Highlights
Previous studies have showed conflicting results regarding the association between atopic dermatitis (AD) and venous thromboembolism (VTE) events.
Based on our systematic review of 6 cohort studies, patients with AD have an increased risk of VTE events.
These findings provide key evidence-based estimates to inform decision-making that VTE is a comorbidity of AD.
Abstract
Atopic dermatitis (AD) is a prevalent chronic inflammatory skin disease. While various inflammatory conditions have been linked to venous thromboembolism (VTE), the risk of VTE among patients with AD remains unclear. We sought to systematically review and meta-analyze population-based studies to determine the association between AD and incident VTE. A systematic review was performed of published studies in PubMed, Web of Science, Embase and Cochrane library from their inception to 27 May 2024. At least two reviewers conducted title/abstract, full-text review and data extraction. Cohort studies examining the association of AD with incident VTE were included. Quality of evidence was assessed using the Newcastle-Ottawa Scale. Six cohort studies, encompassing a total of 10,186,861 participants, were included. The meta-analysis revealed a significantly increased risk for incident VTE among AD patients (pooled hazard ratio (HR), 1.10; 95% CI, 1.00–1.21), with an incidence rate of VTE at 3.35 events per 1000 patient-years. Individual outcome analyses suggested that AD was associated with higher risks of deep vein thrombosis (pooled HR, 1.15; 95% CI, 1.04–1.27) but not pulmonary embolism (pooled HR, 0.99; 95% CI, 0.87–1.13). This systematic review and meta-analysis indicated an increased risk of incident VTE among patients with AD. Future studies are necessary to elucidate the underlying pathophysiology of the association between AD and VTE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atopic dermatitis (AD) is a chronic immune-mediated inflammatory skin disorder with a lifetime prevalence of up to 20% [1, 2]. The pathophysiology is intricate, involving genetic, immunologic, and environmental factors that precipitate in epidermal barrier dysfunction and immune system dysregulation [3]. Growing evidence indicates that AD extends beyond localized skin inflammation, representing a systemic disorder. Moreover, the severity of AD is positively correlated with an increased risk of cardiovascular outcomes [4, 5].
Venous thromboembolism (VTE), encompassing deep vein thrombosis (DVT) and pulmonary embolism (PE), is a relatively common circulatory disease associated with high recurrence rates and notable mortality [6, 7]. Increasing evidence underscores the role of both inflammation and immunity in fostering a prothrombotic state and elevating the risk of VTE [8]. Elevated levels of inflammatory and prothrombotic markers, such as β-thromboglobulin and platelet factor 4, integral to VTE pathophysiology, are also observed in patients with AD [9, 10].
Despite efforts in epidemiologic studies to assess the risk of VTE in patients with AD, the results remain controversial. A cross-sectional analysis of US hospitalizations reported a significant 22% increase in VTE risk associated with AD [11]. Notably, a previous systematic review in 2022, limited to two cohort articles, concluded no significant association between AD with incident VTE [12]. However, a retrospective cohort study using a US Claims Database found a significant difference in VTE risk between patients with AD and non-AD controls in 2024 [13].
This study conducted a comprehensive systematic review and meta-analysis to explore the association between AD and incident VTE events, including DVT and PE.
Method
This systematic review was prepared in accordance with the Meta- analysis of Observational Studies in Epidemiology (MOOSE) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The protocol for this systematic review was registered in PROSPERO (CRD42023479539) before the data extraction.
Literature search
The relevant articles from network databases including PubMed, Web of Science, Embase and Cochrane library were searched by two investigators (Yifei Wang and Zhiqiang Chen) independently (up to May 27, 2024). Detailed lists of the search terms were available in Supplementary Material (Supplementary Table 1). Additionally, we meticulously reviewed the reference lists of eligible articles and explored gray literature databases recommended by the Cochrane handbook. In case where the manuscripts lacked the required data, we proactively reached out to the corresponding authors for clarification. Articles without accessible data after author contact were subsequently excluded from the analysis.
Inclusion and exclusion criteria
Before filtrating articles, we engaged in a thorough discussion in regards to the inclusion and exclusion criteria. The inclusion criteria were defined as follows: (I) cohort studies examining the temporal association between AD and incident VTE; (II) studies with a participant population consisting individuals with a confirmed diagnosis of AD, determined based on the accepted diagnostic criteria at the time of study, and with a reference comparison group; (III) availability of risk estimates for clinical outcomes, including VTE, DVT, or PTE incidence; (IV) articles written in English. Excluded from consideration were meeting abstracts, reviews, editorials, comments, case reports/series, cross- sectional studies, case-control studies, and animal studies. In instances where the manuscripts did not present the desired data, proactive communication with the primary authors was initiated. And studies lacking the requisite data following author contact were subsequently excluded from the analysis.
Data selection and extraction
Two independent investigators (Yifei Wang and Zhiqiang Chen) conducted a comprehensive review of all the full texts of eligible articles and extracted data using a predefined standard form. All discrepancies between the two investigators were resolved through discussion and a consensus was reached on all items. The systematically extracted data included the following: first author, publication date, study design, study period, and study population characteristics (sample size, age and sex), definition of AD and outcomes of interest (risk estimates or the number of VTE events).
Quality assessment
Two reviewers independently appraised the included cohort studies by using the Newcastle-Ottawa Scale (NOS), which included three parameters regarding patients’ selection, comparability, and exposure or outcome of interest. A high-quality study was defined as having a score of ≥ 6 stars. Any disagreements were resolved by third researcher (Chen Shen).
Statistical analysis
The hazard ratio (HR) and its 95% confidence interval (CI) were calculated to determine the incidence of VTE among AD patients. Data from different studies were pooled using an inverse variance-weighted approach, which determined the weight assigned to each enrolled study. Statistical heterogeneity was examined through the calculation of I2 value and the Cochran’s Q test. In cases of high heterogeneity (I2 > 50%), a random-effect model was applied; otherwise, a fixed-effect model was used. Moreover, potential heterogeneity was explored through sensitivity analysis, involving the individual deletion of each study. If 10 or more studies assessed a particular outcome, funnel plots would be used to evaluate the publication bias. If the number of studies was less than 10, due to providing little power, publication bias could not be explored with funnel plots [14]. These analyses were performed using STATA 15.0 with all P-values being 2-tailed and a significance level of 0.05.
Results
Description of studies included
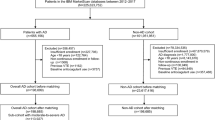
Initially, 872 studies were identified from the database search. After the removal of 122 duplicated articles and the exclusion of 714 irrelevant articles based on the title and abstract, 36 publications were progressed to a full-text review. Following the second round of screening, 6 articles met the predefined inclusion criteria and were included in the analysis. The study selection process, along with reasons for exclusion, is illustrated in the PRISMA flow chart (Fig. 1).
Characteristics of included studies
The included cohort studies were published between 2021 and 2024, and spanned multiple countries and areas, including USA [13, 15, 16], UK [17, 18] and Taiwan [19]. The definition of AD was based on validated International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes. In the included studies, there were 3,689,515 individuals with AD (54.70% female) and 6,497,346 controls. Participants were primarily adults; although two studies by Wan et al [17] and Zirpel et al [16] included both pediatric and adult populations. The length of follow-up varied from 1 to 20 years across the included studies. All included studies adjusted for a set of conventional risk factors, including the age, sex and relevant comorbidities. Key characteristics of the included studies are summarized in Table 1.
Risk of bias assessment
Table 2 provided the detailed information on the quality of the studies based on the Newcastle Ottawa Scale. All cohort studies were considered to have a high quality of evidence. Although no studies were identified as having a high risk of bias, there was heterogeneity in the association estimates, definitions of exposure, outcomes, and populations, accompanied by wide CIs.
Relative risk of VTE, DVT, and PE events in patients with AD
In the primary analysis of the HRs for VTE in patients with AD compared with non-AD controls, five studies were included [13, 15, 16, 18, 19]. As illustrated in Fig. 2A, the risk for incident VTE significantly increased among patients with AD compared with non-AD controls (pooled HR, 1.10; 95% CI 1.00–1.21). Substantial heterogeneity was observed in the HR estimates across the studies (I2 = 95.5%, p < 0.001).
When focusing on the HR of DVT (Fig. 2B) and PE (Fig. 2C) in patients with AD compared with the general population, five studies were identified [15,16,17,18,19]. The pooled risk of DVT in patients with AD was 1.15 times higher than that of non-AD controls (pooled HR 1.15, 95% CI 1.04–1.27). Substantial heterogeneity was observed in the HR estimates across the studies (I2 = 93.7%, p < 0.001). The pooled HR estimate for PE was 0.99 (95% CI 0.87–1.13; I2 = 93.5%, p < 0.001), indicating the risk for incident PE did not significantly increase among patients with AD.
Incidence rate of VTE, DVT, and PE events in patients with AD
Four studies were included in the analysis of the incidence rate (IR) of VTE events in patients with AD [13, 15, 17, 18]. The overall incidences of VTE in patients with AD varied from 1.05 to 7.30 per 1000 person-yesars (PY). Pooling the results showed that the IR of VTE among patients with AD was 3.35 per 1000 PY (95% CI 0.11–6.58; I2 = 100.0%, p < 0.001) (Fig. 3A). Three studies provided estimates for the IR of DVT and PE events in patients with AD [15, 18, 19]. The incidence of DVT and PE was 1.56 per 1000 PY (95% CI 0.79–2.32) and 0.64 per 1000 PY (95% CI 0.20–1.08), respectively. Substantial heterogeneity was observed (p < 0.001, I2 = 99.7 and 99.6%) (Fig. 3B and C).
Subgroup evaluation of sex and age
Two studies by Chen et al. [19] and Warren et al. [18]. calculated the HR of VTE events in patients with AD, compared with non-AD controls, stratified by sex. The association between AD and increased VTE risk remained significant for both sexes. Similar findings were observed in the analyses for DVT. However, Chen et al. [19]. found a significantly increased risk of incident PE compared with adults without AD in both sexes, while the study of Warren et al. [18] did not observe this (Supplementary Fig. 1).
The study by Wan et al. [17]. calculated the HR of VTE events among children and adults with AD. Among the pediatric cohort, children with AD demonstrated a 23% higher risk of DVT (HR, 1.23; 95% CI 1.02–1.48), but no increased risk for PE (HR, 0.78; 95% CI 0.58–1.05). The HRs for VTE were similar for adults with AD (DVT : HR, 1.14; 95% CI 1.11–1.18 & PE: HR, 0.99; 95% CI 0.95–1.03). In the study by Schneeweiss et al. [20], no significant difference in VTE risk was observed between patients with and without AD, who were otherwise comparable in those aged 18 to 49 years (HR, 1.00; 95% CI, 0.81–1.25) or 50 years or older (HR, 1.01; 95% CI, 0.88–1.14). Interestingly, Chen et al. [19] and Warren et al. [18] found an increased risk of incident VTE and DVT in adults with AD 45 years or older but not in those younger than 45 years (Supplementary Fig. 2).
Subgroup evaluation of disease severity
Four studies evaluated heterogeneity in AD-associated VTE risk by AD severity (Supplementary Table 2) [15, 17,18,19]. The studies conducted by Chen et al. [19] and Warren et al. [18] observed a consistently increased risk of VTE across mild, moderate and severe AD, with HRs vs. controls. Meyers et al. [15] also noted a similar trend for VTE in patients with moderate-to-severe AD (HR 1.24; 95% CI 1.13–1.36).
Chen et al.17 and Wan et al. [17] reported that the incidence of DVT was significantly higher in individuals with mild, moderate or severe adult AD, compared to controls, with the highest risk observed in those with severe AD. Similarly, DVT risk was 28% greater among patients with moderate-to-severe AD (HR 1.28, 95% CI 1.16–1.42) in the study of Meyers et al. [15].
In the study of Chen et al. [19], adults with mild and severe AD exhibited an increased risk of PE compared to controls. Wan et al. [17] found that PE risk was 39% highter among patients with severe AD (HR 1.39, 95% CI 1.21–1.60), while mild AD was conversely associated with a 6% lower PE risk compared to those without AD (HR 0.94, 95% CI 0.89–0.99). However, there was no difference for moderate AD. Meyers et al. [15] did not detect significant difference in PE risk among individuals with moderate-to-severe AD.
Discussion
Our systematic review and meta-analysis revealed a statistically significant association between AD and incident VTE in cohort studies. The overall incidence rate of VTE was 3.35 events per 1000 PY among individuals with AD. Notably, this study represented the first meta-analysis to comprehensively investigate the association between VTE and factors including sex, age and lesion severity.
In our study, AD was associated with a modest increase around 10% in the relative risk of VTE compared with individuals without AD. The heightened VTE risk was specifically attributed to an increased risk of DVT and not PE. The precise reason for this selectivity is not clear. In contrast to a previous systemic review that reported no significant association between AD and VTE [12], our study incorporates a more extensive set of relevant studies and expands beyond the confines of merely 2 US-based studies. Emerging epidemiological evidence has established associations between VTE and various immune-mediated chronic inflammatory conditions, such as systemic lupus erythematosus, inflammatory bowel disease and psoriasis [21,22,23]. Moreover, recent guidelines acknowledge a subtle association between AD and various cardiovascular conditions [24]. Chronic systemic inflammation in AD is believed to stimulate vascular inflammation and oxidative stress [25]. In addition, elevated eosinophil counts could contribute to increased blood viscosity and hypercoagulability [26]. The elevated platelet activation and prolonged fibrinolysis, identified in AD patients, may lead to increased risk of thrombosis [27]. Therefore, these findings offer potentially explanations for the elevated risks of VTE associated with AD.
In the stratified analysis, Chen et al. [19] and Warren et al. [18] found a higher risk of incident VTE and DVT in adults aged above 45 years, while Schneeweiss et al. [20]. did not identify a significant difference. Recent research has shown higher levels of systemic inflammatory markers in older individuals with AD [28], which may been linked to VTE disease. In contrast, our results suggested that AD-associated risk does not differ by sex. Descriptive analyses suggest that a potent increased in VTE risk in severe AD, compared with controls, but our findings do not provide evidence of a further VTE risk increase compared with mild or moderate AD. The different definitions of AD severity can partly be explanatory. Current studies showed the systemic immune abnormalities in patients with severe AD, characterized by increased expression of proinflammatory cytokines and cardiovascular risk proteins [29, 30], whereas these abnormalities were not pronounced in those with mild AD [31]. A retrospective cohort study also highlighted an increased risk of cardiovascular events in AD patients, with the risk escalating with the severity of AD [32]. Further studies are still needed to clarify the effects of AD severity on the VTE risk.
Formal exploration of causes of heterogeneity was not undertaken due to the low number of studies providing little power to detect them. Publication bias could not be explored with funnel plots due to the presence of high heterogeneity, which precludes the expectation of a plot of estimates against their Standard Errors to have a funnel shape [14].
Strengths and limitations
This systematic review and meta-analysis has several strengths. We preregistered and published protocol, increasing the transparency of our study. Besides, the inclusion of multiple population-based cohort studies conducted across different countries contributed to the provision of up-to-date and robust evidence. In addition, we introduced the subgroup analysis based on sex, age and AD severity, enriching the conclusions. As comorbidities among AD are not comprehensively established, this study potentially provides insights into paying attention to the risk of VTE in patients with AD, based on population-based cohort studies.
This study has a few limitations. Firstly, the diagnosis of both AD and VTE relied on ICD codes. Although these codes have undergone validation, the potential for misclassification bias cannot be entirely ruled out. Secondly, substantial heterogeneity was observed in the meta-analysis regarding the association between AD and VTE. These included cohort and database studies derived from medical records or registries introduced variability in terms of data completeness, accuracy, and population coverage, thus serving as a potential source of variation across studies. Thirdly, the severity of AD was mostly defined according to a treatment pattern instead of indices used in clinical trials, such as total body surface area, Investigator Global Assessment score, and Eczema Area and Severity Index score [3, 33]. Finally, the data on AD treatment was scarce, which might influence the correlation between disease severity and VTE risk. Some patients might have low severity of AD due to high corticosteroid use (which has been associated with VTE).
Conclusion
The findings from this comprehensive systematic review and meta-analysis indicate a significant association between AD and the incident VTE, more specifically, DVT and not PE, compared with individuals without AD. Subsequent research endeavors should focus on elucidating the underlying mechanisms contributing to DVT in AD. Additionall, identifying specific subgroups within the AD population at highest risk is crucial for implementing targeted management strategies for individuals with AD.
Data availability
The data of this study are available from the corresponding author upon reasonable request.
References
Langan SM, Irvine AD, Weidinger S (2020) Atopic dermatitis. Lancet 396(10247):345–360. https://doi.org/10.1016/s0140-6736(20)31286-1
Weidinger S et al (2018) Atopic dermatitis. Nat Rev Dis Primers 4(1). https://doi.org/10.1038/s41572-018-0001-z
Eichenfield LF et al (2014) Guidelines of care for the management of atopic dermatitis: Sect. 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 70(2):338–351. https://doi.org/10.1016/j.jaad.2013.10.010
Ascott A et al (2019) Atopic eczema and major cardiovascular outcomes: a systematic review and meta-analysis of population-based studies. J Allergy Clin Immunol 143(5):1821–1829. https://doi.org/10.1016/j.jaci.2018.11.030
Silverwood RJ et al (2018) Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: population based cohort study. BMJ (clinical research ed. 361. https://doi.org/10.1136/bmj.k1786
Arshad N et al (2017) Recurrence and mortality after first venous thromboembolism in a large population-based cohort. J Thromb Haemostasis: JTH 15(2):295–303. https://doi.org/10.1111/jth.13587
Cohen AT et al (2017) Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thromb Haemost 117(1):57–65. https://doi.org/10.1160/TH15-08-0686
Riva N, Donadini MP, Ageno W (2015) Epidemiology and pathophysiology of venous thromboembolism: similarities with atherothrombosis and the role of inflammation. Thromb Haemost 113(6):1176–1183. https://doi.org/10.1160/TH14-06-0563
Tamagawa-Mineoka R et al (2008) Elevated platelet activation in patients with atopic dermatitis and psoriasis: increased plasma levels of beta-thromboglobulin and platelet factor 4. Allergology International: Official J Japanese Soc Allergology 57(4):391–396. https://doi.org/10.2332/allergolint.O-08-537
Piazza G, Ridker PM (2015) Is venous thromboembolism a chronic inflammatory disease? Clin Chem 61(2):313–316. https://doi.org/10.1373/clinchem.2014.234088
Shaheen MS, Silverberg JI (2021) Association of inflammatory skin diseases with venous thromboembolism in US adults. Arch Dermatol Res 313(4):281–289. https://doi.org/10.1007/s00403-020-02099-6
Chen T-L et al (2022) Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and Meta-analysis. JAMA Dermatology 158(11):1254–1261. https://doi.org/10.1001/jamadermatol.2022.3516
Merola JF et al (2024) Venous thromboembolism risk is lower in patients with atopic dermatitis than other immune-mediated inflammatory diseases: a retrospective, observational, comparative cohort study using US claims data. J Am Acad Dermatol 90(5):935–944. https://doi.org/10.1016/j.jaad.2023.12.027
Afonso J et al (2023) The perils of Misinterpreting and Misusing Publication Bias in Meta-analyses: an Education Review on Funnel plot-based methods. Sports Medicine (Auckland, N.Z.) https://doi.org/10.1007/s40279-023-01927-9
Meyers KJ et al (2021) Risk of venous thromboembolism among patients with atopic dermatitis: a Cohort Study in a US administrative claims database. Dermatology Therapy 11(3):1041–1052. https://doi.org/10.1007/s13555-021-00538-4
Zirpel H et al (2024) Atopic dermatitis is associated with an increased risk of cardiovascular diseases: a large-scale, propensity-matched US-based retrospective study. Clin Exp Dermatol. https://doi.org/10.1093/ced/llae164
Wan J et al (2023) Incidence of Cardiovascular Disease and venous thromboembolism in patients with atopic dermatitis. The Journal of Allergy and Clinical Immunology. Practice 11(10). https://doi.org/10.1016/j.jaip.2023.08.007
Warren RB et al (2023) The risk of venous thromboembolism in atopic dermatitis: a matched cohort analysis in UK primary care. Br J Dermatol 189(4):427–436. https://doi.org/10.1093/bjd/ljad212
Chen T-L et al (2023) Risk of venous thromboembolism among adults with atopic dermatitis. JAMA Dermatology 159(7):720–727. https://doi.org/10.1001/jamadermatol.2023.1300
Schneeweiss MC et al (2021) Incidence of venous thromboembolism in patients with dermatologist-diagnosed chronic inflammatory skin diseases. JAMA Dermatology 157(7):805–816. https://doi.org/10.1001/jamadermatol.2021.1570
Bello N et al (2023) Systematic Literature Review and Meta-analysis of venous thromboembolism events in systemic Lupus Erythematosus. Rheumatol Therapy 10(1). https://doi.org/10.1007/s40744-022-00513-1
Grainge MJ, West J, Card TR (2010) Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet (London England) 375(9715):657–663. https://doi.org/10.1016/S0140-6736(09)61963-2
Chen T-L et al (2022) Association of Psoriasis With Incident venous thromboembolism and peripheral vascular disease: a systematic review and Meta-analysis. JAMA Dermatology 158(1):59–67. https://doi.org/10.1001/jamadermatol.2021.4918
Davis DMR et al (2022) American Academy of Dermatology Guidelines: awareness of comorbidities associated with atopic dermatitis in adults. J Am Acad Dermatol 86(6). https://www.jaad.org/article/S0190-9622(22)00080-9/fulltext
Brunner PM et al (2017) The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci Rep 7(1):8707. https://doi.org/10.1038/s41598-017-09207-z
Réau V et al (2021) Venous thrombosis and predictors of relapse in eosinophil-related diseases. Sci Rep 11(1):6388. https://doi.org/10.1038/s41598-021-85852-9
Nastałek M, Wojas-Pelc A, Undas A (2010) Plasma fibrin clot properties in atopic dermatitis: links between thrombosis and atopy. J Thromb Thrombolysis 30(2):121–126. https://doi.org/10.1007/s11239-010-0478-0
Wollenberg A et al (2020) Summary of efficacy, impact on work productivity and activity, and safety of baricitinib in moderateto-severe atopic dermatitis from two monotherapy phase III trials. British Journal of Dermatology, 183(SUPPL 1): p. 66.https://doi.org/10.1111/bjd.18968
Del Duca E et al (2023) Proteomic characterization of atopic dermatitis blood from infancy to adulthood. J Am Acad Dermatol 88(5):1083–1093. https://pubmed.ncbi.nlm.nih.gov/36773824/
Pavel AB et al (2020) The proteomic skin profile of moderate-to-severe atopic dermatitis patients shows an inflammatory signature. J Am Acad Dermatol 82(3):690–699. https://doi.org/10.1016/j.jaad.2019.10.039
He H et al (2021) Mild atopic dermatitis lacks systemic inflammation and shows reduced nonlesional skin abnormalities. J Allergy Clin Immunol 147(4):1369–1380. https://doi.org/10.1016/j.jaci.2020.08.041
Hedderson MM et al (2022) Rates of cardiovascular events among patients with moderate-to-severe atopic dermatitis in an integrated health care system: a retrospective cohort study. PLoS ONE 17(11):e. https://doi.org/10.1371/journal.pone.0277469
Silverberg JI et al (2020) Validation of five patient-reported outcomes for atopic dermatitis severity in adults. Br J Dermatol 182(1):104–111. https://doi.org/10.1111/bjd.18002
Acknowledgements
We thank Professor Adam J Adler for critically reading the manuscript.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities, Grant/Award Numbers: YCJJ20230213.
Author information
Authors and Affiliations
Contributions
Data curation: Yifei Wang, Zhiqiang Chen, Ting He, Changzheng Huang, Chen Shen. Methodology: Yifei Wang, Zhiqiang Chen, Ting He, Changzheng Huang, Chen Shen. Formal analysis and investigation: Yifei Wang, Zhiqiang Chen, Ting He, Changzheng Huang, Chen Shen. Writing – original draft preparation: Yifei Wang, Zhiqiang Chen, Chen Shen, Changzheng Huang. Writing – review and editing: Yifei Wang, Chen Shen, Changzheng Huang. Funding acquisition: Yifei Wang. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
The manuscript has been read and approved by all the authors. The requirements for authorship as stated earlier in this document have been met. All the authors believe that the manuscript represents honest.
Conflict of interest
The authors declare no conflict of interest regard- ing the publication of this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Chen, Z., He, T. et al. Risk of incident venous thromboembolism in patients with atopic dermatitis: systematic analysis of the literature and meta-analysis. J Thromb Thrombolysis (2024). https://doi.org/10.1007/s11239-024-03038-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s11239-024-03038-2