Abstract
Objective
This study aimed to summarize the clinical outcomes of endovascular treatment in patients with basilar artery occlusion (BAO) with different pathologic mechanisms.
Methods
Two independent reviewers searched PubMed/MEDLINE, Embase and Cochrane Library database up to December 2022, patients with different BAO pathological mechanisms (BAO with in situ atherosclerosis vs. embolism alone without vertebral artery steno-occlusion vs. embolism from tandem vertebral artery steno-occlusion) were collected and analyzed. We calculated the odds ratios (ORs) and 95% confidence intervals (CIs) to assess the associations between clinical outcomes and BAO pathological mechanisms.
Results
A total of 1163 participants from 12 studies were identified. Compared with embolism alone, patients with in situ atherosclerotic BAO had a lower favorable outcome rate (modified Rankin score [mRS] 0–2: 34.5% vs. 41.2%; OR 0.83, 95% CI 0.70–0.98; P = 0.03) and moderate outcome rate (mRS 0–3: 45.8% vs. 55.4%; OR 0.65, 95% CI 0.47–0.90; P = 0.01) at 3 months and a higher risk of mortality (29.9% vs. 27.2%; OR 1.31, 95% CI 0.96–1.79, P = 0.09; adjusted OR 1.46, 95% CI 1.08–1.96). Tandem BAO had a comparable mortality risk to that of in situ atherosclerotic BAO (OR 1.37, 95% CI 0.84–2.22; P = 0.48) or embolism alone (OR 1.44, 95% CI 0.65–3.21; P = 0.43), and there were no significant differences in favorable or moderate outcomes between tandem BAO and each of the other two BAO mechanisms.
Conclusion
Among BAO patients with endovascular treatment, embolism mechanism had better clinical outcomes than in situ atherosclerosis, and atherosclerotic mechanism was associated with a higher mortality at 3 months. RCTs are needed to further confirm clinical outcomes of BAO by different mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute basilar artery occlusion (BAO) is an emergency neurological defect and has a very poor clinical prognosis if timely treatment is not received. More than one-third of BAO patients do not survive, and the remaining patients often present functional dependence [1,2,3]. Recently, two randomized trials—ATTENTION (Endovascular Treatment for Acute Basilar Artery Occlusion) and BAOCHE (Basilar Artery Occlusion Chinese Endovascular Trial)—found that endovascular thrombectomy led to better functional outcomes than best medical treatment but had a higher risk of intracerebral hemorrhage and procedural complications. Moreover, stroke subtype analyses revealed that large-artery atherosclerosis and cardioembolism generally responded best to thrombectomy treatment [4, 5].
We all know that cardiac embolism and artery atherosclerosis are the common causes of infarction in the basilar artery occlusion, atherosclerotic mechanisms leading to stroke include artery-to-artery embolism, in situ thrombosis, hemodynamic impairment, perforator occlusion, and mixed causes. Nevertheless, posterior circulation occlusion and anterior circulation occlusion have inherent differences in vascular anatomy, clinical presentation, and clinical course. Furthermore, an underlying atherosclerotic lesion with/without tandem steno-occlusion is a confounding factor for thrombectomy outcomes. A better understanding of the underlying mechanisms may therefore help clinicians to select eligible patients, plan therapeutic strategies, and improve patient prognosis [6,7,8].
Recently, several studies have investigated the associations between the underlying mechanisms of posterior circulation occlusion, especially BAO, and endovascular treatment in stroke; however, they have reported inconsistent results, and their single-center designs and small sample sizes likely compromised their conclusions [9,10,11,12,13,14]. Here, we conducted a meta-analysis to compare the clinical outcomes of endovascular treatment in patients with BAO with different pathologic mechanisms.
Materials and methods
The research and reporting methods of this review were according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [15].
Search strategy
A comprehensive literature search of PubMed/MEDLINE databases, Cochrane Library database, and Embase was designed and carried out by two independent experienced reviewers with the study procedure. The key words ‘posterior circulation stroke’, ‘basilar artery occlusion’, ‘vertebral artery occlusion’, ‘endovascular thrombectomy’, ‘mechanical thrombectomy’, ‘intra-arterial thrombectomy’, ‘revasculization’, ‘pathologic mechanism’, ‘intracranial atherosclerotic disease’, ‘embolism’, ‘thromboembolism’, ‘cardioembolism’, ‘stroke etiology’ were used in both ‘AND’ and ‘OR’ combinations. The search was limited to articles published from beginning to December 2022 in the English language only. Furthermore, the reference lists of retrieved and recent reviews were also reviewed, we could contact with the authors if necessary.
Study selection
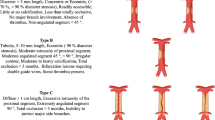
The inclusion criteria were as follows: (1) included a series of > 50 patients; (2) comparison of BAO patients with different pathologic mechanisms, divided into two or three groups (in situ atherosclerotic thrombosis [Group A], embolism alone without vertebral artery [VA] steno-occlusion [Group B], and/or tandem BAO with a proximal VA stenosis [Group C]); and (3) had available data on clinical and periprocedural evaluations and angiographic outcomes. When multiple studies were performed by the same center or authors, we selected the newest or largest sample article in the final analysis. Two reviewers (XJ Shang and LY Pan) independently screened the collected studies on the basis of the predetermined selection criteria, and any disagreements were resolved by discussion or consultation with a third reviewer (ZM Zhou).
Data extraction and management
Two reviewers independently extract the following data for each eligible study: the last name of the first author, years of publication and recruitment, study design and populations, demographic data (age and sex), vascular risk factors, time intervals, intravenous thrombolysis rates, recanalization rates, 3-month clinical outcome and mortality. Recanalization rate was defined as a modified Thrombolysis in Myocardial Infarction (mTIMI) score of 2b or 3. Clinical outcome data included number of mortality and the modified Rankin Scale (mRS range: 0 [no symptoms] to 6 [death]) at 3 months. We resolved disagreements by consensus.
Primary outcomes were the rate of favorable clinical outcome (mRS: 0–2) and moderate outcome (mRS: 0–3) at 3 months. Secondary outcome measures mainly included 3-month mortality.
Quality assessment in included studies
Quality assessment of case-control or cohort studies was according to the Newcastle-Ottawa scale. This scale assigns a maximum score of 4, 2 and 3 for subject selection, comparability, and exposure, respectively. A score of 9 is the best and reflects the highest quality. Independent reviewers evaluated the quality of the included study with the Newcastle-Ottawa scale for studies between groups. The third reviewer was consulted to address the discrepancies.
Statistical analysis
This study calculated a pooled odds ratio (OR) and 95% confidence interval (CI) of clinical outcomes or mortality between different groups treated with endovascular treatment, and using RevMan software (Version 5.4, The Cochrane Collaboration, Oxford, United Kingdom). We chose P < 0.05 as the level of significance. We used forest plots to determine whether there was a statistical association between the groups and to assess the heterogeneity of the collected studies. Heterogeneity was quantified using the I2 statistic, which yielded a range from 0 to 100%. We considered a value greater than 50% as representing substantial heterogeneity [16], if heterogeneity existed, the random effects model was used; otherwise, the fixed model was used. The risk of publication bias was assessed by visual inspection of the funnel plots of included studies, and the Egger weighted linear regression test with the Comprehensive Meta-Analysis software (Version 3). If Egger’s test shows significant difference suggesting small study bias existing, we would conduct a non-parametric analysis of ‘trim and fill’, which yields an effect adjusted for funnel plot asymmetry [17].
Results
Identification of eligible studies
Of the 29 articles identified initially, 12 studies—which included a total of 1163 participants—with available outcome data that met the inclusion criteria were collected (Fig. 1). Totally, there were 442 (38.0%) participants in Group A, 590 (50.7%) participants in Group B, and 131 (11.3%) participants in Group C.
Characteristics of included studies
The details of the study design and the available data were summarized in the characteristics of the included studies (Table 1). Eight studies were performed in Asian population, four in South Korea [18,19,20,21], three in Chinese [11,12,13] and one in Japanese population [9], the remaining each in Switzerland [22], American [23], Dutch [10] and French [14] population. All studies were designed as case-control studies, six of the studies were designed to compare in situ atherosclerotic thrombosis with embolism alone in BAO patients [9, 10, 19,20,21, 23], of note, the participants with embolism alone mechanism had those with tandem intracranial or extracranial VA lesion; and the remaining 6 studies were carried out to compare clinical outcomes and mortality between the three predisposed BAO groups [11,12,13,14, 18, 22]. All cases were defined as in situ atherosclerosis or embolism mechanism with/without tandem VA stenosis by radiographic manifestations and revascularization procedure. One study initially excluded patients with tandem lesion, and patients with atherosclerotic or embolic disease were defined by the revascularization procedure [23]. Another two study classified overall participants as large artery atherosclerosis, cardioembolism, dissection, and embolic stroke of undetermined source according to the TOAST criteria, we combined cardioembolism and embolic stroke of undetermined source as single embolic BAO in the pooled analysis [10, 14]. Additionally, six studies collected participants who received endovascular treatment during the last ten years, the other six studies recruited more earlier treated patients.
Quality of included studies
The quality assessment of 12 published studies is shown in online supplemental Tables 1, two studies were rated at 5 stars (low quality), five studies at 6 stars (moderate quality), and five studies at 7 stars (high quality). The most common selection bias was the recruitment of participants from hospitalization. There was inadequate comparability between the studied groups of included studies on the basis of different design. Ascertainment of exposure was the most exposure bias in the studies.
Comparison 1: in situ atherosclerotic BAO ( group A) vs. embolism alone BAO(group B)
Figure 2 shows the estimated pooled OR of mRS 0–2 of group A compared with group B after endovascular treatment. Because there was no significant heterogeneity (I2 = 47%), a fixed effects model was used. The pooled OR of the available data from 11 studies was 0.83 (95% CI: 0.70–0.98, P = 0.03), and atherosclerotic BAO patients had a 34.5% (137/397) favorable outcome compared with 41.2% (226/548) for embolic causes. The funnel plot was symmetrical, indicating no publication bias existed, the Egger linear regression also detected no evidence of publication bias among the studies of pathologic mechanisms and favorable outcome benefits (Egger t = 0.038, P = 0.97).
The pooled OR of mRS 0–3 in Group A compared with Group B after endovascular treatment is shown in Fig. 3. Data from seven included studies were available, and the pooled OR was 0.65 (95% CI: 0.47–0.90, P = 0.01), and atherosclerotic BAO patients had a 45.8% (149/325) moderate outcome compared with 55.4% (177/319) for embolic causes. The analysis had no heterogeneity (I2 = 42%) and no publication bias (Egger t = 0.029, P = 0.98).
The pooled OR of mortality in Group A (29.9%) compared with Group B (27.2%) at 3-month follow-up was 1.31 (95% CI: 0.96–1.79, P = 0.09), and there was no significant heterogeneity between them (I2 = 5%, Fig. 4). However, the funnel plot was unsymmetrical, suggesting that there was publication bias, and Egger linear regression also detected publication bias among the eleven included studies (Egger t = 2.238, P = 0.05). Then we found 3 possible missing studies with the ‘trim and fill’ method, the adjusted OR 1.46 (95% CI: 1.08–1.96) including the 3 missing studies was significantly different from our estimated pooled OR value.
Comparison 2: tandem BAO with a proximal VA stenosis (group C) vs. embolism alone BAO (group B)
The estimated pooled OR (95%CI) of mRS0-2, mRS0-3, and mortality of Group C compared with Group B were 0.87(0.38–1.98), 1.07(0.25–4.55) and 1.44(0.65–3.21), respectively. Due to the significant heterogeneity (I2 > 50%), all three pooled analyses used the random-effect model. There were no publication biases for mRS0-2, mRS0-3 and mortality (Figs. 1, 2 and 3 in supplemental materials).
Comparison 3: tandem BAO with a proximal VA stenosis (group C) vs. in situ atherosclerotic BAO (group A)
The estimated pooled OR (95%CI) of mRS0-2, mRS0-3, and mortality of Group C compared with Group A were 1.11(0.67–1.84), 1.08(0.28–4.22) and 1.37(0.84–2.22), respectively. Due to the significant heterogeneity (I2 > 50%), the pooled analyses of mRS0-3 used the random-effect model. There were significant differences in publication biases, analysis of mRS0-2 (Egger t = 3.59, P = 0.04), when including the two missing studies by the ‘trim and fill’ method, their adjusted OR and 95% CI were changed to 1.11(0.69–1.76) (Figs. 4, 5 and 6 in supplemental materials).
Discussion
Endovascular treatment for BAO patients is better than standard medical therapy, but increasing the risk of bleeding and procedural complications. Considering the differences in the etiology and mechanism of BAO, current studies have failed to further explicit the outcome differences of intravascular treatment for different mechanisms of BAO, especially atherosclerosis and embolism. The present meta-analysis first found that in situ atherosclerotic BAO resulted in poorer clinical outcomes than embolism alone, with lower favorable and moderate outcome rates, and a higher mortality rate.
Most of the studies explored the associations between atherosclerotic and embolic mechanisms and clinical outcomes after thrombectomy. The intravenous thrombolysis rates ranged from 18.2 to 47.1% and successful reperfusion rates ranged from 78.0 to 99.0% (excluding one study with no reported data). All but one [22] study found that patients with atherosclerosis had poorer functional independence compared with those with an embolic mechanism at 3 months after thrombectomy. Furthermore, patients with tandem occlusion had the worst outcomes of the three groups despite the existence of variable conclusions between studies and having the smallest number of patients. However, there were substantial differences in mortality comparisons after treatment in all included studies; the inconsistent conclusions need to be further clarified in a meta-analysis.
There is evidence that in people with BAO with an in situ atherosclerotic mechanism, endovascular treatment is associated with a higher risk of death and/or unfavorable outcomes than in patients with embolic lesion [10, 18, 21]. There are many possible causes of this excess risk associated with in situ atherosclerotic thrombosis. First, there was a significant interaction between procedural length and the effects of treatment on 3-month outcomes among the published study data [14, 18, 21]. Atherosclerotic occlusion required a longer procedural time than embolism alone because it generally received more multimodal endovascular treatments—such as balloon angioplasty/permanent stenting and intra-artery thrombolysis—when existing remnant stenosis (≥ 70%) and stenotic lesions were repeatedly occluded or the flow was not rescued after thrombectomy treatment. Second, embolism alone was associated with distal BAO, whereas in situ atherosclerotic BAO always had proximal and middle occlusion [10, 11]; this can be associated with extensive pons ischemia and lead to more severe conditions (such as locked-in syndrome). Third, in situ atherosclerotic BAO may have a lower recanalization rate than embolic BAO; recanalization is the most important prognostic factor and improves survival [18]. Finally, a higher number of passes during endovascular procedures in the atherosclerotic group may be associated with a higher risk of basilar artery reocclusion by inducing damage of the vessel wall and promoting local thrombosis, thus resulting in worse outcomes [11, 14, 24].
BAO combined with extracranial or intracranial vertebral artery stenosis—also known as a tandem BAO lesion—is a relatively less common mechanism of vertebral BAO [25, 26]. Higher thrombus load, challenging anatomical conditions, and related prolongation of procedural time were all associated with tandem BAO [12, 13, 18], ultimately leading to worse clinical outcomes. However, several studies have also reported that tandem BAO results in better outcomes than other pathologic mechanisms; this depends on minimizing the time to recanalization using retrograde techniques and reducing the risk of reocclusion in tandem lesions [22]. In the present meta-analysis, compared with atherosclerotic and embolic mechanisms, tandem BAO lesion had no differences in clinical outcomes or mortality. These findings indicate that the use of modern thrombectomy devices and advanced therapeutic strategies may achieve a better outcome.
This study had the following limitations. Although we included 12 eligible published studies, most were retrospective analysis in design, which carries a risk of selection bias for a meta-analysis. Furthermore, the pooled participant sample remained limited in size, especially for the tandem BAO group, possibly because some studies included these patients in the embolism alone stroke group. Moreover, the classification of pathologic mechanisms was not unified in the included studies. Atherosclerotic lesion is the most common cause of ischemic stroke in Asian populations but not Western populations; thus, the present conclusions cannot be generalized. Differences in endovascular strategies, first-line contact aspiration or stent-retriever thrombectomy, and even complex angioplasty procedures and intra-arterial tirofiban infusion among different studies also complicate the interpretation of the study outcomes. Furthermore, the recruitment period of study patients was wide (2010–2020) and likely led to differences in thrombectomy devices and surgeons’ experiences, which may also bias the pooled results.
This study was to conduct a meta-analysis on the thrombectomy outcomes of BAO patients with different mechanisms. Patients with atherosclerotic mechanism faced a higher risk of disability and short-term death, which had a suggestive significance for patients’ treatment selection and clinical conversation in the future. Specific treatment measures would be taken to reduce the risk of disability and death for patients with atherosclerotic mechanism.
Conclusion
Clinical outcomes in patients with BAO treated with endovascular strategies were significantly different among patients with different pathologic mechanisms. Embolic BAO without tandem VA steno-occlusion had better functional outcomes and safety than BAO with in situ atherosclerosis. Randomized studies with larger samples and comparative studies with tailored therapeutic strategies between in situ atherosclerotic and embolic BAO are necessary.
References
Singer OC, Berkefeld J, Nolte CH et al (2015) Mechanical recanalization in basilar artery occlusion: the ENDOSTROKE study. Ann Neurol 77:415–424. https://doi.org/10.1002/ana.24336
Schonewille WJ, Wijman CA, Michel P et al (2009) Treatment and outcomes of acute basilar artery occlusion in the basilar artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol 8:724–730. https://doi.org/10.1016/S1474-4422(09)70173-5
Mattle HP, Arnold M, Lindsberg PJ, Schonewille WJ, Schroth G (2011) Basilar artery occlusion. Lancet Neurol 10:1002–1014. https://doi.org/10.1016/S1474-4422(11)70229-0
Jovin TG, Li C, Wu L et al (2022) Trial of Thrombectomy 6 to 24 hours after Stroke due to basilar-artery occlusion. N Engl J Med 387:1373–1384. https://doi.org/10.1056/NEJMoa2207576
Tao C, Nogueira RG, Zhu Y et al (2022) Trial of Endovascular Treatment of Acute basilar-artery occlusion. N Engl J Med 387:1361–1372. https://doi.org/10.1056/NEJMoa2206317
Weber R, Minnerup J, Nordmeyer H et al (2019) Thrombectomy in posterior circulation stroke: differences in procedures and outcome compared to anterior circulation stroke in the prospective multicentre REVASK registry. Eur J Neurol 26:299–305. https://doi.org/10.1111/ene.13809
Baik SH, Jung C, Kim BM, Kim DJ (2020) Mechanical thrombectomy for Tandem Vertebrobasilar Stroke: characteristics and treatment outcome. Stroke 51:1883–1885. https://doi.org/10.1161/STROKEAHA.120.029503
Sun X, Zhang J, Tong X et al (2022) A comparison between acute large vessel occlusion in the posterior circulation and anterior circulation after endovascular treatment: the ANGEL-ACT registry experience. Stroke Vasc Neurol:svn-2021-001093. https://doi.org/10.1136/svn-2021-001093
Katsumata M, Ota T, Tsuruta W et al (2021) Comparisons of characteristics and outcomes after mechanical thrombectomy for vertebrobasilar occlusion with cardioembolism or atherosclerotic brain infarction: data from the Tokyo-Tama-Registry of Acute Endovascular Thrombectomy (TREAT). World Neurosurg 148:e680–e88. https://doi.org/10.1016/j.wneu.2021.01.071
Pirson F, Boodt N, Brouwer J et al (2022) Etiology of large vessel occlusion posterior circulation stroke: results of the MR CLEAN Registry. Stroke 53:2468–2477. https://doi.org/10.1161/STROKEAHA.121.038054
Yang W, Zhang L, Li Z et al (2021) Endovascular treatment for Acute Basilar artery occlusion: a comparison of arteriosclerotic, embolic and Tandem Lesions. Cardiovasc Intervent Radiol 44:1954–1963. https://doi.org/10.1007/s00270-021-02994-z
Jiang L, Yang JH, Ruan J et al (2021) A single-center experience of endovascular treatment in subtypes of basilar artery occlusion: embolization caused by Tandem Vertebral Artery Stenosis May be Associated with Better Outcomes. World Neurosurg 151:e918–e26. https://doi.org/10.1016/j.wneu.2021.05.011
Sun X, Raynald, Tong X et al (2021) Analysis of treatment Outcome after Endovascular Treatment in different pathological subtypes of basilar artery occlusion: a single Center experience. Transl Stroke Res 12:230–238. https://doi.org/10.1007/s12975-020-00833-w
Abdelrady M, Derraz I, Dargazanli C et al (2022) Outcomes following mechanical thrombectomy in different etiological subtypes of Acute Basilar artery occlusion: Stroke etiology and outcome after EVT in BAO. Clin Neuroradiol Online ahead of print. https://doi.org/10.1007/s00062-022-01217-3
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. https://doi.org/10.1371/journal.pmed.1000097
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. https://doi.org/10.1002/sim.1186
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455 – 63. https://doi.org/10.1111/j.0006-341x.2000.00455.x
Baik SH, Park HJ, Kim JH et al (2019) Mechanical thrombectomy in subtypes of basilar artery occlusion: relationship to recanalization rate and clinical outcome. Radiology 291:730–737. https://doi.org/10.1148/radiol.2019181924
Baek JH, Kim BM, Heo JH et al (2019) Endovascular and clinical outcomes of Vertebrobasilar Intracranial atherosclerosis-related large vessel occlusion. Front Neurol 10:215. https://doi.org/10.3389/fneur.2019.00215
Lee YY, Yoon W, Kim SK et al (2017) Acute Basilar artery occlusion: differences in characteristics and outcomes after endovascular therapy between patients with and without underlying severe atherosclerotic stenosis. AJNR Am J Neuroradiol 38:1600–1604. https://doi.org/10.3174/ajnr.A5233
Kim YW, Hong JM, Park DG et al (2016) Effect of intracranial atherosclerotic disease on endovascular treatment for patients with Acute Vertebrobasilar occlusion. AJNR Am J Neuroradiol 37:2072–2078. https://doi.org/10.3174/ajnr.A4844
Piechowiak EI, Kaesmacher J, Zibold F et al (2020) Endovascular treatment of tandem occlusions in vertebrobasilar stroke: technical aspects and outcome compared with isolated basilar artery occlusion. J Neurointerv Surg 12:25–29. https://doi.org/10.1136/neurintsurg-2019-014825
Sefcik RK, Tonetti DA, Desai SM et al (2021) Does stroke etiology influence outcome in the posterior circulation? An analysis of 107 consecutive acute basilar occlusion thrombectomies. Neurosurg Focus 51:E7. https://doi.org/10.3171/2021.4.FOCUS2189
Marto JP, Strambo D, Hajdu SD et al (2019) Twenty-four-hour reocclusion after successful mechanical thrombectomy: Associated factors and long-term prognosis. Stroke 50:2960–2963. https://doi.org/10.1161/STROKEAHA.119.026228
Caplan LR, Wityk RJ, Glass TA et al (2004) New England Medical Center posterior circulation registry. Ann Neurol 56:389–398. https://doi.org/10.1002/ana.20204
Cohen JE, Leker RR, Gomori JM et al (2016) Emergent revascularization of acute tandem vertebrobasilar occlusions: endovascular approaches and technical considerations-confirming the role of vertebral artery ostium stenosis as a cause of vertebrobasilar stroke. J Clin Neurosci 34:70–76. https://doi.org/10.1016/j.jocn.2016.05.005
Funding
This work was supported by 2022jc73 of Wuhu Municipal Science and Technology Bureau, Anhui Province, China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shang, X., Pan, L., Xu, Y. et al. Effect of endovascular treatment on patients with basilar artery occlusion presenting with different pathologic mechanisms: a systematic review and meta-analysis. J Thromb Thrombolysis 57, 124–131 (2024). https://doi.org/10.1007/s11239-023-02884-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-023-02884-w