Abstract
Prediction of recurrence in patients with unprovoked venous thromboembolism (VTE) remains a challenge. Studies of atherosclerosis suggest a protective role of transforming growth factor (TGF)-β. However, the role of TGF-β has not been studied in VTE. The aim of this study was to investigate TGF-β as a predictive marker of recurrent VTE in patients with a first episode of unprovoked VTE. Patients in the Malmö Thrombophilia Study (MATS) were followed after the discontinuation of anticoagulant treatment until the diagnosis of recurrent VTE or the end of the study in December 2008 (mean ± SD 38.5 months ± 27). Among patients with a first episode of unprovoked VTE, we identified 42 patients with recurrent VTE during the follow-up period. Two age- and sex-matched control subjects without recurrent VTE were selected for each patient (n = 84). Plasma levels of the three isoforms of TGF-β (TGF-β1, TGF-β2 and TGF-β3) were quantified simultaneously by TGF-β 3-plex immunoassay. Compared to controls, plasma levels of TGF-β1 and TGF-β2 were significantly lower in patients with recurrent VTE (p < 0.05), whereas no difference was found for TGF-β3. In a multivariate Cox regression analyses, adjusted for inherited thrombophilia, age, sex and BMI, low levels of TGF-β1 [hazard ratio (HR) = 2.2, 95 % confidence interval (CI) 1.1–4.3; p = 0.02] and TGF-β2 (HR = 2.4, 95 % CI 1.2–4.7; p = 0.01) were independently associated with a higher risk of recurrent VTE. We propose TGF-β1 and TGF-β2 as potential predictive markers for recurrence in patients with unprovoked VTE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Venous thromboembolism (VTE) is a common cardiovascular disease (CVD) that frequently recurs and is associated with significant numbers of deaths annually [1]. Multiple acquired factors (older age, malignancy, trauma, major surgery, immobilization, female hormone therapy, pregnancy) [2, 3] and genetic factors (Factor V Leiden (rs6025) and prothrombin G20210A (rs1799963) mutations, protein C, protein S and antithrombin deficiencies) [4, 5] have been identified as risk factors for VTE. However, there is an increased risk of recurrence in patients with no identifiable acquired risk factor associated with the first event (unprovoked VTE), compared to patients with known risk factors (provoked VTE) [6]. The cumulative risk of recurrent VTE after a first episode of deep vein thrombosis (DVT) has been shown to be about 17 % at 2 years of follow-up and 30 % at 8 years [7]. Treatment with oral anticoagulants such as vitamin K antagonists or new oral anticoagulants (NOAC) such as Rivaroxaban, Dabigatran and Apixaban prevents most episodes of recurrence, albeit at the cost of an increased risk of bleeding [4]. Therefore, thrombotic risk assessment is necessary for continuation of anticoagulant treatment in patients with high risk of recurrence and to limit the treatment duration in patients with a lower risk of recurrence. However, the risk of recurrent thrombosis after discontinuation of treatment is not easily predicted, despite the knowledge of a number of identified risk factors, such as male sex, elevated D-dimer levels and residual thrombosis [4, 8].
An association between VTE and risk factors for atherosclerotic vascular diseases is emerging and may help in identifying new risk factors for venous thrombosis [9]. TGF-β is a secreted protein that has been shown to be a target molecule for development of CVDs and metabolic syndrome [2, 3, 6]. It exists in three subtypes in humans, TGF-β1, TGF-β2 and TGF-β3, of which TGF-β1 is recognized as the potentially most pivotal TGF-β isoform for the cardiovascular system [10]. In general, TGF-β1 is considered as an anti-inflammatory cytokine in the vessel wall [11]. Clinical and experimental studies suggest an important role for TGF-β1 in various cardiac pathologies [12–14]. In atherosclerosis, TGF-β1 is considered to be an antiatherogenic factor and, in hemostasis, it has been suggested to be an antifibrinolytic factor [15, 16]. However, the roles of TGF-β1, TGF-β2 and TGF-β3 in VTE are not well defined. In this study, we analyzed all three isoforms of TGF-β and investigated their potential roles in predicting recurrent VTE in patients with unprovoked VTE in the Malmö Thrombophilia Study (MATS).
Subjects and methods
Study population
Participants were selected from the Malmö Thrombophilia Study (MATS), a prospective population-based study conducted at Skåne University Hospital in Sweden from March 1998 to December 2008 on 1,465 consecutive patients diagnosed with VTE [17]. Patients for whom complete information on recurrence was missing (n = 51) and those who had thrombotic events before inclusion (n = 25), who had recurrence during anticoagulant therapy (n = 281) or who gave post-treatment samples during treatment (n = 382) were excluded. Of the remaining 726 patients who had a recorded history of an acquired risk factor for VTE, such as surgical intervention, immobilization or cast therapy within the last month, malignancies diagnosed prior to or at diagnosis of the first VTE event, use of contraceptives pills, use of hormonal therapy, pregnancy or postpartum period (first 6 weeks after delivery) were excluded (n = 283). Among the remaining 443 patients (unprovoked VTE without any acquired risk factors at the time of inclusion), 43 (9.7 %) suffered from recurrent VTE. However, for one patient there was not enough sample available for analysis and that patient was excluded. Control samples were selected from the remaining patients at a ratio of 1:2 and were matched for age, sex and time of sampling. In total, 42 unprovoked recurrent VTE patients and 84 matched controls (unprovoked non-recurrent VTE) were analyzed in the study. The primary end-point of the study was a confirmed diagnosis of DVT and/or pulmonary embolism (PE) during the follow-up period. Patients were censored if they were free of DVT and/or PE throughout the follow-up period. All participants provided written informed consent and the study was approved by the Ethics Committee of Lund University.
Diagnosis of DVT and PE was objectively confirmed by phlebography, duplex ultrasonography, computed tomography (CT), lung scintigraphy or magnetic resonance imaging (MRI) [17]. All patients were initially treated with low molecular weight heparin (LMH) or unfractionated heparin (UFH). Warfarin was used as an oral anticoagulant (OAC) for 3–6 months. Thrombophilia was defined as presence of the Factor V Leiden mutation (rs6025) or the prothrombin G20210A mutation (rs1799963), or levels below the laboratory reference range of free protein S (women < 0.5 kIE/L, men < 0.65 kIE/L), protein C (<0.7 kIE/L) or antithrombin (<0.82 kIE/L).
Laboratory methods
Whole blood samples were collected into citrate-treated tubes and were centrifuged at 2,000×g for 15 min at 4 °C to collect platelet-depleted plasma. Samples were transferred to new polypropylene tubes and, to completely remove platelets and precipitates, samples were centrifuged again at 10,000×g for 10 min at 4 °C. The samples were then aliquoted and stored at −80 °C prior to analysis. All three TGF-β isoforms (TGF-β1, TGF-β2 and TGF-β3) were quantified simultaneously using a Bio-Plex Pro™ TGF-β 3-plex immunoassay kit (Bio-Rad Inc., Hercules, CA, USA) according to the manufacturer’s instructions. Briefly, the samples (25 μL) were activated with 1 N HCL (5 μL) and neutralized with 5 μL of 1.2 N NaOH/0.5 M HEPES. They were then diluted 1:16 in the dilution buffer provided with the kit and incubated with magnetic beads coupled to specific antibodies. TGF-β isoforms were detected with premixed detection antibody. Protein levels were quantified using a Bio-Plex Suspension Array System and the data obtained were analyzed using Bio-Plex Manager™ software (version 4.0). Absolute concentrations were measured from a standard curve generated from eight serially diluted standards provided with the kit. Each sample was analyzed in duplicate. Values are presented in ng/mL for TGF-β1 and in pg/mL for TGF-β2 and TGF-β3. Each run included controls of known concentration (spiked-in samples) for each isoform and a blank. Intra-assay coefficients of variation (CV) values for TGF-β1, TGF-β2 and TGF-β3 were 2.1, 7.9 and 5.5 %, respectively; inter-assay CV values for TGF-β1, TGF-β2 and TGF-β3 were 3.8, 6.4 and 7.1 %, respectively (values were calculated from six different experiments).
DNA mutations for factors II and V were analyzed by TaqMan allele discrimination using gene-specific assays for these two factors (Applied Biosystems, Life Technologies Corporation, Carlsbad, CA, USA) [18].
Statistical analysis
Nonparametric tests were used in this study. Two-sided p values of <0.05 were considered significant. Baseline differences in age, BMI, lab markers, thrombophilia, duration of anticoagulation and TGF-β levels were analyzed by Mann–Whitney U test and differences in sex and type of thrombosis were anlysed by Fisher’s exact test and Chi square test respectively. Time to event analysis was performed by Kaplan–Meier analysis. The log-rank test was used to compare survival. Uni- and multi-variate regression analyses were performed using the Cox proportional hazards model. The fit of the proportional hazards model was checked visually by plotting the recurrence rates over time and by calculating Schoenfeld (partial) residuals. Schoenfeld residuals were used as the dependent variable and time as the independent variable in order to assess the proportional hazards assumption. A possible interaction between thrombophilia and TGF-β was explored by adding interaction terms to the multivariate model. Statistical analyses were performed using IBM SPSS 21 (IBM, Armonk, NY, USA). Descriptive data are presented as the mean ± standard deviation (SD) but medians were used in the statistical models.
Results
Baseline characteristics are presented in Table 1. Of the patients with thrombophilia, 64 % developed recurrent VTE, compared to 36 % of those without thrombophilia. Of the patients with thrombophilia, 42 % were free of recurrent VTE, compared to 58 % of those without thrombophilia (p = 0.02). Patients with non-recurrent VTE had lower hemoglobin (mean ± SD, 138 ± 11) levels compared to those with recurrent VTE (mean ± SD, 146 ± 16), p = 0.02. There were no significant differences (p = >0.05) in age, sex, BMI, C-reactive protein, creatinine, platelets, cholesterol, triglycerides, HDL, LDL, duration of anti-coagulant treatment or type of thrombosis between patients with non-recurrent and recurrent VTE (Table 1).
Compared to non-recurrent VTE (mean ± SD, 5.2 ± 1.8 ng/mL), TGF-β1 levels were significantly decreased in patients with recurrent VTE (mean ± SD, 4.6 ± 1.9 ng/mL; p = 0.017). Similarly, TGF-β2 levels were also significantly decreased in patients with recurrent VTE (mean ± SD, 487 ± 73 pg/mL) compared to patients with non-recurrent VTE (mean ± SD, 517 ± 78 pg/mL; p = 0.02). In contrast, compared to non-recurrent VTE (mean ± SD, 23 ± 13 pg/mL), TGF-β3 levels tended to be lower in patients with recurrent VTE (mean ± SD, 20 ± 12 pg/mL); however, this difference was not statistically significant (p = 0.6) (Table 1).
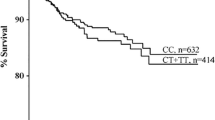
To investigate the predictive value of TGF-β in recurrent VTE, patients were divided into two groups according to median levels of each TGF-β isoform: high (above the median, n = 63) and low (median or below, n = 63). Median levels were chosen because TGF-β values were not normally distributed. These two groups were then subjected to survival analysis by the Kaplan–Meir method. Recurrent VTE-free survival was significantly higher in patients with high levels of TGF-β1 (>4.5 ng/mL) compared to patients with low levels (≤4.5 ng/mL) (log rank test, χ2 = 8.1; p = 0.005) (Fig. 1a). Similarly, patients with high levels of TGF-β2 (>503 pg/mL) had significantly higher recurrent VTE-free survival compared to those with low levels of TGF-β2 (≤503 pg/mL) (log rank test, χ2 = 9.7; p = 0.002) (Fig. 1b). There was no difference in recurrent VTE-free survival between patients with high levels of TGF-β3 (>21 pg/mL) and those with low levels (≤21 pg/mL) (log rank test, χ2 = 0.02; p = 0.9) (Fig. 1c).
Univariate Cox regression analysis comparing low and high levels of TGF-β isoforms showed that lower levels of TGF-β1 [hazard ratio (HR) = 2.5, 95 % confidence interval (CI) 1.3–4.6] and TGF-β2 (HR = 2.7, 95 % CI 1.5–5.0) were associated with higher risk of recurrent VTE. No difference in risk of recurrent VTE was found between patients with low and high levels of TGF-β3 (HR = 1.0, 95 % CI 0.5–1.8) (Table 2).
In order to investigate the role of thrombophilia in predicting recurrent VTE in the study population, all patient were divided into two groups according to their thrombophilia status. Univariate Cox regression analysis comparing patients positive and negative for thrombophilia showed a significantly higher risk of recurrent VTE in patients positive for thrombophilia compared to those who were negative for thrombophilia (HR = 2.0, 95 % CI 1.1–3.7; p = 0.03; Table 2).
Multivariate Cox regression analysis on the whole study population was performed by including each TGF-β isoform, thrombophilia status, age, sex and BMI. Models including TGF-β1 or TGF-β2 showed that lower levels of both TGF-β1 (HR = 2.2, 95 % CI 1.1–4.3; p = 0.02) and TGF-β2 (HR = 2.4, 95 % CI 1.2–4.7; p = 0.01) were associated with increased risk of recurrent VTE independent of thrombophilia, age, sex and BMI. However, in the model where TGF-β3 was included, thrombophilia (HR = 1.9, 95 % CI 1.04–3.8; p = 0.039) was the only significant predictor of recurrent VTE. No association between level of TGF-β3 and recurrent VTE was observed (HR = 0.85, 95 % CI 0.45–1.6; p = 0.62), (Table 3).
Discussion
For the first time, we identified TGF-β1 and TGF-β2 as potential predictive markers for recurrent VTE in patients with an unprovoked first episode of VTE. Our results show associations between higher risks of recurrent VTE and lower levels of TGF-β1 and TGF-β2. Furthermore, these associations were independent of inherited genetic risk factors associated with increased risk of recurrent VTE (factor V Leiden and factor II mutations, protein S, protein C and antithrombin deficiencies) and BMI.
During recent years, efforts have been made to identify risk factors for thrombotic risk assessment. However, it remains a challenge to predict the individual risk for recurrence of VTE, especially in unprovoked VTE. Recent epidemiological studies suggest that arterial and venous CVDs share common risk factors [9, 19] and TGF-β1 has been suggested as a predictive marker for arterial CVDs. Notably, increased plasma levels of TGF-β1 are significantly associated with extended survival and reduced incidence of coronary events [20]. However, to the best of our knowledge, no previous study has investigated the role of this major player in vascular function and hemostasis in VTE. We found significantly lower levels of TGF-β1 and TGF-β2 in recurrent VTE compared to non-recurrent VTE, suggesting that they could be used as potential biomarkers of recurrent VTE.
Cell culture studies suggest that TGF-β protects against atherosclerosis by inhibiting the proliferation of vascular smooth muscle cells [21, 22]. Preclinical studies also indicate a protective role for TGF-β1 in atherogenesis. Furthermore, suppression of TGF-β1 activity accelerates atherosclerosis and the switch from a stable matrix-rich plaque phenotype to an unstable proinflammatory plaque at risk of rupture [23]. In agreement with these studies, we found a significant association between lower levels of TGF-β1 and TGF-β2 and an increased risk of recurrent VTE. In contrast, other studies have shown an association between TGF-β and the development of metabolic syndrome and cardiovascular damage [24, 25]. TGF-β was also weakly associated with factor VII in patients with hypercholesterolemia [26]. Furthermore, it has been reported that TGF-β has multi-faceted effects on numerous immune functions, which are dependent on the cellular and environmental context [27]. Therefore, it is possible that TGF-β may play a dual role in cardiovascular diseases dependent on the cellular and molecular environment.
VTE recurrence rates are reported to be higher in patients with inherited genetic defects [5, 28, 29]. However, the clinical importance of these inherited genetic defects in recurrent VTE risk is controversial [8, 30]. Consistent with previous research [5], we also found an increased risk of recurrent VTE in patients with thrombophilia compared to those without thrombophilia. In the multivariate Cox regression analyses, including thrombophilia, BMI and the TGF-β isoforms, we demonstrate that lower levels of TGF-β1 and TGF-β2 were associated with a higher risk of recurrent VTE, independent of thrombophilia and BMI. Interestingly, thrombophilia was not an independent predictor of recurrent VTE when TGF-β1 or TGF-β2 were included in the model. These results suggest that the levels of TGF-β1 and TGF-β2 may be of importance when evaluating the predictive role of thrombophilia.
The main limitation of the study is the relatively small number of cases. The results need to be confirmed in a larger number of patients. A potential limitation is that the present study did not detect any mechanisms, although that was not the scope of the study. It is possible that TGF-β may not be involved in the pathogenesis of recurrent VTE. Furthermore, hemoglobin levels were different between patients with non-recurrent and recurrent VTE. To the best of our knowledge, hemoglobin is not known to be associated with recurrent VTE. Higher levels of hematocrit, however, which is associated with hemoglobin, have been shown to be associated with recurrent VTE but only in women with the highest hematocrit values [29]. However due to the missing values (44 % missing values) we were unable to adjust our results with hemoglobin as well as hematocrit. Nevertheless, our results show that TGF-β, in this group of patients, predicts recurrent VTE independent of thrombophilia, age, sex and BMI. A key strength of the study is that only unprovoked cases with a first event of VTE were included. Moreover, all events of VTE were confirmed by objective methods such as phlebography, duplex ultrasonography, computed tomography (CT), and/or lung scintigraphy.
In conclusion, our results show that patients with high levels of TGF-β1 and TGF-β2 have a decreased risk of recurrent VTE. We propose TGF-β1 and TGF-β2 as potential predictive markers of recurrent VTE.
References
Anderson FA Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, Forcier A, Dalen JE (1991) A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 151:933–938
Eichinger S, Heinze G, Jandeck LM, Kyrle PA (2010) Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 121:1630–1636
Rodger MA, Kahn SR, Wells PS, Anderson DA, Chagnon I, Le Gal G, Solymoss S, Crowther M, Perrier A, White R, Vickars L, Ramsay T, Betancourt MT, Kovacs MJ (2008) Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 179:417–426
Anderson FA Jr, Spencer FA (2003) Risk factors for venous thromboembolism. Circulation 107:I9–I16
Sveinsdottir SV, Saemundsson Y, Isma N, Gottsater A, Svensson PJ (2012) Evaluation of recurrent venous thromboembolism in patients with Factor V Leiden mutation in heterozygous form. Thromb Res 130:467–471
Lijfering WM, Rosendaal FR, Cannegieter SC (2010) Risk factors for venous thrombosis—current understanding from an epidemiological point of view. Br J Haematol 149:824–833
Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E, Prins MH (1996) The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 125:1–7
Heit JA (2005) Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost 3:1611–1617
Sorensen HT, Horvath-Puho E, Sogaard KK, Christensen S, Johnsen SP, Thomsen RW, Prandoni P, Baron JA (2009) Arterial cardiovascular events, statins, low-dose aspirin and subsequent risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost 7:521–528
Annes JP, Munger JS, Rifkin DB (2003) Making sense of latent TGFbeta activation. J Cell Sci 116:217–224
Lebastchi AH, Khan SF, Qin L, Li W, Zhou J, Hibino N, Yi T, Rao DA, Pober JS, Tellides G (2011) Transforming growth factor beta expression by human vascular cells inhibits interferon gamma production and arterial media injury by alloreactive memory T cells. Am J Transpl 11:2332–2341
Cambien F, Ricard S, Troesch A, Mallet C, Generenaz L, Evans A, Arveiler D, Luc G, Ruidavets JB, Poirier O (1996) Polymorphisms of the transforming growth factor-beta 1 gene in relation to myocardial infarction and blood pressure. The Etude Cas-Temoin de l’Infarctus du Myocarde (ECTIM) Study. Hypertension 28:881–887
Leask A (2010) Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res 106:1675–1680
Schultz Jel J, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, Kimball TR, Doetschman T (2002) TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest 109:787–796
Grainger DJ (2004) Transforming growth factor beta and atherosclerosis: so far, so good for the protective cytokine hypothesis. Arterioscler Thromb Vasc Biol 24:399–404
Vayalil PK, Olman M, Murphy-Ullrich JE, Postlethwait EM, Liu RM (2005) Glutathione restores collagen degradation in TGF-beta-treated fibroblasts by blocking plasminogen activator inhibitor-1 expression and activating plasminogen. Am J Physiol Lung Cell Mol Physiol 289:L937–L945
Isma N, Svensson PJ, Gottsater A, Lindblad B (2009) Prospective analysis of risk factors and distribution of venous thromboembolism in the population-based Malmo Thrombophilia Study (MATS). Thromb Res 124:663–666
Saemundsson Y, Sveinsdottir SV, Svantesson H, Svensson PJ (2013) Homozygous factor V Leiden and double heterozygosity for factor V Leiden and prothrombin mutation. J Thromb Thrombolysis 36:324–331
Franchini M, Mannucci PM (2008) Venous and arterial thrombosis: different sides of the same coin? Eur J Intern Med 19:476–481
Tashiro H, Shimokawa H, Sadamatu K, Yamamoto K (2002) Prognostic significance of plasma concentrations of transforming growth factor-beta in patients with coronary artery disease. Coron Artery Dis 13:139–143
Bjorkerud S (1991) Effects of transforming growth factor-beta 1 on human arterial smooth muscle cells in vitro. Arterioscler Thromb Vasc Biol 11:892–902
Owens GK, Geisterfer AA, Yang YW, Komoriya A (1988) Transforming growth factor-beta-induced growth inhibition and cellular hypertrophy in cultured vascular smooth muscle cells. J Cell Biol 107:771–780
Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamate C, Merval R, Fradelizi D, Tedgui A (2001) Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res 89:930–934
Sciarretta S, Ferrucci A, Ciavarella GM, De Paolis P, Venturelli V, Tocci G, De Biase L, Rubattu S, Volpe M (2007) Markers of inflammation and fibrosis are related to cardiovascular damage in hypertensive patients with metabolic syndrome. Am J Hypertens 20:784–791
Surendar J, Aravindhan V, Rao MM, Ganesan A, Mohan V (2011) Decreased serum interleukin-17 and increased transforming growth factor-beta levels in subjects with metabolic syndrome (Chennai Urban Rural Epidemiology Study-95). Metabolism 60:586–590
Porreca E, Di Febbo C, di Castelnuovo A, Baccante G, Amore C, Angelini A, Di Nisio M, Donati M, Cuccurullo F, Iacoviello L (2002) Association of factor VII levels with inflammatory parameters in hypercholesterolemic patients. Atherosclerosis 165:159–166
Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA (2006) Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 24:99–146
De Stefano V, Simioni P, Rossi E, Tormene D, Za T, Pagnan A, Leone G (2006) The risk of recurrent venous thromboembolism in patients with inherited deficiency of natural anticoagulants antithrombin, protein C and protein S. Haematologica 91:695–698
Eischer L, Tscholl V, Heinze G, Traby L, Kyrle PA, Eichinger S (2012) Hematocrit and the risk of recurrent venous thrombosis: a prospective cohort study. PLoS ONE 7:e38705
Middeldorp S (2011) Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 31:275–281
Acknowledgments
We would like to thank Anna Hedelius for excellent technical support, Sven-Arnne Olsson for his help in collecting clinical data and science editor Stephen Gilliver for critical reading of the manuscript. This work was supported by grants awarded to Dr Bengt Zöller by the Swedish Heart–Lung Foundation and Region Skåne (REGSKANE-124611); ALF funding awarded to Drs Bengt Zöller, Kristina Sundquist, and Jan Sundquist by Region Skåne; grants awarded to Drs Kristina and Jan Sundquist by the Swedish Research Council (K2009-70X-15428-05-3 and K2012-70X-15428-08-3); and Grants awarded to Dr Jan Sundquist by the Swedish Council for Working Life and Social Research (2007-1754), King Gustaf V and Queen Victoria’s Foundation of Freemasons and FORTE, Swedish Reserch Council for health, Working life and welfare.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Memon, A.A., Sundquist, K., Wang, X. et al. Transforming growth factor (TGF)-β levels and unprovoked recurrent venous thromboembolism. J Thromb Thrombolysis 38, 348–354 (2014). https://doi.org/10.1007/s11239-013-1047-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-013-1047-0