Abstract
Anticoagulant therapy is a mainstay in the management of patients with cardiovascular disease. The use of conventional anticoagulants carries potential side effects, mainly bleeding. Drugs targeting Factor XI (FXI) have been investigated in randomized controlled trials as a new option with more favorable outcomes. A comprehensive literature search was conducted to identify relevant studies comparing FXI inhibitors to placebo or standard therapy. The primary outcomes were incidence of all bleeding events, major bleeding, and thromboembolism. Secondary outcomes included incidence of all adverse events (AE), serious AE, and all-cause mortality. A total of 11 studies involving 10,536 patients were included. FXI inhibitors were associated with a trend toward reduction of bleeding events and incidence of thromboembolism compared to the control group (placebo/standard therapy). There was no statistically significant difference between both groups in terms of adverse events and all-cause mortality. When compared to enoxaparin, FXI inhibitors significantly reduced the risk of bleeding events (RR = 0.42, 95% CI: 0.23–0.76, P = 0.004) and thromboembolism (RR = 0.59, 95% CI: 0.44–0.77, P = 0.001). On the other hand, when compared to DOACs, FXI inhibitors were associated with a significant reduction in bleeding events but not thromboembolism. Whereas, compared to placebo, FXI inhibitors did not increase the risk of bleeding events, adverse events, or all-cause mortality (P > 0.05). FXI inhibitors could be a safer and more potent option for prevention of thromboembolism than conventional therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of anticoagulants has become a pivotal strategy in preventing thromboembolism in many conditions such as atrial fibrillation (AF) [1], end-stage renal disease (ESRD) [2, 3], venous thromboembolism (VTE) prophylaxis [4,5,6,7], strokes [8, 9], and myocardial infarction (MI) [10]. Direct oral anticoagulants (DOACs) have replaced the traditional vitamin K antagonists (VKAs) in many indications in clinical practice because of their several advantages including selectivity, more rapid action, and no need for laboratory monitoring [11]. Despite their widespread application, apprehensions persist regarding the safety profile of anticoagulants, specifically the occurrence of bleeding events as an adverse outcome [12]. The transition to DOACs has not eliminated hemorrhagic events in clinical practice, prompting the quest for safer therapeutic strategies. These events can be attributed to the direct impact of factor X and factor II inhibition on hemostasis [12].
In recent years, factor XI (FXI) inhibitors have emerged as a novel class of anticoagulants, aiming to achieve improved efficacy with a more favorable bleeding profile. This proposition was based on the fundamental role of FXI in thrombus amplification through the activation of thrombin (factor II) within a cyclic process, while exhibiting a minor role in hemostasis [13, 14]. Importantly, hemostasis initiation is a self-limited process with no additional propagation and is mainly dependent on the small amount of fibrin produced rather than FXI activation [14]. This physiological perspective aligns with observations in congenital FXI deficiency (hemophilia C), characterized by post-traumatic bleeding rather than spontaneous bleeding, and a decreased incidence of venous thromboembolism and strokes in the affected individuals [15,16,17]. Interestingly, a recent genetic study using Mendelian randomization (MR) analysis showed an association between reduced levels of FXI and the lower risk of ischemic strokes and VTE without a similar association with increased bleeding tendency [18].
While several phase II randomized clinical trials (RCTs) have explored the potential efficacy of FXI inhibitors in comparison to DOACs and low molecular weight heparin (LMWH), the limitations of sample size and patient selection in these trials necessitate caution in drawing definitive conclusions. Consequently, to address these concerns, we performed a systematic review and meta-analysis encompassing 11 phase II RCTs. The objective of our meta-analysis is to question the previously suggested positive correlations indicating the potential of FXI inhibitors to decouple thrombosis and hemostasis, thereby offering a safe and effective anticoagulant option for the target population.
Methods
Search strategy & inclusion criteria
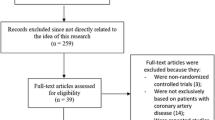
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19]. We systematically searched the literature for the RCTs investigating the safety and efficacy of FXI inhibitors in preventing thromboembolism. The search strategy and keywords are available in the supplementary data. Multiple databases were searched, including PubMed, Scopus, and ScienceDirect. Inclusion criteria involved only English-language articles with an available full text. Two reviewers conducted abstract and full-text screenings using Rayyan software [20], with any discrepancies resolved by a third author. Articles were included if they were RCT exploring outcomes in adult patients (≥ 18 years old) at an increased risk of thromboembolism, comparing FXI inhibitors to placebo or standard therapy. Exclusion criteria encompassed observational studies, case series, case reports, or reviews. The primary outcomes included incidence of all bleeding events, major bleeding, and thromboembolism. Secondary outcomes included the incidence of all adverse events (AE), serious AE (SAE), and all-cause mortality. The complete PRISMA flow chart illustrating article inclusion is presented in Fig. 1.
Data extraction
Included articles were thoroughly explored by two reviewers to extract study characteristics (First author, publication year, study population, intervention group, control group, and the study outcomes) and patients’ characteristics (clinical indication, average age, female percentage, BMI, comorbidities, prior medications, baseline APTT, and FXI activity level). The primary and secondary endpoints for this meta-analysis were also extracted from each of the included studies.
Quality assessment
The quality of the included RCTs was evaluated independently by two reviewers using the Modified Cochrane Risk of Bias Assessment Tool [21]. This tool assessed various domains of methodological quality in the trials, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcomes assessment, attrition bias due to missing data, reporting bias, and other sources of bias [21]. Each domain was assigned a rating of low risk, high risk, or unclear risk. Additionally, publication bias was examined through the assessment of funnel plot asymmetry.
Statistical analysis
The statistical analysis was conducted using Review Manager software (RevMan) version 5.3. Outcomes were included in the analysis if they were reported in two or more studies. Effect estimates were assessed as mean differences and relative risks, employing the random-effect model. Sensitivity analysis was performed using the leave-one-out test. Measures of heterogeneity, including the I2 and Chi2 indices, were utilized, with a threshold of greater than 50% indicating high heterogeneity. Subgroup analyses were conducted for relevant outcomes based on the control group and clinical indication if a minimum of two studies were reported per the subgroup arm. A two-sided p-value of < 0.05 was deemed significant.
Results
Eligible studies
A total of 590 studies were identified through databases. Duplicates were removed and the screening process was done. A total of 18 articles were retrieved for full-text screening. Finally, 11 RCTs were included in this systematic review representing a total of 10,536 participants (7625 patients in the FXI inhibitors group and 2911 patients in the control group).
Studies and population characteristics
Four studies compared different FXI inhibitors to enoxaparin [4,5,6,7]. Two studies compared FXI inhibitors to apixaban [1, 6] while another study compared them to rivaroxaban (AZALEA-TIMI 71 clinical trial). Five studies compared FXI inhibitors to placebo [2, 3, 8,9,10]. Four studies investigated the effect and safety of FXI inhibitors in patients undergoing total knee arthroplasty (TKA) [4,5,6,7] while two studies examined the drugs in patients with ESRD [2, 3]. Two studies had a population of patients with AF [1], one had patients with recent MI [10], and two studies had patients with ischemic stroke [8, 9]. A background antiplatelet therapy was given in three studies; in AXIOMATIC-SSP, dual antiplatelet therapy (DAPT) for 21 days followed by aspirin alone until 90 days; in PACIFIC-Stroke trial, 43% of participants received DAPT for a mean duration of 70 days; in PACIFIC-AMI trial, 79% of participants received DAPT until 6 months and 63% until 12 months of follow-up. The results of all included clinical trials were published except for the AZALEA-TIMI 71 clinical trial for which the results were reported from the American Heart Association Scientific Session 2023. The primary outcomes were all bleeding events, major bleeding events, and incidence of new or recurrent thromboembolism. A summary of the included studies consisting of the first author, year of publication, population, control group, intervention group, and study endpoints is available in Table 1. Baseline characteristics and detailed information about the included studies are summarized in Supplementary Table S1.
Risk of bias assessment
The risk of bias graph is illustrated in Fig. 2. The overall possibility of bias in the selected studies was low. There were some concerns regarding randomization and allocation concealment in some studies due to the open-label design. Nevertheless, this potential bias was decreased by the blinding of outcomes evaluators. The risk of bias summary is illustrated in Supplementary data (Figure S1). The overall risk of publication bias was considered low as shown by the Funnel Plots in Supplementary Figure S2.
Meta-analysis
-
A.
Effect of FXI inhibitors on all-bleeding events:
The meta-analysis of the included 11 studies showed that FXI inhibitors were associated with a trend toward reduction of all-bleeding events when compared to the control group (enoxaparin/DOACs/placebo) in the studies populations (RR = 0.72, 95% CI: 0.5–1.05, P = 0.09). The pooled results showed significant heterogeneity (I2 = 73%) Fig. 3(A). Leave-one-out sensitivity analysis was performed and did not resolve the heterogeneity.
-
B.
Effect of FXI inhibitors on major bleeding events:
11 studies were included and showed no statistically significant difference between FXI inhibitors and the control group (RR = 0.68, 95% CI: 0.33–1.41, P = 0.3), and there was moderate heterogeneity among the included studies (I2 = 44%) Fig. 3(B).
-
C.
Effect of FXI inhibitors on thromboembolism incidence:
9 studies were included in the meta-analysis. FXI inhibitors were found to be associated with a trend toward reduction in the risk of thromboembolism when compared to the control group (RR = 0.82, 95% CI: 0.65–1.04, P = 0.1). The pooled studies showed significant heterogeneity with I2 = 68%; Fig. 3(C). Leave-one-out sensitivity analysis was performed; however, the heterogeneity was not resolved.
-
D.
Effect of FXI inhibitors on the incidence of all adverse events:
10 studies reported the all-adverse events as an outcome. Pooled analysis showed that FXI inhibitors did not increase the risk of adverse events when compared to the control group (RR = 1.03, 95% CI: 1.00–1.06, P = 0.08), and no statistical heterogeneity was found (I2 = 0%) Fig. 4(A).
-
E.
Effect of FXI inhibitors on the incidence of serious adverse events:
9 of the included studies reported serious adverse events as an outcome. Pooled analysis showed that there was no statistically significant difference between FXI inhibitors and the control group (RR = 0.93, 95% CI: 0.84–1.04, P = 0.21), and no significant heterogeneity was found among studies ((I2 = 0%) Fig. 4(B).
-
F.
Effect of FXI inhibitors on all-cause mortality:
6 studies were included in the meta-analysis. The overall risk ratio of all-cause mortality showed that there was no statistically significant difference between FXI inhibitors and the control group (RR = 1.03, 95% CI: 0.71–1.48, P = 0.88), and there was no heterogeneity among the included studies (I2 = 0%) Fig. 4(C).
Subgroup analysis based on the control group
FXI inhibitors vs. enoxaparin
Four studies involving 2589 patients compared FXI inhibitors to enoxaparin. One study involved IONIS-FXIRx [5], one study involved osocimab [6], one study involved abelacimab [4], and one study involved milvexian [7] in comparison to enoxaparin. The pooled analysis demonstrated that FXI inhibitors were associated with a 58% reduction in the incidence of bleeding events when compared to enoxaparin group (RR = 0.42, 95% CI: 0.23–0.76, P = 0.004), and no heterogeneity was found among the included studies (I2 = 0%). Whereas, no difference was found between the two groups in terms of major bleeding events (RR = 0.39, 95% CI: 0.06–2.45, P = 0.31), all-adverse events (RR = 1.07, 95% CI: 0.93–1.23, P = 0.34), and serious adverse events (RR = 0.67, 95% CI: 0.39–1.15, P = 0.14). Additionally, FXI inhibitors were associated with a 41% reduction in the incidence of thromboembolism when compared to enoxaparin group (RR = 0.59, 95% CI: 0.44–0.77, P = 0.001) Fig. 5.
FXI inhibitors vs. DOACs
Three studies involving 2722 patients compared FXI inhibitors to DOACs. The FOXTROT [6] and PACIFIC-AF [1] trials compared osocimab and asundexian to apixaban. Whereas AZALEA-TIMI 71 study compared abelacimab to rivaroxaban. FXI inhibitors were associated with a 62% reduction in the incidence of bleeding events (RR = 0.38, 95% CI: 0.19–0.74, P = 0.005) and a 77% reduction in the incidence of major bleeding events (RR = 0.23, 95% CI: 0.1–0.55, P = 0.001). Moreover, both groups were comparable without significant difference in terms of all-adverse events (RR = 1.03, 95% CI: 0.98–1.09, P = 0.23) and serious adverse events (RR = 0.93, 95% CI: 0.8–1.09, P = 0.37). On the other hand, no difference in the incidence of thromboembolism was found between the two groups (RR = 1.35, 95% CI: 0.83–2.17, P = 0.22) Fig. 6.
FXI inhibitors vs. placebo
Five studies involving 5810 patients compared FXI inhibitors to placebo. One study involved IONIS-FXIRx [2], one study involved Xisomab 3G3 [3], one study involved milvexian [9], and two studies involved asundexian [8, 10] in comparison to placebo. FXI inhibitors were not associated with an increased risk of all-bleeding (RR = 1.09, 95% CI: 0.86–1.39, P = 0.47) or major bleeding events (RR = 1.13, 95% CI: 0.61–2.06, P = 0.7). Moreover, no difference was found between the two groups in terms of all-adverse events (RR = 1.02, 95% CI: 0.97–1.07, P = 0.47), serious adverse events (RR = 0.95, 95% CI: 0.82–1.11, P = 0.54), all-cause mortality (RR = 1.24, 95% CI: 0.77–1.98, P = 0.38), or incidence of thromboembolism (RR = 1.02, 95% CI: 0.88–1.18, P = 0.79) Fig. 7.
Subgroup analysis based on the clinical indication
Total knee arthroplasty (TKA)
Four studies involving 2689 patients compared FXI inhibitors (IONIS-FXIRx, osocimab, abelacimab, and milvexian) to enoxaparin [4,5,6,7] and apixaban [6] in patients undergoing TKA. FXI inhibitors were found to be associated with a trend toward reduction in the incidence of all-bleeding events, major bleeding events, and serious adverse events. Furthermore, FXI inhibitors were associated with a significant reduction of 34% in the incidence of thromboembolism compared to the control group (RR = 0.66, 95% CI: 0.54–0.79, P = 0.001) supplementary figure S3.
Ischemic stroke
Two studies involving 4142 patients compared FXI inhibitors (milvexian and asundexian) to placebo in patients with non-cardioembolic stroke [8, 9]. No significant difference was found between both groups with regards to all-bleeding events, major bleeding events, thromboembolism, and all-cause mortality (P > 0.05) supplementary figure S4.
Atrial fibrillation
Two studies involving 2037 patients compared FXI inhibitors (asundexian and abelacimab) to apixaban [1] and rivaroxaban (AZALEA-TIMI 71) in patients with AF. FXI inhibitors were associated with a significant reduction in all-bleeding events (RR = 0.29, 95% CI: 0.18–0.48, P = 0.001) and major bleeding events (RR = 0.22, 95% CI: 0.09–0.53, P = 0.001) when compared to DOACs. Whereas, no statistically significant difference was found between the two groups in terms of the incidence of thromboembolism, all-adverse events, serious adverse events, or all-cause mortality (P > 0.05) supplementary figure S5.
Discussion
In our meta-analysis, we examined eleven phase 2 RCTs involving 10,536 participants. This study sheds light on FXI inhibitors’ effectiveness as anticoagulants. According to the major results, FXI inhibitors do not affect bleeding episodes or significant bleeding events when compared to control groups (DOACs, enoxaparin, and placebo). More importantly, FXI inhibitors showed higher efficacy in reducing the incidence of thromboembolism. In terms of distinguishing thrombosis and hemostasis, FXI inhibitors outperform traditional anticoagulants. The ability of FXI inhibitors to minimize the likelihood of thromboembolic events while not significantly increasing the risk of bleeding resolves a fundamental safety problem associated with anticoagulant treatment.
Various comparisons produced intriguing results in the subgroup analysis. FXI inhibitors have demonstrated a significant decrease in the occurrence of bleeding events and thromboembolism compared to enoxaparin, indicating a superior effectiveness and safety profile in this particular situation. On the other hand, when compared to DOACs, FXI inhibitors were associated with a significant reduction in bleeding events but not thromboembolism. Nevertheless, the disparities did not exhibit statistical significance in comparison to placebo, suggesting that the advantages of FXI inhibitors may change based on the comparator and patient cohort.
Therapeutic anticoagulation has been one of the most studied topics in the history of medicine with continuous evolutions and recommendations [22]. The history of therapeutic anticoagulation has evolved significantly over the years, with the initial use of vitamin K antagonists (VKAs) such as warfarin, which served as the cornerstone of anticoagulation therapy for several decades [23]. However, the introduction of direct oral anticoagulants (DOACs) marked a significant shift in anticoagulation therapy and has replaced the traditional VKAs in many clinical scenarios, offering advantages such as consistent and predictable anticoagulation, oral administration, and a good safety profile [11, 24]. More recently, the focus has shifted to Factor XI inhibitors, which have shown promise in early clinical trials, demonstrating increased safety and efficacy compared with traditional anticoagulants [12, 13].
Emerging evidence demonstrates a differential role of FXI in hemostasis and thrombosis. On one hand, Hemostasis commences with the interaction between tissue factor and activated factor VII (TF-FVIIa) in the tissue factor pathway. This interaction then leads to a series of events that eventually lead to fibrin and hemostatic clot formation [13, 14]. FXI has a minor role in hemostasis limited to its activation to FXIa by thrombin which in turn stabilizes the final hemostatic clot by activation of FIX [14, 25]. On the other hand, FXI plays a fundamental role in thrombosis. Pathological thrombosis is initiated through two primary pathways: the contact pathway and the tissue factor pathway. In both scenarios, FXI is activated by the generated thrombin during the initial phase which further motivates thrombus growth and propagation [12, 25]. Moreover, individuals with elevated FXI levels showed increased susceptibility to venous thromboembolism (VTE) [26], whereas those with FXI deficiency exhibited a lower risk of thrombosis, including DVT and less prominent activation of the hemostatic system compared to the general population [17, 27]. Considering the different roles of FXI in hemostasis and thrombosis outlined earlier, the inhibition of FXI presents a compelling strategy to separate the positive therapeutic outcomes from the adverse effects associated with anticoagulant treatments. In essence, targeting FXI offers the potential to decrease the risk of thrombotic complications without concomitantly elevating the probability of bleeding, a significant concern with alternative anticoagulants.
Several phase 2 RCTs investigating the efficacy of FXI inhibitors in the prevention of thromboembolism and their safety profile in comparison to the current standard of care (DOACs and enoxaparin) or placebo have been published. These trials included patients from various clinical scenarios including TKA, AF, MI, ischemic stroke, and ESRD.
Implications of subgroup analysis
FXI inhibitors vs. enoxaparin
The subgroup analysis, specifically comparing FXI inhibitors to enoxaparin in patients undergoing TKA, demonstrates that FXI inhibitors effectively decrease the occurrence of bleeding events and thrombosis. This discovery is particularly noteworthy because patients undergoing major surgeries such as TKA are prone to both bleeding and thromboembolic complications. The observed decrease of 58% in bleeding events and 41% in thromboembolism incidence indicates that FXI inhibitors can offer a safer and more efficient method of anticoagulation in this surgical setting. The absence of notable disparities in major bleeding and serious adverse events, however, calls for prudence and highlights the necessity for further investigation into the risk-benefit characteristics of FXI inhibitors in surgical contexts. Our results are consistent with a recent study that showed a significant reduction in the incidence of bleeding events and thromboembolism among patients undergoing major orthopedic surgeries [28].
FXI inhibitors vs. DOACs
The data indicates a decreased occurrence of both all-bleeding and major bleeding episodes with FXI inhibitors in comparison to DOACs. The similarity in the risk of thromboembolism, all-adverse events, and serious adverse events between FXI inhibitors and DOACs indicates that they have a comparable safety profile. Nevertheless, due to the limited availability of evidence and the low number of the included studies in this comparison (3 studies), it is imperative to conduct further direct comparative trials to ascertain the comparative effectiveness and safety of FXI inhibitors in relation to DOACs, especially in patient populations where DOACs such as apixaban and rivaroxaban is currently the preferred therapeutic choice such as AF.
FXI inhibitors vs. placebo
When comparing FXI inhibitors to placebo in a large group of patients, there was no higher occurrence of any bleeding, major bleeding episodes, serious side events, or mortality from any cause. This discovery is noteworthy as it emphasizes the safety of FXI inhibitors, especially in comparison to a group that did not get any treatment. Nevertheless, the absence of a significant decrease in the occurrence of thromboembolism in comparison to a placebo indicates that, although FXI inhibitors are safe, their effectiveness in reducing thromboembolic events in some populations may be lower than anticipated. In this particular comparison, our results were different from a recent meta-analysis which demonstrated a 25% increase in the incidence of all bleeding events with FXI inhibitors when compared to placebo [29]. This underscores the significance of performing more focused investigations to ascertain specific patient cohorts that would derive the greatest advantage from FXI inhibitor treatment.
Specific patient subgroups
TKA
FXI inhibitors demonstrate a propensity for decreasing bleeding events and significantly reducing the occurrence of thromboembolism in individuals undergoing TKA. This finding is particularly significant for the management of patients before, during, and after orthopedic surgeries, as it involves the delicate task of weighing the potential risks of blood clot formation and excessive bleeding. However, it’s noteworthy that in the included trials, the majority of thromboembolism events were identified through screening venography, many of which were asymptomatic [13]; hence, the necessity for larger phase 3 clinical trials focusing on clinical endpoints.
Ischemic stroke
There were no notable disparities in bleeding events, thromboembolism, or overall mortality between FXI inhibitors and placebo in patients with non-cardioembolic stroke. This highlights the necessity for further investigation into the function of FXI inhibitors in the prevention of strokes, specifically in distinguishing between different types of strokes and risk factors that are specific to each patient.
AF
The findings revealed a notable reduction in bleeding events with FXI inhibitors compared to DOACs. However, no difference was observed between the two groups regarding the incidence of thromboembolism, all adverse events, serious adverse events, or all-cause mortality. While these results suggest that FXI inhibitors may offer a favorable bleeding risk profile compared to DOACs, this should be interpreted cautiously due to the limited number of the included studies (2 studies); therefore, further investigation is warranted to fully elucidate their efficacy and safety profile in atrial fibrillation management.
Limitations
Although the meta-analysis offers valuable insights, it is not devoid of limitations. The presence of diverse patient categories and indications in the studies examined, such as TKA, ESRD, AF, and ischemic stroke, introduces heterogeneity that could affect the applicability of the findings. The presence of heterogeneity was particularly evident in the examination of the occurrence of thromboembolism, with notable differences observed among the studies. Another limitation arises from the potential for bias in the selection of patients in a few studies because of the open-label design of the trial. Additionally, all the included studies were phase 2 trials with a relatively small sample size. The conclusions are constrained by the small number of participants in some subgroup analyses. Moreover, the omission of studies published or unpublished in languages other than English may have led to a bias in the published research.
Future considerations
Subsequent to this, it is recommended that further extensive phase 3 trials be carried out to authenticate these discoveries and examine the safety and effectiveness of FXI inhibitors in a wider array of patient cohorts and clinical scenarios. This research would also help to clarify the mechanism by which FXI inhibitors produce anticoagulant effects and uncover any potential long-term harmful consequences. Notably, several ongoing phase 3 clinical trials are investigating various FXI inhibitors such as milvexian, asundexian, and abelacimab in different indications including AF, ischemic stroke, and cancer-associated VTE. It is noteworthy that the OCEANIC-AF study, a phase III trial comparing asundexian with apixaban in AF patients, was terminated early due to lack of efficacy. This excerpt underscores the challenges in developing new effective medications and highlights the importance of ongoing trials to confirm the initial findings about FXI inhibitors, their cost-effectiveness, and their potential clinical implications.
Conclusion
Ultimately, this meta-analysis and its subgroup analyses offer a thorough and detailed understanding of the possible role of FXI inhibitors as anticoagulants. In summary, the results suggest that FXI inhibitors have the capacity to be a safe and efficient option, specifically in terms of diminishing the likelihood of thromboembolism without significantly increasing concerns about bleeding. This is particularly promising in certain therapeutic situations, such as TKA, where they demonstrate a reduction in both bleeding and thromboembolic events. Nevertheless, the diversity of trials, differences in results across different patient groups, and comparisons with alternative anticoagulants, such as DOACs and placebo, emphasize the complexity of anticoagulant therapy.
These findings, albeit limited by the constraints of the current evidence, such as small sample numbers in specific subgroup comparisons, suggest the need for careful interpretation. They emphasize the necessity for further investigation via larger, more varied phase 3 clinical studies. Such research would help in better understanding of FXI inhibitors’ role as anticoagulant therapy, advocate for personalized medicine approaches, and determine the optimal patient demographics and clinical situations for their application. This approach will guarantee that the prospective advantages of FXI inhibitors are used effectively and safely in all aspects of anticoagulant treatment.
Abbreviations
- FXI:
-
Factor XI
- AE:
-
Adverse events
- SAE:
-
Serious adverse events
- RR:
-
Risk ratio
- TKA:
-
Total Knee arthroplasty
- AF:
-
Atrial fibrillation
- ESRD:
-
End-stage renal disease
- VTE:
-
Venous thromboembolism
- MI:
-
Myocardial infarction
- DOACs:
-
Direct oral anticoagulants
- VKAs:
-
Vitamin K antagonists
- MR:
-
Mendelian randomization
- RCTs:
-
Randomized clinical trials
- LMWH:
-
Low molecular weight heparin
- BMI:
-
Body mass index
- APTT:
-
Activated partial thromboplastin time
- DVT:
-
Deep vein thrombosis
- PE:
-
Pulmonary embolism
- TIA:
-
Transient ischemic attack
- INR:
-
International normalized ratio
- ISTH:
-
International Society on Thrombosis and Hemostasis
- BARC:
-
Bleeding Academic Research Consortium
References
Piccini JP, Caso V, Connolly SJ, Fox KAA, Oldgren J, Jones WS, Gorog DA, Durdil V, Viethen T, Neumann C, Mundl H, Patel MR (2022) Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): a multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet 399(10333):1383–1390. https://doi.org/10.1016/s0140-6736(22)00456-1
Walsh M, Bethune C, Smyth A, Tyrwhitt J, Jung SW, Yu RZ, Wang Y, Geary RS, Weitz J, Bhanot S (2022) Phase 2 study of the factor XI antisense inhibitor IONIS-FXI(rx) in patients with ESRD. Kidney Int Rep 7(2):200–209. https://doi.org/10.1016/j.ekir.2021.11.011
Lorentz CU, Tucker EI, Verbout NG, Shatzel JJ, Olson SR, Markway BD, Wallisch M, Ralle M, Hinds MT, McCarty OJT, Gailani D, Weitz JI, Gruber A (2021) The contact activation inhibitor AB023 in heparin-free hemodialysis: results of a randomized phase 2 clinical trial. Blood 138(22):2173–2184. https://doi.org/10.1182/blood.2021011725
Verhamme P, Yi BA, Segers A, Salter J, Bloomfield D, Büller HR, Raskob GE, Weitz JI (2021) Abelacimab for Prevention of venous thromboembolism. N Engl J Med 385(7):609–617. https://doi.org/10.1056/NEJMoa2105872
Büller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE, Segers A, Verhamme P, Weitz JI (2015) Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med 372(3):232–240. https://doi.org/10.1056/NEJMoa1405760
Weitz JI, Bauersachs R, Becker B, Berkowitz SD, Freitas MCS, Lassen MR, Metzig C, Raskob GE (2020) Effect of Osocimab in preventing venous thromboembolism among patients undergoing knee arthroplasty: the FOXTROT Randomized Clinical Trial. JAMA 323(2):130–139. https://doi.org/10.1001/jama.2019.20687
Weitz JI, Strony J, Ageno W, Gailani D, Hylek EM, Lassen MR, Mahaffey KW, Notani RS, Roberts R, Segers A, Raskob GE (2021) Milvexian for the Prevention of venous thromboembolism. N Engl J Med 385(23):2161–2172. https://doi.org/10.1056/NEJMoa2113194
Shoamanesh A, Mundl H, Smith EE, Masjuan J, Milanov I, Hirano T, Agafina A, Campbell B, Caso V, Mas JL, Dong Q, Turcani P, Christensen H, Ferro JM, Veltkamp R, Mikulik R, De Marchis GM, Robinson T, Lemmens R, Hart RG (2022) Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet 400(10357):997–1007. https://doi.org/10.1016/s0140-6736(22)01588-4
Sharma M, Molina CA, Toyoda K, Bereczki D, Bangdiwala SI, Kasner SE, Lutsep HL, Tsivgoulis G, Ntaios G, Czlonkowska A, Shuaib A, Amarenco P, Endres M, Yoon BW, Tanne D, Toni D, Yperzeele L, von Weitzel-Mudersbach P, Silva S, Hankey G, G. J (2024) Safety and efficacy of factor XIa inhibition with milvexian for secondary stroke prevention (AXIOMATIC-SSP): a phase 2, international, randomised, double-blind, placebo-controlled, dose-finding trial. Lancet Neurol 23(1):46–59. https://doi.org/10.1016/s1474-4422(23)00403-9
Rao SV, Kirsch B, Bhatt DL, Budaj A, Coppolecchia R, Eikelboom J, James SK, Jones WS, Merkely B, Keller L, Hermanides RS, Campo G, Ferreiro JL, Shibasaki T, Mundl H, Alexander JH (2022) A Multicenter, phase 2, Randomized, Placebo-Controlled, Double-Blind, Parallel-Group, dose-finding trial of the oral factor XIa inhibitor asundexian to prevent adverse Cardiovascular outcomes after Acute myocardial infarction. Circulation 146(16):1196–1206. https://doi.org/10.1161/circulationaha.122.061612
Chen A, Stecker E, B AW (2020) Direct oral anticoagulant use: a practical guide to Common Clinical challenges. J Am Heart Assoc 9(13):e017559. https://doi.org/10.1161/jaha.120.017559
Greco A, Laudani C, Spagnolo M, Agnello F, Faro DC, Finocchiaro S, Legnazzi M, Mauro MS, Mazzone PM, Occhipinti G, Rochira C, Scalia L, Capodanno D (2023) Pharmacology and Clinical Development of factor XI inhibitors. Circulation 147(11):897–913. https://doi.org/10.1161/circulationaha.122.062353
Ali AE, Becker RC (2024) Factor XI: structure, function and therapeutic inhibition. Journal of thrombosis and thrombolysis. https://doi.org/10.1007/s11239-024-02972-5
Hsu C, Hutt E, Bloomfield DM, Gailani D, Weitz JI (2021) Factor XI inhibition to uncouple thrombosis from hemostasis: JACC Review topic of the Week. J Am Coll Cardiol 78(6):625–631. https://doi.org/10.1016/j.jacc.2021.06.010
Salomon O, Steinberg DM, Koren-Morag N, Tanne D, Seligsohn U (2008) Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood 111(8):4113–4117. https://doi.org/10.1182/blood-2007-10-120139
Preis M, Hirsch J, Kotler A, Zoabi A, Stein N, Rennert G, Saliba W (2017) Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood 129(9):1210–1215. https://doi.org/10.1182/blood-2016-09-742262
Salomon O, Steinberg DM, Zucker M, Varon D, Zivelin A, Seligsohn U (2011) Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb Haemost 105(2):269–273. https://doi.org/10.1160/th10-05-0307
Daghlas I, Gill D (2023) Leveraging genetic predictors of factor XI levels to anticipate results from clinical trials. Eur J Neurol 30(7):2112–2116. https://doi.org/10.1111/ene.15820
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Mourad, Ouzzani Hossam, Hammady Zbys, Fedorowicz Ahmed, Elmagarmid (2016) Rayyan—a web and mobile app for systematic reviews Systematic Reviews 5(1) https://doi.org/10.1186/s13643-016-0384-4
Chapter 8 Assessing risk of bias in a randomized trial | Cochrane Training. https://training.cochrane.org/handbook/current/chapter-08
Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G (2012) Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis. Pract Guidelines Chest 141(2 Suppl). 9th edn.https://doi.org/10.1378/chest.11-2292. American College of Chest Physicians Evidence-Based Clinicale44S-e88S
Zirlik A, Bode C (2017) Vitamin K antagonists: relative strengths and weaknesses vs. direct oral anticoagulants for stroke prevention in patients with atrial fibrillation. J Thromb Thrombolysis 43(3):365–379. https://doi.org/10.1007/s11239-016-1446-0
Hinojar R, Jiménez-Natcher JJ, Fernández-Golfín C, Zamorano JL (2015) New oral anticoagulants: a practical guide for physicians. Eur Heart J Cardiovasc Pharmacother 1(2):134–145. https://doi.org/10.1093/ehjcvp/pvv002
Woodruff RS, Sullenger B, Becker RC (2011) The many faces of the contact pathway and their role in thrombosis. J Thromb Thrombolysis 32(1):9–20. https://doi.org/10.1007/s11239-011-0578-5
Meijers JC, Tekelenburg WL, Bouma BN, Bertina RM, Rosendaal FR (2000) High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med 342(10):696–701. https://doi.org/10.1056/nejm200003093421004
Kyrle PA, Eischer L, Šinkovec H, Eichinger S (2019) Factor XI and recurrent venous thrombosis: an observational cohort study. J Thromb Haemost 17(5):782–786. https://doi.org/10.1111/jth.14415
Presume J, Ferreira J, Ribeiras R, Mendes M (2022) Achieving higher efficacy without compromising safety with factor XI inhibitors versus low molecular weight heparin for the prevention of venous thromboembolism in major orthopedic surgery-systematic review and meta-analysis. J Thromb Haemost 20(12):2930–2938. https://doi.org/10.1111/jth.15890
Galli M, Laborante R, Ortega-Paz L, Franchi F, Rollini F, D’Amario D, Capodanno D, Tremoli E, Gibson CM, Mehran R, Angiolillo DJ (2023) Factor XI inhibitors in early clinical trials: a Meta-analysis. Thromb Haemost 123(6):576–584. https://doi.org/10.1055/a-2043-0346
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ali, A.E., Awad, M.K., Ali, K. et al. Factor XI as a new target for prevention of thromboembolism in cardiovascular disease: a meta-analysis of randomized controlled trials. J Thromb Thrombolysis (2024). https://doi.org/10.1007/s11239-024-02986-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s11239-024-02986-z