Abstract
The efficacy and safety of direct oral anticoagulants (DOACs) versus low-molecular-weight heparin (LMWH) are still debated in the treatment of patients with cancer, and the optimal duration of therapy remains uncertain. Electronic databases (PubMed, Embase, and Cochrane Library) were searched to retrieve studies on the efficacy and safety of DOACs versus LMWH in treating patients with cancer from January 1980 to October 2018. The primary efficacy and safety endpoints were recurrent venous thromboembolism (VTE) and major bleeding. Our study included two randomized controlled trials (RCTs) and nine observational studies, together comprising 4509 patients with cancer. The pooled estimates indicated that DOACs led to a modest reduction recurrent VTE in the RCTs [RR: 0.63, 95% confidence interval (CI), 0.42–0.96, P = 0.03] and in the observational studies (RR: 0.74, 95% CI, 0.58–0.93, P = 0.011), without increasing the risk of major bleeding for observational studies (P = 0.805), but increased for RCTs (P = 0.017). The same trends were observed in the rivaroxaban subgroup. Moreover, subgroup analyses according to the treatment duration indicated that DOACs significantly reduced the incidence of recurrent VTE (P = 0.006 at 6 months; P < 0.001 at 12 months) without significant differences in major bleeding compared with LMWH at 6 or 12 months. Patients with cancer who received DOACs exhibited a significant reduction in recurrent VTE with no increased risk of major bleeding compared with LMWH. DOACs may be an alternative choice for long-term anticoagulant therapy in patients with cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Patients with cancer who received DOACs, particularly rivaroxaban, had significantly reduced risk of recurrent VTE with no significant effect on the risk of major bleeding, compared with those who received LMWH.
-
DOACs can be used as an alternative strategy for longer treatment duration (6 or 12 months) of cancer-associated VTE.
-
In future, it is necessary to need larger, well-designed RCT and real-world studies to assess the efficacy and safety of DOACs in patients with cancer, especially concerned with the issue of drug–drug interactions between DOACs and chemotherapeutic agents.
Introduction
Venous thromboembolism (VTE) is a common complication in patients with cancer, occurring in 20–30% of patients [1, 2]. In past years, international guidelines recommended Low-molecular-weight heparin (LMWH) for 3 to 6 months as the first-line treatment for cancer-associated VTE [3,4,5,6]. However, subcutaneous injection of LMWH results in poor adherence for patients who require a longer duration of anticoagulant treatment [7, 8]. A large retrospective analysis showed fewer patients persist with injectable anticoagulants than oral anticoagulants due to concerns of cost and self-injection [9]. Only 13% of patients remained on injectables at 6 months [10]. Therefore, the guidelines for antithrombotic therapy in patients with cancer who required long-term anticoagulation are still poorly followed in clinical practice. Reluctance to impose daily injections is one of the important reasons for poor adherence to guidelines in clinical practice [11].
In recent years, direct oral anticoagulants (DOACs) such as apixaban, edoxaban, rivaroxaban, and dabigatran, with the advantages of oral administration and no laboratory monitoring, have been widely used in the treatment of VTE in patients without cancer. The NCCN guideline has updated that edoxaban (level 1) and rivaroxaban (level 2A) are considered preferred for treatment of cancer-associated thrombosis (CAT) [12]. International Society on Thrombosis and Haemostasis (ISTH) also recommended DOACs are an acceptable alternative to LMWH for treatment of CAT in patients with a low risk of bleeding [13]. However, the guidelines suggested DOACs for the treatment of cancer associated VTE based on data from two randomized controlled trials (RCTs) [14, 15] and limited subgroup analyses of patients with cancer from landmark RCTs [16,17,18,19]. The efficacy and safety of DOACs are still debated in the treatment of patients with cancer. Prior meta-analyses studies that compared DOACs with LMWH followed by warfarin showed DOACs seem to be as effective and safe as conventional treatment for the prevention of cancer-associated VTE [20,21,22,23,24]. However, evidence comparing DOACs to LMWH, to date, remains limited. The published meta-analyses of head-to-head comparisons between DOACs and LMWH seem to suggest DOACs was better safety and efficacy than LMWH [25,26,27]. Moreover, two studies have been published in the 2 years since the meta- analyses were published. Streiff et al. showed that DOAC had significantly lower risk of recurrent VTE without increasing the risk of bleeding compared with LMWH [28]. However, the study for Simmons et al. indicated that while DOAC appears to offer a reasonably effective therapy, bleeding complications may be higher compared to LMWH [29]. So we included the two recently published observational studies and updated it. In addition, patients with cancer-associated VTE are at a high risk of becoming thrombosis, and prolonging the duration of anticoagulant therapy could reduce the incidence of recurrent VTE, albeit simultaneously increasing the risk of bleeding. The specific meta-analyses on the duration of DOACs in patients with cancer are limited. The optimal duration of anticoagulant therapy for DOACs and LMWH remains uncertain. Assessment of the optimum duration for anticoagulant therapy has been mainly concerned with balancing the incidence of ischemic complications (such as recurrent VTE) with bleeding complications. Therefore, we performed a new meta-analysis to investigate (1) whether DOACs has the same efficacy and safety as LMWH and (2) the optimal duration of therapy for DOACs and LMWH in patients with cancer.
Methods
Data sources, search strategy, and selection criteria
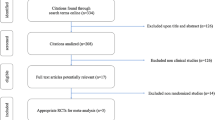
We conducted and reported this systematic review and meta-analysis in accordance with the Providing Innovative Service Models and Assessment criteria (PRISMA) and the Cochrane Handbook. To identify all the eligible studies of DOACs versus LMWH in patients with cancer, we performed a systematic search, without language restrictions, on PubMed, Embase, and Cochrane Library from January 1980 to October 2018. The following keywords were used as search terms: (“Oral Factor Xa Inhibitor” OR “direct oral anticoagulants” OR “rivaroxaban” OR “Dabigatran” OR “Apixaban” OR “edoxaban”) AND (“Low Molecular Weight Heparin” OR “dalteparin”) AND (“Cancer” OR “Tumor”) AND (“Venous Thromboembolism” OR “VTE”) (Fig. 1 for the search strategy). We also performed a manual search of the reference lists of studies, reviews, and pertinent meta-analyses on this topic.
The literature search was independently performed by two authors (Y.D. and Y.W.) using a standardized approach. Any disagreements between the two authors were settled by the primary author (R.L.M) until a consensus was reached. The studies included fulfilled the following inclusion criteria: (1) studies were case–control or cohort studies or RCTs; (2) studies compared DOACs with LMWH; (3) risk estimates and 95% confidence intervals (CIs) were reported, and the VTE recurrences and/or bleeding outcomes required to calculate them were available; (4) patients with cancer were enrolled in the studies; and (5) studies included outcomes measured in a follow-up period of ≥ 1 month. The primary efficacy and safety endpoints were recurrent VTE and major bleeding (defined according to the studies concerned), respectively. The studies that met the following criteria were excluded: (1) repeated publication; (2) incomplete original data or relevant data cannot be obtained by contacting authors; and (3) basic science studies, review, or case reports.
Data extraction and quality assessment
Independent data selection, extraction, and evaluation by the two researchers (Y.D. and Y.W.) were designed in accordance with the inclusion and exclusion criteria. The following details were recorded from each study: general data (study design, year of publication), population characteristics (number, mean age, sex, country), and treatment (therapeutic indication, type of drug, does, duration) (Table 1). The Newcastle–Ottawa Scale (NOS) for observational studies was used to assess the methodological quality of the included studies [30]. The quality of the included RCTs was assessed using the Cochrane risk of bias assessment [31].
Outcomes assessed
The primary analysis focused on assessing VTE recurrence and major bleeding in patients with cancer who received LMWH or treatment with DOACs. Taking into account the effect of the potential heterogeneity, we conducted some subgroup analyses for different factors, such as study design, drug and the follow-up duration. Firstly, we performed subgroup analyses based on study design (cohort study or RCT). Moreover, a subgroup analysis of rivaroxaban in patients with cancer was performed. Finally, we examined the relationship between the duration of DOACs and the risk of the endpoints. LMWH for 3–6 months was considered as the preferred option for the treatment of cancer-associated VTE with a high-grade recommendation [12, 32, 33]. However, American society of clinical oncology (ASCO) and French guidelines suggested that LMWH should be extended beyond 6 months as long as cancer is active and the risk of VTE recurrence persists [34, 35]. Simultaneously, DOACs have been recommended for the treatment of VTE in cancer patients in recent years [12, 13, 33], but the duration of treatment is different (Table 2). In our study, through a systematic screening of the literature, we found that this study focused on short-term (3 months), mid-term (6 months) and long-term (12 months) interventions for anticoagulant therapy, without reporting 6–12 months or beyond 12 months interventions. So a subgroup meta-analysis was performed according to the duration of treatment with LMWH and DOACs. We explored efficacy and safety of LMWH and DOACs in short-term (3 months), mid-term (6 months) and long-term (12 months) anticoagulation durations, respectively.
Statistical analysis
In the presence of heterogeneity, we used a random-effects model because its assumptions account for the presence of variability among studies. The Q test and I2 statistic were used to investigate heterogeneity among the studies [36]; a P value of < 0.05 for the Q test was considered indicative of significant heterogeneity [37]. The adjusted effect estimates of odds ratio, RR, and hazard risk between DOACs and LMWH were extracted. The reported event frequencies were used to calculate RRs with 95% CI in each study. The endpoint outcomes were relatively uncommon and the odds ratios in the case–control studies were close to 1; hence, the odds ratios were considered approximations of RR [38]. We calculated the absolute risk reduction (ARR), 95% CI, and number needed to treat (NNT) of the endpoint events. In addition, we performed a sensitivity analysis by removing each individual study from the meta-analysis and used qualitative Egger’s [39] or Begg’s [40] test to check for potential publication bias. All the reported P-values are two sided, and a P-value < 0.05 was considered statistically significant. STATA 12.0 software (StataCorp LP, College Station, TX) was used to perform statistical analysis. Our study was registered with PROSPERO, number CRD42019122535.
Results
Literature search
We identified 208 potentially eligible articles in our initial electronic search. In total, 38 duplicate articles were eliminated, and 151 irrelevant citations were excluded by reading the abstract. A total of 19 studies were evaluated in detail. Finally, 11 studies [14, 15, 28, 29, 41,42,43,44,45,46,47] were included in our meta-analysis after excluding eight studies (two studies compared between oral anticoagulant and parenteral anticoagulant in patients with cancer [48, 49], one study compared between LMWH and DOACs in patients with cancer-related stroke [50], one study compared the treatment duration between injectable and oral anticoagulants [51], four studies compared between LMWH for treatment with followed by VKAs and DOACs in patients with cancer [16,17,18,19]). A manual search did not identify any new eligible studies. Figure 2 shows the flowchart of the studies.
Study characteristics
A total of 4509 patients from 11 studies were included (1868 patients who received DOAC and 2641 patients who received LMWH). Two RCTs met the inclusion criteria (one on edoxaban [14], one on rivaroxaban [15]), and nine observational studies were included (six on rivaroxaban, three on other DOAC) [28, 29, 41,42,43,44,45,46,47]. Among the included studies, 1 study was performed in multi-country [14], 1 study in Britain [15], 1 study in Brazil [47] and 8 studies in America [28, 29, 41,42,43,44,45,46]. The methodological quality of the RCTs and cohort studies was assessed using the Cochrane risk of bias assessment (Table S1) and NOS (Table S2), respectively. The mean score of the RCTs included in the analysis was 6.5. Of the cohort studies, six studies were of high quality (NOS ≥ 7) [28, 29, 41, 42, 45, 46], whereas three studies were of low quality (NOS ≤ 6) [43, 44, 47] (Table S1). The mean score of the nine observational studies was 6.9. The follow-up period of the studies was > 1 month. The main characteristics of the studies are shown in Table 1, including previous pulmonary embolism (PE), DVT, duration of follow-up, type of cancer, and methodological quality assessment scores.
Global analysis of DOACs versus LMWH in patients with cancer
The global analysis included all studies. DOACs decreased VTE recurrence by 21% from 11.45% to 9.01% (pooled RR: 0.72, 95% CI, 0.60–0.85, P < 0.001; ARR: 2.44%, 95%CI, 0.62%–4.26%, NNT = 41, I2 = 0%) compared with LMWH. Similarly, RCTs subgroup (pooled RR: 0.63, 95% CI, 0.42–0.96, P = 0.03, I2 = 13.2%) and observational studies subgroup (pooled RR: 0.74, 95% CI, 0.58–0.93, P = 0.011, I2 = 3.3%) indicated that DOACs reduced the risk of VTE recurrence compared with LMWH.
Safety analysis showed that there was no significant difference in major bleeding for global analysis (pooled RR: 1.21, 95% CI, 0.94–1.55, P = 0.143; ARR: 1.36%, 95%CI, 0%–2.72%, NNT = 74, I2 = 0%) and observational studies subgroup (pooled RR: 1.04, 95% CI, 0.77–1.39, P = 0.789, I2 = 0%). However, the result of RCTs subgroup showed that DOACs increased in major bleeding (pooled RR: 1.78, 95% CI, 1.11-2.87, P = 0.017, I2 = 0%) compared with LMWH. Figure 3 shows the risk of recurrent VTE and major bleeding in patients with cancer receiving DOACs and LMWH.
No significant heterogeneity was observed in the evaluated endpoints (P ≥ 0.28). When we sequentially omitted each study from the analysis, the results were not affected. All the results were confirmed by the fixed-effects model.
Rivaroxaban versus LMWH in patients with cancer
In this analysis, 3746 patients with cancer were included from seven studies [15, 28, 29, 41,42,43, 47]. Of these, one study was RCT [15] and six studies were observational studies [28, 29, 41,42,43, 47]. The results showed that rivaroxaban caused a significant reduction in VTE recurrence (pooled RR: 0.74, 95% CI, 0.60–0.91, P = 0.005, I2 = 1.4%). Compared with LMWH, rivaroxaban was associated with a nonsignificant reduction in major bleeding (pooled RR: 1.08, 95% CI, 0.80–1.46, P = 0.615, I2 = 0%). Figure 4 shows the risk of recurrent VTE and major bleeding in patients with cancer receiving rivaroxaban and LMWH.
No significant heterogeneity was observed in the evaluated endpoints (P ≥ 0.41). Sensitivity analysis showed that the result was not affected after excluding each study. All results were confirmed by the fixed-effects model.
Effect of duration of DOACs versus LMWH in patients with cancer
Considering the effect of the anticoagulation duration, we conducted a subgroup analysis for different periods. It was found that the studies focused on intervention durations of 3 [29, 42, 46], 6 [14, 15, 28, 41,42,43,44, 46, 47], and 12 [14, 28, 29, 44,45,46] months for anticoagulation treatment.
The 3-month subgroup analysis included three studies [29, 42, 46] with overall 623 patients (n = 253, DOACs group; n = 370, LMWH group). There was no difference in VTE recurrence (P = 0.056, I2 = 0%) or major bleeding (P = 0.751, I2 = 0%). However, for the 6-month [14, 15, 28, 41,42,43,44, 46, 47] and 12-month [14, 28, 29, 44,45,46] subgroup analyses, the DOACs group showed a moderate reduction in VTE recurrence at 6 months (pooled RR: 0.74, 95% CI, 0.60–0.92, P = 0.006, I2 = 2.1%) and a significant reduction in VTE recurrence at 12 months (pooled RR: 0.72, 95% CI, 0.59–0.86, P<0.001, I2 = 0%). There was no significant effect on the risk of major bleeding in the 6-month subgroup (P = 0.200, I2 = 0%) or 12-month subgroup (P = 0.067, I2 = 0%) compared with treatment with LMWH. Figure 5 shows the risk of recurrent VTE and major bleeding in patients with cancer receiving DOACs and LMWH according to different duration.
All the results were confirmed using the fixed-effects model. No heterogeneity was observed in the analysis of each result (all P > 0.38). Moreover, when we sequentially excluded each study from all the pooled analyses, the results were not affected.
Publication bias
Visual inspection of the funnel plots did not show any evidence of obvious asymmetry for VTE recurrence or major bleeding in the DOACs versus LMWH group or the rivaroxaban versus LMWH group. Egger’s and Begg’s tests revealed no significant publication bias for study outcomes for DOACs versus LMWH (VTE recurrence Egger’s test P = 0.306, Begg’s test P = 0.102; major bleeding Egger’s test P = 0.896, Begg’s test P = 0.243) or for rivaroxaban versus LMWH (VTE recurrence Egger’s test P = 0.818, Begg’s test P = 0.453; major bleeding Egger’s test P = 0.936, Begg’s test P = 0.293) (Fig. 6).
Discussion
In this comprehensive meta-analysis of two RCTs and nine observational studies, we compared the efficacy and safety of DOACs and LMWH for treating cancer-associated VTE in 4509 patients. Our results indicated that DOACs, particularly rivaroxaban, were associated with a significantly lower risk of recurrent VTE and no increased risk of major bleeding in patients with cancer than LMWH. Moreover, the administration of DOACs provided significant reductions in recurrent VTE than LMWH, with no differences in the risk of major bleeding in patients with cancer for the 6-month and 12-month subgroups.
Currently, some guidelines recommend LMWH as the first-line treatment of cancer-associated VTE [3,4,5]. LMWH have several advantages over VKA, including fewer drug–drug interactions with chemotherapeutic agents, predictable dose response, no need for therapeutic drug monitoring, and shorter half-life allowing a greater flexibility during periprocedural management. Similarly, DOACs offer all of these advantages, with the addition of an oral route to preclude injections [20]. Data on the efficacy and safety of DOACs compared with LMWH in patients with cancer was based on the two direct head-to-head RCTs. The SELECT-D trial, which was a randomized, open-label, multicenter pilot trial, compared rivaroxaban with dalteparin to assess their efficacy in the treatment of cancer-associated VTE [15]. The results showed that rivaroxaban reduced the rate of recurrent VTE but increased the risk of bleeding. The Hokusai VTE Cancer trial enrolled 1050 cancer patient with VTE to receive edoxaban or dalteparin for 6–12 months. Edoxaban (7.9%) was lower rate of recurrent venous thromboembolism than dalteparin (11.3%) but with higher rate of major bleeding than dalteparin (14.6% vs. 11.1%) [14]. Both trials reported the higher rates of gastrointestinal bleeding. The two RCTs are included in our global analysis. Our meta-analysis for RCTs subgroup indicated that DOACs decreased recurrent VTE (P = 0.03) but increased the risk of major bleeding (P = 0.017). The increased bleeding may be attributed to enrolling a high proportion of patients with gastrointestinal cancer [43.9% (177/403) for SELECT-D trial and 29.2% (305/1046) for Hokusai VTE Cancer trial] [15, 52]. The real-world study findings DOACs were significantly reduced risk of recurrent VTE (P = 0.011) and no increased risk of major bleeding (P = 0.805) in patients with cancer than LMWH. Therefore, DOACs may be an effective alternative to LMWH for the treatment of cancer-associated VTE in patients without gastrointestinal cancer. Simultaneously, several RCTs (NCT02744092, NCT02585713, and NCT02583191) are ongoing to compare the safety and efficacy of DOACs with those of LMWH in patients with cancer. The information obtained will empower patients with cancer and physicians to make more informed choices about anticoagulation strategies to manage VTE.
The optimal duration of anticoagulation treatment in patients with cancer-associated thrombosis (CAT) remains uncertain. In patients with VTE and active cancer, practice guidelines recommended extended anticoagulant therapy for at least 3 [3] or 3–6 [4] months. Some reports have discussed the length of anticoagulation to treat CAT [53, 54]. The evidence-based recommendations are lacking, particularly for DOACs. To address the optimal duration of anticoagulation, our subgroup analyses according to the treatment duration indicated that DOACs significantly reduced the incidence of recurrent VTE without increasing the risk of major bleeding in the 6-month and 12-month subgroups. A population-based cohort study showed that the VTE recurrence per 100 person-years in patients with active cancer was 54.0 for 1–2 months, 15.1 for 3–6 months, 6.1 for 1–2 years, and 1.7 for 5–10 years [55]. Our study provided evidence for the duration of anticoagulation with DOACs for the treatment of cancer-associated VTE. The longer treatment duration with DOACs may be required.
When analyzing the outcomes of VTE recurrence and major bleeding, our study combined different DOACs. However, the results showed no heterogeneity. Presently, compared with other DOACs, rivaroxaban is used more for the treatment of cancer-associated VTE. Therefore, we performed a subgroup analysis of rivaroxaban in patients with cancer. The pooled results showed a significant reduction in recurrence VTE (P = 0.005), without increasing the risk of major bleeding (P = 0.615), compared with LMWH, which is consistent with the DOACs analysis. The results of previous meta-analyses have indicated that rivaroxaban was not inferior to LWMH for the treatment and prevention of cancer-associated VTE [27, 56]. In addition, after the previously published meta-analysis, two observational studies were published [28, 29], but the results were not consistent. Streiff et al. showed rivaroxaban had significantly lower risk of recurrent VTE and bleeding compared to those treated with LMWH in cancer patients with VTE [28]. However, the other study indicated the risk of recurrent VTE and bleeding were no differences between rivaroxaban and LMWH at 12 months [29]. So we included the two published studies to perform an updated meta-analysis. The results supported that rivaroxaban may be more effective and safer than LMWH.
Three strengths of our study should be highlighted. First, we included RCTs and “real-world” studies to evaluate the efficacy and safety of DOACs, respectively. Although the majority of observational studies introduce potential unidentified confounders and selection bias, the overall incidence of recurrent VTE in these “real-world” studies were consistent with the result of our RCT subgroup. Moreover, subgroup analysis based on the anticoagulation duration was performed to reduce bias. In addition, individual DOAC analysis was completed to assess the efficacy and safety of rivaroxaban in patients with cancer.
Several limitations of our study should be considered. First, the current evidence from RCTs is not specifically designed to assess the effects on VTE and major bleeding of DOACs in patients with cancer, and the data included in our study predominantly comprised the results of subgroup analyses. Therefore, differences in the baseline characteristics of patients may introduce bias when randomly assigned. Second, the definition of active cancer was not consistent across included studies. Third, not all studies classified the types or stages of cancer, or the type of VTE. Therefore, it was not possible to aggregate data to complete the subgroup analysis. The risk of thrombosis in different types of cancer may affect the outcome of the endpoint in each study [57]. Fourth, as an aggregated data meta-analysis based on study subgroup, we could not adjust for race/ethnicity due to the evidence in the Asian population was limited. A trial with rivaroxaban to compare steady-state trough (Cmin, ss) and peak (Cmax, ss) concentrations between Asians and Caucasians found Asians had lower Cmin, ss and Cmax, ss than Caucansians [58]. Therefore, further clinical trials are needed to evaluate the safety and efficacy of DOACs in Asian patients with cancer. Finally, drug–drug interactions for DOACs were not reported in the included studies.
Conclusion
Patients with cancer who received DOACs, particularly rivaroxaban, had significantly reduced risk of recurrent VTE with no significant effect on the risk of major bleeding, compared with those who received LMWH. In addition, treatment with DOACs for 6–12 months may be more effective for the prevention of recurrent VTE in patients with cancer than LMWH. DOACs may be an alternative choice for long-term anticoagulant therapy in patients with cancer.
References
Prandoni P, Falanga A, Piccioli A (2005) Cancer and venous thromboembolism. Lancet Oncol 6:401–410
Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC (2013) Epidemiology of cancer-associated venous thrombosis. Blood 122:1712–1723
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H et al (2016) Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 149:315–352
Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N et al (2014) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 35:3033–3069k
Lyman GH, Bohlke K, Falanga A (2015) Venous thromboembolism prophylaxis and treatment in patients with cancer: American society of clinical oncology clinical practice guideline update. J Oncol Pract 11:e442–e444
Farge D, Bounameaux H, Brenner B, Cajfinger F, Debourdeau P, Khorana AA et al (2016) International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 17:e452–e466
Khorana AA, Yannicelli D, McCrae KR, Milentijevic D, Crivera C, Nelson WW et al (2016) Evaluation of US prescription patterns: are treatment guidelines for cancer-associated venous thromboembolism being followed? Thromb Res 145:51–53
van der Wall SJ, Klok FA, den Exter PL, Barrios D, Morillo R, Cannegieter SC et al (2017) Continuation of low-molecular-weight heparin treatment for cancer-related venous thromboembolism: a prospective cohort study in daily clinical practice. J Thromb Haemost 15:74–79
Patel HK, Khorana AA (2019) Anticoagulation in cancer patients: a summary of pitfalls to avoid. Curr Oncol Rep 21:18
Khorana AA, Yannicelli D, McCrae KR, Milentijevic D, Crivera C, Nelson WW et al (2016) Evaluation of US prescription patterns: are treatment guidelines for cancer-associated venous thromboembolism being followed? Thromb Res 145:51–53
Mahe I, Chidiac J, Helfer H, Noble S (2016) Factors influencing adherence to clinical guidelines in the management of cancer-associated thrombosis. J Thromb Haemost 14:2107–2113
Streiff MB, Holmstrom B, Angelini D, Ashrani A, Bockenstedt PL, Chesney C et al (2018) NCCN guidelines insights: cancer-associated venous thromboembolic disease J Natl Compr Cancer Netw 16(11):1289–1303
Khorana AA, Noble S, Lee AYY, Soff G, Meyer G, O’Connell C et al (2018) Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost 16:1891–1894
Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D et al (2018) Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med 378:615–624
Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C et al (2018) Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized TRIAL (SELECT-D). J Clin Oncol 36:2017–2023
Agnelli G, Buller HR, Cohen A, Gallus AS, Lee TC, Pak R et al (2015) Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost 13:2187–2191
Prins MH, Lensing AW, Brighton TA, Lyons RM, Rehm J, Trajanovic M et al (2014) Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol 1:e37–e46
Raskob GE, van Es N, Segers A, Angchaisuksiri P, Oh D, Boda Z et al (2016) Edoxaban for venous thromboembolism in patients with cancer: results from a non-inferiority subgroup analysis of the Hokusai-VTE randomised, double-blind, double-dummy trial. Lancet Haematol 3:e379–e387
Schulman S, Goldhaber SZ, Kearon C, Kakkar AK, Schellong S, Eriksson H et al (2015) Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost 114:150–157
Di Minno MND, Ageno W, Lupoli R, Conte G, van Es N, Buller HR et al (2017) Direct oral anticoagulants for the treatment of acute venous thromboembolism in patients with cancer: a meta-analysis of randomised controlled trials. Eur Respir J 50(3):1701097
Vedovati MC, Germini F, Agnelli G, Becattini C (2015) Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest 147:475–483
Brunetti ND, Gesuete E, De Gennaro L, Correale M, Caldarola P, Gaglione A et al (2017) Direct oral anti-coagulants compared with vitamin-K inhibitors and low-molecular-weight-heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol 230:214–221
Posch F, Konigsbrugge O, Zielinski C, Pabinger I, Ay C (2015) Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res 136:582–589
Al Yami MS, Badreldin HA, Mohammed AH, Elmubark AM, Alzahrani MY, Alshehri AM (2018) Direct oral anticoagulants for the treatment of venous thromboembolism in patients with active malignancy: a systematic review and meta-analysis. J Thromb Thrombolysis 46:145–153
Kahale LA, Hakoum MB, Tsolakian IG, Matar CF, Terrenato I, Sperati F et al (2018) Anticoagulation for the long-term treatment of venous thromboembolism in people with cancer. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858
Li A, Garcia DA, Lyman GH, Carrier M (2018) Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta-analysis. Thromb Res 173:158–163
Xing J, Yin X, Chen D (2018) Rivaroxaban versus enoxaparin for the prevention of recurrent venous thromboembolism in patients with cancer: a meta-analysis. Medicine 97:e11384
Streiff MB, Milentijevic D, McCrae K, Yannicelli D, Fortier J, Nelson WW et al (2018) Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol 93:664–671
Simmons B, Wysokinski W, Saadiq RA, Bott-Kitslaar D, Henkin S, Casanegra A et al (2018) Efficacy and safety of rivaroxaban compared to enoxaparin in treatment of cancer-associated venous thromboembolism. Eur J Haematol 101(2):136–142
GA W, Shea B OCD, Peterson J WV. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. 2012;Ottawa, Ontario: Department of Epidemiology and Community Medicine
JP H, S G. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0). Available at: http://www.cochrane-handbookorg
Mandala M, Falanga A, Piccioli A, Prandoni P, Pogliani EM, Labianca R et al (2006) Venous thromboembolism and cancer: guidelines of the Italian Association of Medical Oncology (AIOM). Crit Rev Oncol Hematol 59:194–204
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H et al (2016) Antithrombotic therapy for VTE disease. Chest 149:315–352
Lyman GH, Bohlke K, Khorana AA, Kuderer NM, Lee AY, Arcelus JI et al (2015) Venous thromboembolism prophylaxis and treatment in patients with cancer: American society of clinical oncology clinical practice guideline update 2014. J Clin Oncol 33:654–656
Farge D, Bosquet L, Kassab-Chahmi D, Mismetti P, Elalamy I, Meyer G et al (2010) 2008 French national guidelines for the treatment of venous thromboembolism in patients with cancer: report from the working group. Crit Rev Oncol Hematol 73:31–46
Demets DL (1987) Methods for combining randomized clinical trials: strengths and limitations. Stat Med 6:341–350
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Grant RL (2014) Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ 348:f7450
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Signorelli JR, Gandhi AS (2017) Evaluation of rivaroxaban use in patients with gynecologic malignancies at an academic medical center: a pilot study. J Oncol Pharm Pract 25(2):362–368
Chaudhury A, Balakrishnan A, Thai C, Holmstrom B, Nanjappa S, Ma Z et al (2017) The efficacy and safety of rivaroxaban and dalteparin in the treatment of cancer associated venous thrombosis. Indian J Hematol Blood Transfus 34(3):530–534
Nicklaus MD, Ludwig SL, Kettle JK (2018) Recurrence of malignancy-associated venous thromboembolism among patients treated with rivaroxaban compared to enoxaparin. J Oncol Pharm Pract 24:185–189
Ross JA, Miller MM, Rojas Hernandez CM (2017) Comparative effectiveness and safety of direct oral anticoagulants (DOACs) versus conventional anticoagulation for the treatment of cancer-related venous thromboembolism: a retrospective analysis. Thromb Res 150:86–89
Xiang E, Ahuja T, Raco V, Cirrone F, Green D, Papadopoulos J (2018) Anticoagulation prescribing patterns in patients with cancer. J Thromb Thrombolysis 45:89–98
Alzghari SK, Seago SE, Garza JE, Hashimie YF, Baty KA, Evans MF et al (2017) Retrospective comparison of low molecular weight heparin versus warfarin versus oral Xa inhibitors for the prevention of recurrent venous thromboembolism in oncology patients: the Re-CLOT study. J Oncol Pharm Pract 24(7):494–500
Xavier FD, Hoff PMG, Braghiroli MI, Paterlini A, Souza KT, Faria L et al (2017) Rivaroxaban: an affordable and effective alternative in cancer-related thrombosis? J Global Oncol 3:15–22
Jean GW, Kelly K, Mathew J, Larumbe E, Hughes R (2017) Venous thromboembolism treatment outcomes in cancer patients and effect of third-party payers on anticoagulant choice. Support Care Cancer 25:59–66
McClinton DA, Breen KA, Thomson GA, McDonald V (2016) Outcomes of patients treated for cancer or chemotherapy associated venous thromboembolism and effectiveness of secondary prevention. Br J Haematol 173:69–70
Nam KW, Kim CK, Kim TJ, An SJ, Oh K, Ko SB et al (2017) Treatment of cryptogenic stroke with active cancer with a new oral anticoagulant. J Stroke Cerebrovasc Dis 26:2976–2980
Khorana AA, McCrae K, Milentijevic D, Yannicelli D, Fortier J, Nelson WW et al (2016) Duration of anticoagulant therapy and VTE recurrence in patients with cancer. J Clin Oncol. https://doi.org/10.1007/s00520-019-4661-3
Kraaijpoel N, Di Nisio M, Mulder FI, van Es N, Beyer-Westendorf J, Carrier M et al (2018) Clinical impact of bleeding in cancer-associated venous thromboembolism: results from the hokusai VTE cancer study. Thromb Haemost 118:1439–1449
Lee AYY (2017) When can we stop anticoagulation in patients with cancer-associated thrombosis? Blood 130(23):2484–2490
Elalamy I, Mahe I, Ageno W, Meyer G (2017) Long-term treatment of cancer-associated thrombosis: the choice of the optimal anticoagulant. J Thromb Haemost 15:848–857
Chee CE, Ashrani AA, Marks RS, Petterson TM, Bailey KR, Melton LJ 3rd et al (2014) Predictors of venous thromboembolism recurrence and bleeding among active cancer patients: a population-based cohort study. Blood 123:3972–3978
Li A, Garcia DA, Lyman GH, Carrier M (2019) Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): a systematic review and meta-analysis. Thromb Res 173:158–163
Thodiyil PA, Kakkar AK (2002) Variation in relative risk of venous thromboembolism in different cancers. Thromb Haemost 87:1076–1077
Ng Tsai HO, Goh JJN, Aw JWX, Lin Y, Fong AYY, Tiong LL et al (2018) Comparison of rivaroxaban concentrations between Asians and Caucasians and their correlation with PT/INR. J Thromb Thrombolysis 46:541–548
Funding
This study was supported by grants from the Natural Science Foundation (No.81460066) of PR China, the Natural Science Foundation (No.2018MS08099) of Inner Mongolia province, Science technology millions of projects of Inner Mongolia Medical University and doctoral Sustentation Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
All authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, Y., Wang, Y., Ma, RL. et al. Efficacy and safety of direct oral anticoagulants versus low-molecular-weight heparin in patients with cancer: a systematic review and meta-analysis. J Thromb Thrombolysis 48, 400–412 (2019). https://doi.org/10.1007/s11239-019-01871-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-019-01871-4