biotic and abiotic stresses adversely influence development of fiber, yield, and quality in cotton. Conventional plant breeding methods have been extensively applied to improve these traits. However, these approaches have been limited by the lack of sufficient genetic variability in the existing germplasm pool (Wu et al. 2004). In this context, genetic engineering offers tremendous potential to introduce foreign genes isolated from any organism directly into crop plants to complement breeding programs. Genetic transformation has been applied in cotton to express insecticidal proteins from bacterial source for improvement of insect resistance. Although several methods have been described for the transformation of cotton, somatic embryogenesis-based transformation offers several advantages over other transformation methods, because of the single-cell origin of the somatic embryo (Merkle et al. 1995), thus reducing the development of chimeric plants, which is often the main disadvantage when using the shoot tip method of transformation (Wilkins et al. 2004). Genetic transformation through somatic embryogenesis (SE) has been reported by several groups using Coker or its derived germplasm lines (Kumar et al. 1998). These Coker lines are the basis of the current generation of commercial transgenic cottons, though they are agronomically very poor. As a consequence, the insecticidal protein coding gene(s) were first introduced into Coker genotype and later transferred into commercial genotypes through laborious and time-consuming back-crossing programs (Sakhanokho et al. 2001). Although these hybrids/cultivars are resistant to lepidopoteran pests, they are still susceptible to several other secondary insect pests, especially sucking pests, and the farmer still need to spray pesticides to control these pests, which are now emerging as major pests (Wang et al. 2008, 2009). Therefore, extending transformation methods to introduce foreign genes of agronomic importance directly into elite genotypes will be of great importance in cotton improvement programs. In addition, the ability to transform an elite cultivar or any parental line used for hybrid seed production directly will enable the required time to be reduced by avoiding back-crossing, and characterization of the genes involved in the elite nature of the genotype through gene knockout, gene complementation, and other gene discovery and functional genomics approaches.
Regeneration in Gossypium hirsutum was first obtained via spontaneous somatic embryogenesis of cotyledon tissues (Davidonis and Hamilton 1983). Since then, somatic embryogenesis and regeneration of plants in cotton have been reported by several authors (Trolinder and Goodin 1987; Trolinder and Xhixian 1989; Firoozabady and DeBoer 1993; Sakhanokho et al. 2001). Media and culture conditions were shown to play a crucial role in regeneration of plantlets through somatic embryogenesis (Sunilkumar and Rathore 2001; Kumria et al. 2003). However, like in other species, somatic embryogenesis response in cotton is highly genotype specific (Trolinder and Xhixian 1989). Although more than 70 genotypes that are capable of differentiating from callus into SEs have now been identified (Wilkins et al. 2000; Rangan and Rajasekaran 1993; Cousins et al. 1991; Rajasekaran 1996; Kumar et al. 1998; Zhang et al. 2001; Kumria et al. 2003; Mishra et al. 2003), the conversion rate of somatic embryos into mature plants is still very low in the majority of non-Coker genotypes, which is a major impediment to the genetic transformation approach. In addition, prolonged culture period, high frequency of abnormal development of embryos, and lack of shoot elongation or root induction are other common problems associated with cotton regeneration. Furthermore, the number of commercial cultivars and elite germplasm lines that have superior fiber and agronomic traits and that can undergo complete plant regeneration still remains very low.
India has the largest area (9 million hectares) under cotton cultivation in the world. Almost 90% of the area is covered by cultivars/hybrids that belong to G. hirsutum. The Indian cotton genotypes are generally regarded as recalcitrant to somatic embryogenesis. To date, there has been only a single report on somatic embryogenesis, in the cultivar MCU-5, with large variation in the frequency of SE and plantlet regeneration (0–85%) among progeny derived from the selfed seed of a single plant (Kumar and Pental 1998). So far, no transformation of MCU-5 or any other genotype has been reported using this protocol. Herein, we report on the development of a highly efficient and reproducible regeneration system based on somatic embryogenesis in Narasimha (NM), also referred to as NA1325, a popular cultivar with superior fiber qualities and which is also used as a female parent in several commercial hybrid seed production programs (Ravindranath 2009). The efficiency of embryogenic callus induction and the somatic embryo production achieved in this study are comparable to those of high-frequency somatic embryogenesis reported earlier for Coker 310 (Kumria et al. 2003). Most importantly, the media and culture conditions developed for NM were also found to be suitable for high-frequency somatic embryogenesis in Coker 310. In addition, the newly developed regeneration protocol has been successfully tested for genetic transformation through co-cultivation with Agrobacterium. Based on the results presented herein, it is possible to introduce foreign gene(s) directly into elite cultivar Narasimha (NM) with similar efficiency to in traditionally used Coker lines in a relatively short period of time. Development of efficient regeneration and transformation systems as demonstrated here should augment the introduction of new traits directly into cultivated varieties/hybrids, reducing time and costs for seed production, besides aiding genomic research in cotton.
Materials and methods
Plant materials and growth conditions
Seeds of cultivars Narasimha and Coker 310 were surface-sterilized with 0.1% HgCl2 solution and a drop of Tween-20 for 20 min with continuous shaking. Seeds were rinsed with sterile distilled water several times to remove the detergent completely and were blot-dried using sterile tissue papers. The seeds were germinated on 1/5-strength basal salts of MS medium (Murashige and Skoog 1962) supplemented with 1% sucrose and 0.8% agar, and pH was adjusted to 5.8. Cultures of all stages, unless otherwise mentioned, were incubated at 28 ± 1°C under 16/8 h light/dark cycle at light intensity of 40–60 μmol s−1m−2. All chemicals used in the study were procured from Sigma–Aldrich (St. Louis, USA).
Callus induction
Hypocotyl (3–5 mm) and cotyledonary segments (10–16 mm2) were used as explants for callus initiation. Explants were excised from 3- to 4-day-old seedlings. About 6–8 explants were placed in a 15 × 100 mm Petri plate containing approximately 30 ml medium for callus induction. MSDK media, i.e., MS salts supplemented with various concentrations of 2,4-dichlorophenoxyacetic acid (2,4-D) and kinetin, were tested for callus induction. Maltose (3%) was used as sole carbon source. Cultures were incubated at 28 ± 2°C under cool, white fluorescent light (40–60 μmol s−1m−2) with 16/8 h light/dark cycle for callus induction. After 20 days, calli were cut into pieces and transferred to MSDiP medium containing MS medium, 0.01 mg l−1 2,4-D, and 0.5 mg l−1 N6-(2-isopentenyl) adenine (2iP) for proliferation.
Somatic embryogenesis
Well-proliferated friable, light green, soft calli obtained on MSDiP medium were spread on MSO medium (MS medium without any growth regulators). Petri plates were sealed with porous Micropore tape (cat no. 1530-0; Millipore) to enhance dehydration of medium, which was found to play an important role in cotton somatic embryogenesis (Kumria et al. 2003, Leelavathi et al. 2004). Induction and development of somatic embryos was monitored continuously, and care was taken to avoid excessive dehydration that may result in complete drying of the medium. Globular and torpedo-stage embryos obtained on MSO medium were cultured further on MSB5 medium (MS salts supplemented with 750 mg l−1 MgCl2·6H2O, and B5 vitamins; Gamborg et al. 1968). MSB5 was supplemented with 0.1 mg l−1 zeatin (MSB5zt) depending on the development of embryos. In all media, 3% maltose was used as sole carbon source, 0.8% agar as solidifying agent, and pH was adjusted to 5.8.
Development of somatic embryo into plantlets
Embryos that reached cotyledonary stage on MSB5 medium were picked up regularly and placed on H1 medium (Stewart and Hsu 1977) supplemented with 0.5% or 1% sucrose, solidified with phytagel (0.4%). Within 2 weeks the embryos developed into plantlets and were then cultured in bottles or magenta boxes for another 2–4 weeks until they attained height of 4–5 inches with well-developed root system. Rooted plants were transferred to small pots containing a mixture of vermiculite, sand, and peat moss in 1:1:1 ratio. Plantlets were covered with porous polythene bags to maintain high humidity (~80%) and kept in a growth room at 25 ± 1°C for hardening. After a week of hardening, the polythene bag was removed and, after another 1 week, the plants were transferred to pots. Plants were grown in a contained greenhouse facility to maturity for seed collection.
Vector construction, transformation, and analysis of transgenic plants
Agrobacterium tumefaciens strain LBA 4404 harboring binary vector pCAMBIA2300-sGFP (Fig. 4a) was used for transformation. The pCAMBIA2300-sGFP vector was constructed by taking the green fluorescent protein (GFP) coding gene, placed under the control of cauliflower mosaic virus 35S promoter (CaMV 35S) and nopaline synthase gene (nos) polyA signal from pCAMBIA1390-sGFP (Toki et al. 2006) as a HindIII—EcoRI fragment and cloned into pCAMBIA2300 at the same sites. The pCAMBIA2300-sGFP has NPTII gene that confers resistance to kanamycin in transformed plant cells. Essentially, a previously described transformation procedure for Coker 310 was followed for NM also, using embryogenic calli as explant source (Leelavathi et al. 2004). In brief, proliferating embryogenic calli were co-cultured with Agrobacterium carrying pCAMBIA2300-sGFP plasmid for 20 min, and the bacterial suspension was removed by passing through a sterile strainer. Co-culture of infected calli was continued for 36 h in the dark on basal MS medium. Co-cultured calli were transferred to a sterile Whatman filter paper (cat. no. 1004 070) placed on MSKC selection medium containing MS medium without any growth regulators, 3% maltose, 50–100 mg l−1 kanamycin, and 500 mg l−1 cefotaxime. Antibiotics were filter-sterilized before adding to the autoclaved medium. Petri plates were sealed with porous tape and cultured for 3–5 weeks under 16/8 h light/dark cycle period. Globular and torpedo-shaped embryos growing on selection medium were subcultured onto fresh selection medium for another 2 weeks. Embryos that reached cotyledonary stage were grown further, as described previously (Kumria et al. 2003; Leelavathi et al. 2004). Transgenic nature of the kanamycin-resistant plants was confirmed by molecular analysis and expression of the reporter gfp gene. Expression of gfp was visualized under Nikon fluorescence microscope (Nikon SMZ1500) illuminated with long-wavelength ultraviolet (UV) light, and various stages of plantlet development were recorded.
Results and discussion
Production of large numbers of independently transformed transgenic events is essential for any crop improvement program through genetic manipulation. In the case of cotton it is equally important to introduce the foreign genes directly into elite varieties or parental lines to minimize the required time, reduce the costs of seed production, and maintain the genetic purity of the lines. To date, high-frequency somatic embryogenesis has been reported in Coker Trolinder and Xhixian 1989), Acala (Mishra et al. 2003), and a few other genotypes (Cousins et al. 1991; Firoozabady and Deboer 1993, Kumar and Pental 1998; Sakhanokho et al. 2001; Sun et al. 2003, 2006; Zhang et al. 1991, 2001). About 70 cotton genotypes are reported to undergo somatic embryogenesis, but the conversion rate of somatic embryos into normal plantlets is still very low in several genotypes, which is often the main obstacle to the genetic engineering approach. The efficiency of somatic embryogenesis and genetic transformation in cotton is influenced by several factors, such as genotype, explant, method of transformation, selection, and regeneration system. Among these factors, an efficient and reproducible regeneration procedure that can be used to obtain a transgenic line in elite cotton genotypes is very important. In this study we have developed a highly efficient and reproducible procedure to induce somatic embryogenesis and plant regeneration from elite variety Narasimha, which is also used as a female parent in several conventional and transgenic hybrid cottons (Ravindranath 2009). The developed procedure was found to be equally suitable to regenerate plants from traditional Coker lines. In addition, the newly developed regeneration procedure was successfully tested for genetic transformation.
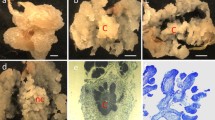
Hypocotyls were found to be suitable for callusing in both genotypes, with NM responding well in the presence of higher levels of kinetin (1.0 mg l−1) along with 0.1 mg l−1 2,4-D (Fig. 1, NM-A) while in Coker 310, callus developed well in the presence of 0.1 mg l−1 2,4-D and 0.5–1.0 mg l−1 kinetin (Fig. 1, Coker-A), similar to in other genotypes of Coker background (Trolinder and Goodin 1988a; Wilkins et al. 2004). MS medium supplementation with 0.1 mg l−1 2,4-D and 1.0 mg l−1 kin (MSDK) was found to be the best combination for induction of embryogenic callus in NM (Table 1). Maltose as carbon source proved to be useful in this study (data not shown) to control secretion of excessive phenolics, similar to our earlier observations using Coker (Kumria et al. 2003). For better proliferation, the callus was subcultured on MSDiP medium (Fig. 1, NM-B, Coker-B). In comparison with MSDK medium, MSDiP medium has low concentrations of 2,4-D (0.01 mg l−1) and kinetin was replaced with 0.5 mg l−1 2iP. 2iP has been proven to be effective for induction of SE in cotton (Trolinder and Goodin 1987; Nobre et al. 2001). After 1 month, callus was spread on MSO medium (MS medium without any growth regulator), resulting in induction of globular embryos (Fig. 1, NM-C, Coker-C). In case of Coker, the globular embryos grow further to torpedo (Fig. 1, Coker-E) and cotyledonary stage (Fig. 1, Coker-F) on the same MSO medium. However, the NM genotype did not respond further on the MSO medium. This is one main difference between NM and Coker genotypes; to overcome this problem we shifted the NM cultures onto MSB5 medium, which promoted growth of globular embryos into torpedo-shaped embryos (Fig. 1, NM-E) and subsequently to cotyledonary stage (Fig. 1, NM-F). At this stage the amount of media poured into each Petri plate was reduced to 20 ml and the callus was spread as a thin layer. Metabolic stress caused due to dehydration was found to play an important role in induction of somatic embryogenesis in cotton (Kumria et al. 2003; Wilkins et al. 2004). Therefore, Petri plates were sealed with porous tape to enhance dehydration, which resulted in better induction of somatic embryogenesis in the present study also. MSB5 proved to be the best medium for embryo induction as well as for their development. Gamborg’s B5 medium is rich in thiamine, and embryos developed quickly in this medium. Use of B5 vitamins in the development and maturation of embryos has been reported earlier (Zhang et al. 2001; Wilkins et al. 2004). Within 2–3 weeks, globular embryos were formed in clusters (Fig. 1g, h). Often, co-development of anthocyanin pigment was observed at this stage (Fig. 1, NM-D, Coker-D). Synthesis of anthocyanin pigments in culture is generally considered an indication of differentiation and regeneration potential of the callus (Mishra et al. 2003). Embryogenesis was asynchronous, and many different stages of embryos were found in the same Petri plate (Fig. 2, NM-A, NM-B). On average, 20 somatic embryos reached cotyledonary stage from the calli derived from each explant when grown on a Petri dish, of which 5–6 reached mature plants that flowered and set seeds. In Coker 310 also, a similar number of cotyledonary embryos was obtained from each Petri dish when following the new procedure; however, the average number of plants that reached maturity was slightly higher (8–10 plants).
Somatic embryogenesis in NM and Coker 310: various developmental stages of somatic embryos from hypocotyl explants of NM (NM-A to NM-F) and Coker 310 (Coker-A to Coker-F). NM-A and Coker-A: callus induced from hypocotyl explants. NM-B and Coker-B: embryogenic callus proliferating on MSDiP medium. NM-C and Coker-C: induction of somatic embryogenesis on MSB5 medium; induction was efficient when calli were placed on filter paper. NM-D and Coker-D: early stage of somatic embryogenesis with globular-stage embryos; note the synthesis of anthocyanin pigment. NM-E and Coker-E: torpedo-stage embryos. NM-F and Coker-F: cotyledonary-stage embryos
Globular embryos grown on MSB5 medium resulted in production of normal plantlets from both NM (Fig. 2, NM-C) and Coker 310. However, at this stage, addition of 0.1 mg l−1 zeatin had a positive effect on normal development of somatic embryos. Zeatin is a potent cytokinin and induces faster cell division. It also balances endogenous cytokinin level (Zhang 2000). Higher-frequency embryo development in medium supplemented with zeatin has been reported earlier (Zhang 2000). An important aspect of the new procedure is that all plantlets developed a good root system without additional manipulation (Fig. 2, NM-C). All rooted plants that were transferred to pots survived, flowered, and set seeds. Following the systematic stepwise procedure outlined in Fig. 3, a high frequency of somatic embryos was obtained from NM as well as from Coker 310, which grew into normal plantlets. The total cycle time, starting from callus induction to maturation of embryos into plantlets, was about 120–140 days. The callus proliferation step on MSDiP medium and induction of somatic embryos on MSB5 were found to be essential for regeneration of plantlets via somatic embryogenesis in Narasimha. The frequency of somatic embryogenesis obtained in this study is comparable to the high frequency reported earlier for Coker 310 (Kumria et al. 2003).
Transformation of NM with Agrobacterium
Agrobacterium-mediated transformation using embryogenic calli as explant was found to be suitable to produce large numbers of independently transformed transgenic lines in cotton (Leelavathi et al. 2004). Therefore, well-proliferated embryogenic callus that constantly produces somatic embryos was chosen for transformation of NM. The callus after co-cultivation with Agrobacterium harboring the pCAMBIA1390-sGFP construct (Fig. 4a; Toki et al. 2006) was placed on sterile filter paper and grown on MSKC selection medium to promote somatic embryogenesis. Simultaneous application of selection pressure combined with dehydration was found to be most suitable for production of large numbers of transformed embryos within 3–4 weeks. Care was taken to pick up independently transformed lines at this stage and to place then on fresh selection medium. The transformed calli/embryos were checked periodically for expression of GFP under fluorescence microscope (Fig. 4b–e). The putative transgenic embryos were grown to plantlets (Fig. 4f–g), using a protocol similar to that followed for regeneration of normal plantlets but containing kanamycin (100 mg l−1) in the medium. The young plantlets were placed on H1 medium (Stewart and Hsu (1977), where they grew again very quickly with well-developed roots. On average, 49.0 ± 12.0 kanamycin-resistant somatic embryos developed on selection medium in a Petri dish, from which 6–8 transgenic mature plants were obtained. Similar results were obtained for Coker 310. The genomic DNA isolated from GFP-expressing plants was found to be positive for presence of gfp and NPTII genes, confirming their true transgenic nature. The transformation procedure was repeated using another vector, where gfp was replaced by a gene coding for a vegetative insecticidal protein (VIP). Stable integration of vip gene was determined using Southern hybridization (data not shown).
Genetic transformation of cotton cultivar Narasimha by Agrobacterium-mediated transformation. a Partial map of the binary vector used for transformation. nptII and sgfp are under separate CaMV 35S promoters. b Embryogenic callus on kanamycin selection after co-cultivation with Agrobacterium. Note the development of freshly growing calli/somatic embryos on selection medium. c Close-up of the putative transgenic somatic embryo developing on kanamycin selection. The embryogenic callus, transformed (right) and untransformed (left), observed under normal light (d) and under UV (e). Note the presence of GFP fluorescence in transgenic calli only. Differentiating somatic embryos taken from kanamycin selection plate (right) and from untransformed plate of the same stage (left) observed under normal light (f) and under UV light (g). Note again the expression of GFP in transformed embryos only. (h) Close-up of cotyledonary-stage somatic embryo expressing GFP; expression of GFP in the well-developed plantlet, just before transfer to soil mix
In conclusion, we have developed a reproducible and highly efficient regeneration system based on somatic embryogenesis in elite cotton cultivar Narasimha, which is also used as a female parent in several hybrid seed production programs. Embryogenic callus was obtained form both hypocotyls and cotyledonary leaves on MS medium containing high concentration of kinetin along with low levels of 2,4-D. Somatic embryogenesis was achieved by subculturing the callus on hormone-free MS medium. Somatic embryos germinated well on MS medium containing B5 vitamins compared with on MSO medium. Addition of zeatin was found to be beneficial for normal development of somatic embryos. The procedure developed for NM was also found to be suitable for Coker 310 genotype. In addition, Agrobacterium-mediated transformation using embryogenic calli as explant was established for NM. Molecular analysis confirmed stable integration and expression of gfp in plantlets growing in the presence of kanamycin selection. The regeneration and transformation method described herein is very simple, efficient, and fast for the introduction of any foreign gene directly into this elite cotton cultivar.
Abbreviations
- NM/NA1325:
-
Narasimha
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- 2iP:
-
N6-(2-isopentenyl) adenine
- SE:
-
Somatic embryogenesis
- GFP:
-
Green fluorescent protein
- VIP:
-
Vegetative insecticidal protein
References
Cousins YL, Lyon BR, Lewellyn DJ (1991) Transformation of an Australian cultivar: prospects for cotton improvement through genetic engineering. Aus J Plant Physiol 18:481–494
Davidonis GH, Hamilton RH (1983) Plant regeneration from callus tissue of Gossypium hirsutum L. Plant Sci Lett 32:89–93
Firoozabady E, Deboer DL (1993) Plant regeneration via somatic embryogenesis in many cultivars of cotton (Gossypium hirsutum L.). In Vitro Cell Dev Biol 299:166–173
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension culture of soybean root cells. Exp Cell Res 50:151–158
Kumar S, Pental D (1998) Regeneration of Indian cotton variety MCU-5 through somatic embryogenesis. Curr Sci 74:538–548
Kumar S, Sharma P, Pental D (1998) A genetic approach to in vitro regeneration of non- regenerating cotton (Gossypium hirsutum L.) cultivars. Plant Cell Rep 18:59–63
Kumria R, Sunnichan VG, Das DK, Gupta SK, Reddy VS, Bhatnagar RK, Leelavathi S (2003) High frequency somatic embryo production and maturation into normal plants in cotton (Gossypium hirsutum) though metabolic stress. Plant Cell Rep 21:635–639
Leelavathi S, Sunnichan VG, Kumria R, Vijaykanth GP, Bhatnagar RK, Reddy VS (2004) A simple and rapid Agrobacterium- mediated transformation protocol (Gossypium hirsutum L.): embryogenic calli as a source to generate large numbers of transgenic plants. Plant Cell Rep 22:465–470
Merkle SA, Parrot WA, Flinn BS (1995) Morphogenetic aspects of somatic embryogenesis. In: Thorpe TA (ed) In vitro embryogenesis in plant. Kluwer Academic, Dordrecht, pp 155–205
Mishra R, Wang HY, Yadav NR, Wilkins TA (2003) Development of highly regenerable elite Acala cotton (Gossypium hirsutum cv. Maxxa)- a step towards genotype independent regeneration. Plant Cell Tissue Organ Cult 73:21–35
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nobre J, Keith DJ, Dunwell JM (2001) Morphogenesis and regeneration from stomatal guard cell complexes of cotton (Gossypium hirsutum L.). Plant Cell Rep 20:8–15
Rajasekaran K (1996) Regeneration of plants from cryopreserved embryogenic cell suspension and callus culture of cotton (Gossypium hirsutum L.). Plant Cell Rep 15:859–864
Rangan TS, Rajasekaran K (1993) Regeneration of cotton plants in suspension cultures. US Patent-5244, 802
Ravindranath K (2009) Narasimha-A versatile Gossypium hirsutum cultivar and proven parent of many superior hybrid cottons in India. J Indian Soc Cotton Improve (Mumbai): 34–37
Sakhanokho HF, Zipf A, Rajasekaran K, Saba S, Sharma GC (2001) Induction of highly embryogenic calli and plant regeneration in upland (G. hirsutum L.) and Pima (G. barbadense L.) cottons. Crop Sci 41:1235–1240
Stewart JM, Hsu CL (1977) In ovulo embryo culture and seedling development of cotton (Gossypium hirsutum L.). Planta 137:113–117
Sun Y, Zhang X, Jin S, Liang S, Nie Y (2003) Somatic embryogeneis and plant regeneration in wild cotton (Gossypium Klotzschianum). Plant Cell Tiss Organ Cult 75:247–253
Sun Y, Zhang X, Huang C, Guo X, Nie Y (2006) Somatic embryogenesis and regeneration from different wild diploid cotton (Gossypium) species. Plant Cell Rep 25(4):289–296
Sunilkumar G, Rathore KS (2001) Transgenic cotton: factors influencing Agrobacterium-mediated transformation and regeneration. Mol Breed 8:37–52
Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H (2006) Early infection of scutellum tissue with Agrobacterium allows high speed transformation of rice. Plant J 47:969–976
Trolinder NL, Goodin JR (1987) Somatic embryogenesis and plant regeneration in G. hirsutum L. Plant Cell Rep 6:231–234
Trolinder NL, Goodin JR (1988a) Somatic embryogenesis in cotton (Gossypium). I. Effects of source of explant and hormone regime. Plant Cell Tiss Organ Cult 12:31–42
Trolinder NL, Goodin JR (1988b) Somatic embryogenesis in cotton (Gossypium). II. Requirements for embryo development and plant regeneration. Plant Cell Tiss Organ Cult 12:43–53
Trolinder NL, Xhixian C (1989) Genotype specificity of the somatic embryogenesis response in cotton. Plant Cell Rep 8:133–136
Wang S, Just DR, Anderson PP (2008) Bt cotton and secondary pests. Int J Biotechnol 10(2–3):113–121
Wang Z, Lin H, Huang JK, Hu RF, Rozelle S, Pry C (2009) Bt cotton in China: are seconday infestations offsetting the benefits in farmer fields? Agric Sci China 8(1):83–90
Wilkins TA, Mishra R, Trolinder NL (2000) Cotton biotechnology. Crit Rev Plant Sci 15:511–550
Wilkins TA, Mishra R, Trolinder NA (2004) Agrobacterium mediated transformation of cotton. Food Agric Env 2(1):179–187
Wu JH, Zhang XL, Nie YC, Jin SX, Ling SG (2004) Factor affecting somatic embryogenesis and plant regeneration from a range of recalcitrant genotypes of Chinese cottons (Gossypium hirsutum L.). In Vitro Cell Dev Biol Plant 40:371–375
Zhang BH (2000) Regulation of plant growth regulators on somatic embryogenesis and plant regeneration. Biochemistry 39:1567
Zhang X, Jizhong S, Jinhan L (1991) Somatic embryogenesis and plant regeneration in upland cotton. Chin J Genet 18:315–322
Zhang BH, Feng R, Li XL, Li FL (1999) Direct induction of cotton somatic embryogenesis. Chin Sci Bull 44:766–767
Zhang BH, Feng R, Liu F, Wang Q (2001) High frequency somatic embryogenesis and plant regeneration of an elite Chinese cotton variety. Bot Bull Acad Sin 42:9–16
Acknowledgments
This work was supported by funds from the Department of Biotechnology (DBT), Government of India, Indian Council of Agricultural Research under the National Agricultural Innovation Project, Component-4, Government of India and the International Centre for Genetic Engineering and Biotechnology, New Delhi, India. The pCAMBIA1390-sGFP vector from Dr. Seiichi Toki, Japan is acknowledged. T.K. is a DBT PDF fellowship recipient.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, T., Reddy, V.S. & Leelavathi, S. High-frequency regeneration via somatic embryogenesis of an elite recalcitrant cotton genotype (Gossypium hirsutum L.) and efficient Agrobacterium-mediated transformation. Plant Cell Tiss Organ Cult 101, 323–330 (2010). https://doi.org/10.1007/s11240-010-9691-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9691-y