Abstract
Oncoceratium n. gen. is proposed to accommodate Oncoceratium amphidactylum n. gen. n. sp. (Monogenoidea, Gyrodactylidae). Although the proposed genus is monotypic, the differences presented in the diagnosis clearly establish it as distinct from species from other genera, such as Gyrodactylus von Nordmann, 1832 and especially those that occur exclusively in the Neotropical region. The new genus is diagnosed by the presence of a bulbous male copulatory organ, with one row of spinelets of similar sizes and shapes, while the only spine usually found in the gyrodactylids is absent or not differentiated; a haptor with anchors presenting points folded outwards, and superficial and deep roots continuous and indistinguishable; the absence of a deep bar; and hooks in two bilateral clusters of eight hooks. Oncoceratium amphidactylum n. gen. n. sp. is described from the tamboatá fish, Hoplosternum littorale (Hancock) (Callichthyidae), and is characterized by the presence of a pair of horn-shaped anchors with the point turned outwards, a superficial bar shield and absent deep bar, hooks disposed in bilateral lobes, and a MCO with no spine or with a row with large spinelets visible in place of the spine. In addition to the morphological features, distance analysis and Bayesian inference, based on 5.8s and partial ITS2, support placing the new species in a new genus, and not including it in a cluster of species of Gyrodactylus and near to Gyrodactyloides bychowskii Albova, 1948, Ieredactylus rivuli Schelkle et al., 2011 and Laminiscus gussevi (Bychowsky & Polyansky, 1953) Pálsson & Beverely-Burton, 1983.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species of Gyrodactylidae van Beneden & Hesse, 1832 (Platyhelminthes: Monogenoidea) from the Neotropical region are known for parasitizing characiforms, cichliforms, cyprinodontiforms, gymnotiforms, mugiliforms, perciforms and siluriforms (Boeger & Vianna 2006; Cohen et al., 2013). The siluriforms are the most diverse group of fish in South America, and are mainly found in Brazil (Reis et al., 2003). Among the Neotropical siluriforms, some species of Auchenipteridae Blekker, 1862, Callichthyidae Bonaparte, 1838, Heptapteridae Gill, 1861 and Loricariidae Rafinesque, 1815 are known to harbor only gyrodactylids (Kritsky et al., 1995; Popazoglo & Boeger, 2000; Bueno-Silva & Boeger, 2009; 2014; Razzolini et al., 2019; Vianna & Boeger 2019). Species of Gyrodactylus von Nordmann, 1832 and Scleroductus Jara & Cone, 1989, both gyrodactylids, are parasites of siluriforms in Brazil. Among the species of Gyrodactylus, eight are parasites of callichthyids. Gyrodactylus anisopharynx Popazoglo & Boeger, 2000, G. corydori Bueno-Silva & Boeger, 2009, and G. superbus (Szidat, 1970) Popazoglo & Boeger, 2000 are parasites of Corydoras paleatus (Jenyns, 1842) and C. ehrhardti Steindachner, 1910, while G. samirae Popazoglo & Boeger, 2000 exclusively parasitize C. ehrhardti (Kritsky et al., 1995; Cone et al., 2010). Gyrodactylus bueni Bueno-Silva & Boeger, 2014, G. major Bueno-Silva & Boeger, 2014, and G. scleromystaci Bueno-Silva & Boeger, 2014 have been found parasitizing Scleromystax macropterus (Regan, 1913), and S. barbatus (Quoy & Gaimard, 1824) (Bueno-Silva & Boeger, 2014). Gyrodactylus polyadenus Vianna & Boeger, 2019 is a parasite of Callichthys callichthys (Linnaeus, 1758) (Vianna & Boeger, 2019).
The Gyrodactylidae family contains 25 valid genera (Přikrylová et al., 2021), 11 of which are monotypic and exhibit the addition, absence, or modification of haptoral sclerites. These include Anacanthocotyle Kritsky & Fritts, 1970, Accessorius Jara, An & Cone, 1991, Citharodactylus Přikrylová, Shinn & Paladini, 2017, Diechodactylus Vianna, Boeger & Silva-Souza, 2008, Gyrdicotylus Vercammen-Grandjean, 1960, Gyrocerviceanseris Cone, Abbott, Gilmore & Burt, 2010, Ieredactylus Schelkle, Paladini, Shinn, King, Johnson, van Oosterhout, Mohammed & Cable, 2011, Mormyrogyrodactylus Luus-Powell, Mashego & Khalil, 2003, and Scutalatus Vianna, Boeger & Dove, 2007. Species of Diplogyrodactylus Přikrylová, Matějusová, Musilová, Gelnar & Harris, 2009 and Tresuncinidactylus Přikrylová, Barson & Shinn, 2021 present hooks with different sizes and shapes, and a male copulatory organ (MCO) consisting of a muscular pouch (Přikrylová et al., 2009; 2021). In addition to the morphological features that support the proposal of new genera, molecular data were used to confirm the status of new species for Gyrocerviceanseris, Ieredactylus, and Tresuncinidactylus (Cone et al., 2010; Schelkle et al., 2011; Přikrylová et al., 2021).
Species of five monotypic genera were proposed from Neotropical hosts: Anacanthocotyle anacanthocotyle Kritsky & Fritts, 1970 and Accessorius peruensis Jara, An & Cone, 1991 parasitizing characiforms; Diechodactylus joaberii Vianna, Boeger & Silva-Souza, 2008 and Scutalatus magniancoratus Vianna, Boeger & Dove, 2007 parasitizing gymnotiforms; and Ieredactylus rivuli Schelkle, Paladini, Shinn, King, Johnson, van Oosterhout, Mohammed & Cable, 2011 parasitizing cyprinodontiforms (Kritsky & Fritts, 1970; Jara et al., 1991; Vianna et al., 2007; 2008; Schelkle et al., 2011).
Oncoceratium n. gen. is the sixth monotypic genus from the neotropical region proposed to accommodate Oncoceratium amphidactylum n. gen. n. sp. from siluriforms, based on the exclusive features of the haptoral structures and the molecular data of rDNA.
Materials and methods
Host collection and morphological analysis
Specimens of the tamboatá fish, Hoplosternum littorale (Hancock, 1828) (Callichthyidae) were collected from February 2014 to February 2018 in the Batalha River, in the municipal district of Reginópolis, and the Peixe River and the Tietê River, in the municipal district of Anhembi, all in the state of São Paulo (coordinates in the respective description).
Fish were captured using nylon monofilament gillnets with different mesh sizes (ranging from 20 to 100 mm internodes). These were placed on slopes or near the river bottom at night, and removed before dawn (10 hours exposure). The fish were removed from the nets, anesthetized with a eugenol solution (clove oil), and submitted to euthanasia through the physical method of medullary section, before being immediately individualized in plastic bags. Part of the hosts were analyzed for parasites while still in the field, while a small number of excess hosts were frozen and taken to the laboratory on the same day, to subsequent analysis. The fish were analyzed within 24 hours after collection
Collections were carried out according to the guidelines of the scientific fishing license, under the authorization of the Chico Mendes Institute of Biodiversity (ICMBio) through the System of Authorization and Information on Biodiversity (SISBIO - authorization nº 40998-2). The research project was submitted to the Ethical Committee on Animal Use (CEUA) of the Centro Universitário Sagrado Coração (the Sagrado Coração University Center) (UNISAGRADO) (authorization nº 3353050417). According to Brazilian laws, species registration for scientific research purposes was carried out at SisGen (A7D0C01)
Specimens of gyrodactylids were collected under a dissecting microscope, preserved individually in 96% ethanol, and mounted on a slide prepared with Gray & Wess mounting medium, while others were stained with Gomori’s trichrome and mounted in Canada balsam (Humason, 1979). Drawings were prepared with the aid of a camera lucida on an Olympus BX51 microscope, equipped with phase contrast. Pictures were taken e câmera digital Olympus DP73, respectivamente acopladas em microscópio Olympus CX41.
Two specimens were processed for confocal laser scanning microscopy using a Leica TCS SP5 confocal microscope, for this, they were fixed with 70% alcohol, then stained with Gomori's trichrome and mounted between slide and cover slip and taken to the confocal microscope. The samples were imaged using laser 488 nm with fluorescence emission collected at 590 nm.
Measurements were made with ImageJ (NIH, Inc.) and are reported in micrometers (µm); the mean is followed by the range and sample size in parentheses (n). Measurements were taken by straight-line distances (Figs. 1–4).
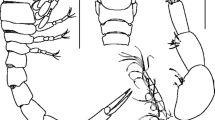
Measurements of the structures of Oncoceratium amphidactylum n. gen. n. sp. (Gyrodactylidae) on the body surface infecting of Hoplosternum littorale (Callichthyidae). 1. Superficial bar: LSB - length of superficial bar; WSB - Width of superficial bar; 2. Male copulatory organ: Diameter; 3. Anchor: LA – Length of anchor; LPA – Length of point of anchor. 4. Hook: LH - length of hooklet; LSH - length of shaft of hook; LFH – Length of filament of hook loop.
Type specimens were deposited in the helminthological collection of Museu de Zoologia da Universidade de São Paulo (the Zoological Museum of the University of São Paulo), São Paulo (MZUSP), Brazil, as described in the respective descriptions.
Molecular analysis
DNA extraction was performed with a blood and tissue kit (Qiagen, Hiden, Germany). The fragments of ITS 1, 5.8S and ITS 2 were amplified and sequenced with ITS 1 (5'-TTTCCGTAGGTGAACCT-3') and ITS 2 (5'-TCCTCCGCTTAGTGATA-3') primers, (Cunningham, 1997). The polymerase chain reaction (PCR) followed the protocol of the PureTaq Ready-to-go PCR Beads kit (GE Healthcare) with the addition of 2 µL of each primer (3.2 M), 3-4 µL of template DNA, and water to complete to a final volume 25 µL. The PCR cycle was performed with initial denaturation at 94 ºC (5 min) followed by 40 cycles as follows: denaturation at 94 °C (1 min), annealing at 50 °C (1 min), extension at 72 °C (1 min), and final extension 72 °C (5 min). The PCR results were confirmed through electrophoresis in 1.5 % agarose gel. The PCR product was purified with QIAquick PCR Purification Kit (Qiagen®, CA, USA). Automated sequencing was performed directly on the purified PCR products from specimens, using a BigDye v.3.1 Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Foster City, CA, USA) for cycle sequencing.
The obtained partial sequences were assembled and edited using Sequencher 4.8 software (Gene Codes Corporation) to obtain consensus sequences. The matrix was constructed with the ITS consensus sequence obtained in this study and those of 27 species, mainly from those genera with additional or modificated haptoral sclerites, available in the Genbank and with sequence obtained in this study (Table 1). All sequences were aligned using the ClustalW algorithm within MEGA X v10.0.5 (1993-2019) (Kumar et al., 2018) for distance analysis, and by ClustalW (Larkin et al., 2007) and standard settings in the Geneious 7.1.3 software (Kearse et al., 2012) for Bayesian inference (BI).
The Distance analysis was performed using the Neighbor-Joining method with 10,000 repetitions based on a Maximum Composite Likelihood (MCL) (Tamura et al., 2004) model and p-distance (Nei & Kumar, 2000) using 5.8s-ITS2 rDNA with bootstrap support. The distance analysis was performed with reduced fragment to represent interspecific distances and not the phylogenetic relationship among species. It was not possible to estimate the intraspecific distance because only one sequence of the new species was obtained.
Bayesian inference was performed using species of Gyrodactylus parasitizing callichthyid hosts as an outgroup. The nucleotide substitution model was selected from 82 possible evolutionary models, based on Akaike's information criterion, using the jModel Test 2.1 (Darriba et al., 2012), which identified the general time-reversible model with gamma distribution and invariant rates (GTR + I + G) as the model of best fit. The Bayesian inference (BI) method was used for phylogenetic analysis. MrBayes 3.1.2 (Ronquist & Huelsenbeck, 2003) was used to run BI using Markov Chain Monte Carlo (MCMC) tree searches. Two parallel runs of four simultaneous searches in the MCMC were carried out for 5 million generations each. The phylogenetic trees were used by MrBayes to estimate the subsequent probability of each node in our phylogenetic reconstruction. The phylogenetic tree obtained was visualized in Figtree 1.4.2 (Rambaut, 2014).
Results
Class Monogenoidea Bychowsky 1937
Subclass Polyonchoinea Bychowsky 1937
Order Gyrodactylidea Bychowsky 1937
Family Gyrodactylidae Van Beneden & Hesse 1863
Oncoceratium n. gen.
Etymology. The generic name originates from Greek and refers to the anchors’ shape that remembers horns (onco = hook; ceratium = diminutive of horn).
Diagnosis. Gyrodactylidae. Body divisible into cephalic region, trunk, peduncle, and haptor. Two cephalic lobes, each with head organ and spike sensillum. Cephalic glands present. Eye-spots absent. Pharynx composed of two subspherical bulbs. Two intestinal caeca not confluent. Gonads tandem; testis postgermarial. Male copulatory organ (MCO) bulbous, with one row of spinelets with similar sizes and shapes; the only spine usually found in the gyrodactylids species is absent; seminal vesicle and prostatic gland not observed. Uterus with up to two consecutive generations of embryos. Haptor with pair of ventral anchors, superficial bar rectangular, 16 hooks; hooks in two anterolateral clusters of eight hooks; the point of the anchor folded to out; deep bar absent; an only root without distinction of superficial and deep root. Parasitic of siluriforms fishes.
Oncoceratium amphidactylum n. gen. n. sp.
(Figs. 5–18)
Type host: Hoplosternum littorale (Hancock, 1828) (Callichthyidae).
Site of infestation: Body surface.
Prevalence and intensity: 22.6% and 3.1 ± 0.7
Type–locality: Batalha River (25° 50, 646′ S, 49° 43, 263′ W), Reginópolis, São Paulo, Brazil, December 2015.
Other localities: Peixe River (22° 49′ 50.7”S - 048° 06′ 37.0″ W) and Tietê River (22° 46′ 07.2″ S - 48° 08′ 27.7″ W), Anhembi municipal district, state of São Paulo.
Specimens studied: Holotype (MZUSP 8040); Paratypes (MZUSP 8041 a-k).
Etymology: The specific name originates from Greek and refers to the hooks, which are reminiscent of fingers, separated on two lobes on the haptor (amphi = on two sides; dactylum = finger).
Description: Body truncated, 558 (421–691, n = 10) long, widest at level of midlength 157 (123–227, n = 10) (Figs. 5, 10, 11). Unicellular cephalic glands, head organ conspicuous. Proximal pharyngeal bulb 83 (56–121, n = 8) in diameter, ovate; distal pharyngeal bulb 63 (51–73, n = 5) diameter, ovate. Male copulatory organ (MCO) 18 (17–19, n = 4) in diameter, bulbous, armed with one row of 5-6 spinelets (Figs. 7, 13). The single spine usually found in gyrodactylid species is absent. Seminal vesicle not observed. Gonads tandem. Testis 48.61 (n = 1) long, 35 (n = 1) wide, ovate, postgermarial and intercaecal. Germarium 29.2 (27.5–31.0, n = 2) in diameter, rounded. Uterus with up to two generations of embryos. Follicles, apparently glandular, posterior to the terminations of the caeca. Haptor 93 (63–137, n = 12) long, 134(83–196, n = 12) wide. Anchor 113 (103–125, n = 8) long; shaft slightly curved; point 24 (20–28, n = 8) long, curved outwards (Figs. 6, 9, 14, 15); superficial and deep roots undifferentiated, forming a single root (Figs. 9, 14, 15). Superficial bar 45 (37–51, n = 6) long, 14 (12–17, n = 6) wide, rectangular (Figs. 6, 14, 16, 17). Shield on the superficial bar and deep bar absent. Hooks of similar size and shape, distributed in two lobes anterolaterally disposed to the anchors, with eight hooks on each (Fig. 8, 12, 18). Hook shank 21 (17–22, n = 9) long; hooklet 7 (6–8, n = 9) long; point and shaft continuous, evenly curved; shelf concave, toe rounded; heel rounded; Filament hook loop 16 (12–23, n = 5) long (Figs. 8, 12, 18).
Photomicroscopy of Oncoceratium amphidactylum n. gen. n. sp. (Gyrodactylidae) on the body surface of Hoplosternum littorale (Callichthyidae), holotype. 10. Wholemount of adult, ventral view. 11. Wholemount ventral view of young with embryo in the uterus. 12. Hook. 13. MCO. 14. The complex of anchors, superficial bar. Objectives used in the photos: Fig. 10-11- 10X magnification; Figs. 12-13 - 100X magnification; Fig. 14 - 40X.
Differential morphological diagnosis
Oncoceratium amphidactylum n. gen. n. sp. is differentiated from other species of gyrodactylids by its distinctive and unique features. The horn-shaped anchor with its point curved outwards has never been observed in gyrodactylid species, including species of Gyrodactylus, which have a recurved point, forming a hook. Obviously, not all species of Gyrodactylus were compared, but in the last descriptions of species of Gyrodactylus from the Neotropical region, all species were described as having an anchor with a recurved point, forming an angle (like-hook) (Popazoglo & Boeger, 2000; Bueno-Silva & Boeger, 2009; Rubio-Godoy et al., 2010; Bueno-Silva & Boeger, 2014; García-Vásquez et al., 2015; 2018; 2019; Razzolini et al., 2019; Vianna & Boeger, 2019).
The MCO of the new species is bulbous, with one row of spinelets of similar sizes and shapes, while the unique spine usually observed in gyrodactylid species is absent or undifferentiated. Species of gyrodactylids from Neotropical region presents MCO bulbous, with spine and one or two rows of spinelets. The only species that presents an arrangement with spinelets of the same size in the only row (18-20 spinelets) is Gyrdicotylus gallieni Vercammen-Grandjean, 1960, a parasite of Xenopus laevis (Daudin) (Anura, Pipidae).
The hooks distributed in two anterolateral lobes differentiate O. amphidactylum n. gen. n. sp. from remaining gyrodactylid species, which possess hooks distributed in the anterior and posterior lobes. In the Neotropical region, species of Accessorius, Diechodactylus, Polyclithrum Rogers, 1967 and Scutalatus were described as having hooks distributed on the anterior and posterior lobes (Rogers, 1967; 1969; Vianna et al., 2007; 2008).
Representative DNA sequences and molecular diagnosis
An ITS (Internal Transcribed Spacer) rDNA sequence from O. amphidactylum n. gen. n. sp. (GenBank accession number: MT345551) was produced from one specimen.
The sequence of the new species has lengths of 691 bp, composed by partial ITS1 (261 bp), 5.8s gene (155 bp) and partial ITS2 (175 bp). The content of the sequence was A=171 (24.75%); C=128 (18.52%); G=140 (20.26%); and T=252 (36.47%); while the content of G+C = 38.78%, and the content of A+T = 61.22%. The GenBank BLAST revealed closest matches with D. martini (AM943008), followed for several species of Gyrodactylus from out of Neotropical region.
Pairwise distance analysis supports new species status for O. amphidactylum n. gen. n. sp. and indicates sufficient interspecific genetic distance to differentiate the new species from those of other genera, including Gyrodactylus. The pairwise distance based on Neighbor-Joining varied between 0.374 – 0.842 using MCL, and between 0.249 – 0.386 using p-distance. The smallest distances obtained were among O. amphidactylum n. gen. n. sp. and non-Gyrodactylus species, while the differences among species of Gyrodactylus were greatest (Table 2).
The phylograms reflect pairwise distance, where the new species clustered near Gyrodactyloides bychowskii Albova, 1948, Laminiscus gussevi (Bychowsky & Polyansky, 1953) Pálsson & Beverely-Burton, 1983 and I. rivuli (Fig. 19). This grouping is inserted in a cluster composed of species with some modification (like the shield of I. rivuli), additions (such as sclerotized plates and ribs), or the absence of haptoral structures (such as a superficial bar and shield). Species of Gyrodactylus were observed at the base of the phylogram (from neotropical callichthyids) or interspersed among Fundulotrema prolongis (Hargis, 1955) Kritsky & Thatcher, 1977, Paragyrodactylus variegatus You, King, Ye & Cone, 2014, and Swingleus ancistrus Billeter, Klink & Maugel, 2000, but distant from O. amphidactylum n. gen. n. sp. The bootstrap support value was low in MCL analysis (44) and high in the p-distance analysis (74) (Fig. 19). However, the phylograms were used to test the status of a new taxon to a new species, and not test phylogenetic position among gyrodactylids.
The Bayesian inference showed O. amphidactylum n. gen. n. sp. near all the selected species with the modification, addition, or absence of haptoral structures, and distant from the cluster composed of species of Gyrodactylus, but with low posterior probability support (0.33). Pairwise distance analysis and Bayesian inference support the new species and distance it from species of Gyrodactylus (Fig. 20).
Discussion
Siluriform fishes from the Neotropical region have been reported as hosts to several species of Gyrodactylidae, of which callichtyid fishes have the highest number of records of parasitosis by gyrodactilids. Callichtyids have been harboring only species of Gyrodactylus (Popazoglo & Boeger, 2000; Bueno-Silva & Boeger, 2009; 2014; Vianna & Boeger, 2019). In this study, Oncoceratium n. gen. is proposed to accommodate O. amphidactylum n. gen. n. sp., described from the callichthyid fish, H. littorale. Oncoceratium amphidactylum n. gen. n. sp. is differentiated from all others species of gyrodactylids by the presence of a horn-shaped anchor with its point curved outward, containing undifferentiated roots that form a continuous region.
Prior to the present study, the anchor pattern found in species of the gyrodactylid genera presented a recurved point forming an angle. Despite the difficulties of revising all Gyrodactylus species due to their high number, all species recently described in the Neotropical region have an anchor with a recurved point, forming an angle between the shaft and the point (Kritsky et al., 1995; Popazoglo & Boeger, 2000; Bueno-Silva & Boeger, 2009; 2014; Rubio-Godoy et al., 2010; García-Vásquez et al., 2015; 2018; 2019; Razzolini et al., 2019; Vianna & Boeger, 2019), as along with species of other genera. The root is one single continuous structure, without evident differences between superficial (ventral) and deep (dorsal) roots, as in species of Archigyrodactylus (Mizelle & Kritsky, 1967). Most species of gyrodactylids possess conspicuous knob-like superficial and deep roots (e.g. species of Gyrodactylus), but few species present modifications. Diechodactylus joaberii and S. magniancoratus, for example, both have a superficial root-like keel-shape structure, and an elongated deep root (Vianna et al., 2007; 2008).
Neighbor-joining phylogram of Oncoceratium amphidactylum n. gen. n. sp. (Gyrodactylidae) and of other neotropical and non-neotropical species based on the Maximum Composite Likelihood (Nei & Kumar, 2004) model (A) and based on p-distance (B) using 5.8s and partial ITS2 rDNA fragment. Bootstrap values (n = 10,000) are presented near the branches.
The most common hook arrangement in gyrodactylids is radial at the edge of the haptor, but the hooks of O. amphidactylum n. gen. n. sp. are the same size and are distributed in two anterolateral lobes. However, in species of ten genera, different numbers of hooks are distributed in the anterior and posterior lobes of the haptor (Bakke et al., 2007), and there is the presence of further sclerites. Species of Macrogyrodactylus Malmberg, 1957 presents one pair of hooks on the anterior lobe and seven pairs on the posterior lobe (1+7). Species of Accessorius, Fundulotrema Kristky & Thatcher, 1977, Gyrocerviceanseris & Swingleus Rogers, 1969 present 3+5, while species of Gyrodactyloides Bychowsky, 1947, Lamniscus Pálsson & Beverly-Burton, 1983, Polyclithrum, and Scutalatus contain 4+4. The only species of Diechodactylus described contained 5+3 (Rogers, 1967; 1969; Kritsky & Thatcher, 1977; Pálsson & Beverley-Burton, 1983; An et al., 1991; Vianna et al., 2007; 2008; Cone et al., 2010). The African Diplogyrodactylus martini Přikrylová, Matějusová, Musilová, Gelnar & Harris, 2009 presents hooks of two different shapes and sizes, whilst the hooks of Tresuncinidactylus wilmienae Přikrylová, Barson & Shinn, 2021 possess three sizes, with the same shape (Přikrylová et al., 2009; 2021).
Oncoceratium amphidactylum n. gen. n. sp. presents an MCO bulbous, with spinelets of similar sizes and shapes distributed in a row, without the only spine usually observed in gyrodactylid species. This arrangement is very similar to that found in G. gallieni, which is a parasite of anuran (Pipidae), and presents only a row (ring) with 18-20 spinelets. Most species of gyrodactylids usually have a muscular bulbous MCO with a single spine and different numbers of rows and spinelets on each (e.g. Gyrodactylus). However, some species have been described presenting different patterns of MCO. African species are the only viviparous species known so far to have a muscular pouch-type MCO, like those of the genera Afrogyrodactylus Paperna, 1968, Citharodactylus, Diplogyrodactylus, Mormyrogyrodactylus, as well as Tresuncinidactylus, the MCO of which is an armed or unarmed muscular duct onto the muscular pouch (Přikrylová et al., 2009; Přikrylová & Luus-Powell, 2014).
Obtaining a single 5.8s and an ITS2 sequence from the new species did not allow phylogenetic position among the gyrodactylids to be established. Despite the analysis of distance not providing enough bootstrap support (Figs. 19 and 20), it showed the new species as near G. bychowskii, L. gussevi and I. rivuli, and distant from species of Gyrodactylus. The proximity of the new species to species with the addition or modification of haptoral sclerites was also observed with Bayesian inference. Gyrodactyloides bychowskii, L. gussevi and I. rivuli were observed composing a clade with Scleroductus sp. (not used in the present study) through the use of 18S rDNA (Přikrylová et al., 2021). Similar topology to the analyses made in this study was observed by Schelkle et al. (2011), which identified cluster of species with the addition of haptoral sclerites in the same clade, using only the ITS2 rDNA in Maximum likelihood analysis.
The smallest pairwise distance values were among O. amphidactylum gen. n. sp. n. and non-Gyrodactylus species, suggesting greater proximity between them, but still allowing them to be differentiated from one another. However, distances were bigger among the new species and species of Gyrodactylus (Table 2), indicating that the new species does not belong to Gyrodactylus. A pairwise distance greater than 1% could indicate interspecific differentiation when associated with conspicuous differentiations, especially ecological differentiations (Ziętara & Lumme, 2003).
Oncoceratium n. gen. is monotypic, as are the vast majority of genera in Gyrodactylidae (11 of 25 genera), such as the recently proposed Tresuncinidactylus (Přikrylová et al., 2021). Afrogyrodactylus was considered monotypic for 45 years until A. girgifae Přikrylová & Luus-Powell, 2014 and A. kingi Přikrylová & Luus-Powell, 2014 were described. Three years later, A. ardae Přikrylová & Gelnar, 2017, was described. The same occurred with other species-poor genera proposed as monotypic, for which new species were identified years later, such as Paragyrodactylus Gvosdev & Martechov, 1953, Polyclithrum (proposed in 1967), Swingleus (proposed in 1969), and Scleroductus (proposed in 1989). However, Acanthoplacatus Ernst, Jones & Whittington, 2001, Fundulotrema, Gyrodactylus, and Macrogyrodactylus were proposed with the description of more than one species. Currently, more than one species is known in Afrogyrodactylus (4 spp.), Archigyrodactylus (3), Acanthoplacatus (7), Fundulotrema (6), Gyrodactyloides (4), Gyrodactylus (500+) (Pinacho-Pinacho et al., 2021), Isancistrum de Beauchamp, 1912 (2), Laminiscus Pálsson & Beverley-Burton, 1983 (2), Macrogyrodactylus (6), Paragyrodactylus (3), Polyclithrum (5), Scleroductus (3), and Swingleus (2).
The late description of species in certain monotypic genera of Gyrodactylidae may be the result of the scarcity of taxonomists with an interest in gyrodactylids, insufficient sampling, or the technological difficulty of diagnosis, and can explain the emergence of monotypic genera over several decades. From the 1950s onwards, several species were known for most genera, nevertheless, the use of molecular data in integrative diagnoses began to be used on a large scale from the 2000s, resolving some taxonomic bottlenecks in Gyrodactylidae.
Data availability
The sequence obtained was submitted to GenBank under the following access number: MT345551 (https://www.ncbi.nlm.nih.gov/nuccore/MT345551). Other datasets generated during the current study will also be available from the corresponding author on reasonable request.
References
An, L., Jara, C. A., Cone, D. K. (1991). Five species of Gyrodactylus Nordmann, 1832 (Monogenea) from freshwater fishes of Peru. Canadian Journal of Zoology, 69(5), 1199–1202. https://doi.org/10.1139/z91-171
Bakke, T. A., Cable, J., Harris, P. D. (2007). The biology of gyrodactylid monogeneans: the “Russian-doll killers”. Advances in Parasitology, 64,161–276. https://doi.org/10.1016/S0065-308X(06)64003-7
Boeger, W. A., Vianna, R. T. (2006). Monogenoidea. In V. E. Thatcher (Ed.) Amazon Fish Parasites. (2nd ed., pp 42–116). Pensoft Publishers, Sofia, Bulgaria.
Bueno-Silva, M., Boeger, W. A. (2009). Neotropical Monogenoidea. 53. Gyrodactylus corydori n. sp. and redescription of Gyrodactylus anisopharynx (Gyrodactylidea: Gyrodactylidae), parasites of Corydoras spp. (Siluriformes: Callichthyidae) from southern Brazil. Folia Parasitologica, 56, 13–20. https://doi.org/10.14411/fp.2009.003
Bueno-Silva, M., Boeger, W. A. (2011). Choice matters: incipient speciation in Gyrodactylus corydori (Monogenoidea: Gyrodactylidae). International Journal for Parasitology, 41(6), 657–67. https://doi.org/10.1016/j.ijpara.2011.01.002
Bueno-Silva, M., Boeger, W. A. (2014). Neotropical Monogenoidea. 58. Three new species of Gyrodactylus (Gyrodactylidae) from Scleromystax spp. (Callichthyidae) and the proposal of COII gene as an additional fragment for barcoding gyrodactylids. Folia Parasitologica, 6(3), 213–222. https://doi.org/10.14411/fp.2014.028
Cable, J., Harris, P. D., Tinsley, R. C., Lazarus, C. M. (1999). Phylogenetic analysis of Gyrodactylus spp. (Platyhelminthes: Monogenea) using ribosomal DNA sequences. Canadian Journal of Zoology, 77(9), 1439-1449. https://doi.org/10.1139/z99-069
Cable, J., Oosterhout, C., Barson, N., Harris, P. D. (2005). Gyrodactylus pictae n. sp. (Monogenea: Gyrodactylidae) from the Trinidadian swamp guppy Poecilia picta Regan, with a discussion on species of Gyrodactylus von Nordmann, 1832 and their poeciliid hosts. Systematic parasitology, 60, 159-164. https://doi.org/10.1007/s11230-004-6348-4
Cohen, S. C., Justo, M. C. N., Kohn, A. (2013). South American Monogenoidea parasites of fishes, amphibians and reptiles. Fiocruz, Rio de Janeiro. https://doi.org/10.13140/2.1.2571.9049
Cone, D., Abbott, C., Gilmore, S., Burt, M. (2010). A new genus and species of Gyrodactylidae (Monogenea) from Silver Hake, Merluccius bilinearis, in the Bay of Fundy, New Brunswick. Canadian Journal of Zoology, 96(4), 681–684. https://doi.org/10.1645/GE-2359.1
Cunningham, C. W. (1997). Is congruence between data partitions a reliable predictor of phylogenetic accuracy? Empirically testing an iterative procedure for choosing among phylogenetic methods. Systematic Biology, 46(3), 464–478. https://doi.org/10.1093/sysbio/46.3.464
Darriba, D., Taboada, G. L., Doallo, R., Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9(8), 772. https://doi.org/10.1038/nmeth.2109
García-Vásquez, A., Razo-Mendivil, U., Rubio-Godoy, M. (2015). Morphological and molecular description of eight new species of Gyrodactylus von Nordmann, 1832 (Platyhelminthes: Monogenea) from poeciliid fishes, collected in their natural distribution range in the Gulf of Mexico slope, Mexico. Parasitology Research, 114, 3337-3355. https://doi.org/10.1007/s00436-015-4559-z
García-Vásquez, A., Pinacho-Pinacho, C. D., Guzmán-Valdivieso, I., Salgado-Maldonado, G., Rubio-Godoy, M. (2019). New species of Gyrodactylus von Nordmann, 1832 from native fish from Chiapas, Mexico, studied by morphology and molecular analyses. Acta Parasitologica, 64, 551–565. https://doi.org/10.2478/s11686-019-00088-y
García-Vásquez, A., Rubio-Godoy, M., Guzmán-Valdivieso, I., Razo-Mendivil, U. (2018). Three new species of Gyrodactylus von Nordmann, 1832 described from Goodea atripinnis (Pisces: Goodeidae), an endemic fresh water fish from the central highlands of Mexico. Parasitology Research, 117, 139–150. https://doi.org/10.1007/s00436-017-5680-y
Humason, G. L. (1979). Animal Tissue Techniques. Freeman and Company, San Francisco.
Huyse, T., Pampoulie, C., Audenaert, V., Volckaert, F. A. M. (2006). First report of Gyrodactylus spp. (Platyhelminthes: Monogenea) in the Western Mediterranean sea: Molecular and morphological descriptions. The Journal of Parasitology, 92(4), 682–690. https://doi.org/10.1645/GE-690R.1
Jara, C. A., An, L., Cone, D. K. (1991). Accessorius peruensis gen. et sp. n. (Monogenea: Gyrodactylidea) from Lebiasina bimaculata (Characidae) in Peru. Journal of the Helminthological Society of Washington, 58(2), 164–166.
Jara, C. A., Cone, D. K. (1989). Scleroductus yuncensi gen. et n. sp. (Monogenea) from Pimelodella yuncensis (Siluriformes: Pimelodidae) in Peru. Proccedings of the Helminthological Society of Washington, 56: 125–127.
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Meintjes, P., Drummond, A. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28(12), 1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Kritsky, D. C., Boeger, W. A., Popazoglo, F. (1995). Neotropical Monogenoidea. 22. Variation in Scleroductus species (Gyrodactylidea, Gyrodactylidae) from Siluriformes fishes of Southeastern Brazil. Journal of the Helminthological Society of Washington, 62, 53–56.
Kritsky, D. C., Fritts, T. H. (1970). Monogenetic trematodes from Costa Rica with the proposal of Anacanthocotyle gen. n. (Gyrodactylidae: Isancistrinae). Proccedings of the Helminthological Society of Washington, 37(1), 63-68.
Kritsky, D. C., Thatcher, V. E. (1977). Phanerothecium gen. nov. and Fundulotrema gen. nov., two new genera of viviparous monogenoidea (Gyrodactylidae) with a description of P. caballeroi and a key to the subfamilies and genera of the family, Exerta Parasitologica en memoria del doctor Eduardo Caballero y Caballero, Instituto de Biologie (Mexico). Publicaciones Especiales, 4, 53–60.
Kumar, S., Stecher, G., Li, M., Knyaz, C., Tamura, K. (2018). MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution, 35(6), 1547–1549. https://doi.org/10.1093/molbev/msy096
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H., Valentin, F., Wallace, I. M., Wilm, A., Lopez, R., Thompson, J. D., Gibson, T. J., Higgins, D. G. (2007). Clustal W and Clustal X version 2.0. Bioinformatics, 23(21), 2947–2948. https://doi.org/10.1093/bioinformatics/btm404
Matejusová, I., Gelnar, M., Verneau, O., Cunningham, C.O., Littlewood, D.T.J. (2003). Molecular phylogenetic analysis of the genus Gyrodactylus (Platyhelminthes: Monogenea) inferred from rDNA ITS region: subgenera versus species groups. Parasitology, 127, 603–611. https://doi.org/10.1017/s0031182003004098
Nei, M., Kumar, S. (2000). Molecular Evolution and Phylogenetics. Oxford University Press, New York.
Pálsson, J., Beverley-Burton, M. (1983). Laminiscus n. g. (Monogenea: Gyrodactylidae) from capelin, Mallotus villosus (Müller), (Pisces: Osmeridae) in the northwest Atlantic with redescriptions of L. gussevi n. comb., Gyrodactyloides petruschewskii, and G. andriaschewi. Canadian Journal of Zoology, 61(2), 298–306. https://doi.org/10.1139/z83-040
Pinacho-Pinacho, C. D., Calixto-Rojas, M., García-Vásquez, A., Guzmán-Valdivieso, I., Barrios-Gutiérrez, J. J., Rubio-Godoy, M. (2021). Species delimitation of Gyrodactylus (Monogenea: Gyrodactylidae) infecting the southernmost cyprinids (Actinopterygii: Cyprinidae) in the New World. Parasitology Research, 120, 831–848. https://doi.org/10.1007/s00436-020-06987-8
Popazoglo, F., Boeger, W. A. (2000). Neotropical Monogenoidea. 37. Redescription of Gyrodactylus superbus (Szidat, 1973) comb. n. and description of two new species of Gyrodactylus (Gyrodactylidea: Gyrodactylidae) from Corydoras paleatus and C. ehrhardti (Teleostei: Siluriformes: Callichthyidae) of Southern Brazil. Folia Parasitology, 47(2), 105–110. https://doi.org/10.14411/fp.2000.022
Prikrylová, I., Barson, M., Shinn, A. P. (2021). Description of Tresuncinidactylus wilmienae gen. et sp. n. (Monogenea: Gyrodactylidae), from the gills of the bulldog, Marcusenius macrolepidotus (Peters) from Lake Kariba, Zimbabwe. Folia Parasitologica, 68: 025. https://doi.org/10.14411/fp.2021.025
Prikrylová, I., Luus-Powell, W. J. (2014). Revision of the genus Afrogyrodactylus Paperna, 1968 (Monogenea: Gyrodactylidae) with description of two new species from geographically distant localities. Folia Parasitologica, 61(6), 529–536. https://doi.org/10.14411/fp.2014.066
Prikrylová, I., Matějusová, I., Musilová, N., Gelnar, M., Harris, P. D. (2009). A new gyrodactylid (Monogenea) genus on gray bichir, Polypterus senegalus (Polypteridae) from Senegal (West Africa). The Journal of Parasitology, 95(3), 555–560. https://doi.org/10.1645/GE-1652.1
Přikrylová, I., Shinn, A. P., Paladini, G. (2017). Description of Citharodactylus gagei gen. n. et sp. n. (Monogenea: Gyrodactylidae) from the moon fish, Citharinus citharus (Geoffroy Saint-Hilaire), from Lake Turkana. Parasitology Research, 116, 281–292. https://doi.org/10.1007/s00436-016-5289-6
Přikrylová, I., Vanhove, M. P. M., Janssens, S. B., Billeter, P. A., Huyse, T. (2013). Tiny worms from a mighty continent: high diversity and new phylogenetic lineages. Molecular Phylogenetics and Evolution, 67(1), 43–52. https://doi.org/10.1016/j.ympev.2012.12.017
Rambaut, A. (2014). FigTree v1.4.2, a graphical viewer of phylogenetic trees. Retrieved June 9, 2021, from http://tree.bio.ed.ac.uk/software/figtree/right angle bracket
Razo-Mendivil, U., García-Vásquez, A., Rubio-Godoy, M. (2016). Spot the difference: Two cryptic species of Gyrodactylus von Nordmann, 1832 (Platyhelminthes: Monogenea) infecting Astyanax aeneus (Actinopterygii, Characidae) in Mexico. Parasitology International, 65(5), 389–400. https://doi.org/10.1016/j.parint.2016.05.009
Razzolini, E., Murari, A. L., Baldisserotto, B., Boeger, W. A. (2019). Gyrodactylus lilianae n. sp. (Polyonchoinea: Gyrodactylidae) from Rhamdia quelen (Quoy & Gaimard) (Siluriformes: Heptapteridae) from southern Brazil: a potential nuisance for aquaculture. Systematic Parasitology, 96, 407–415. https://doi.org/10.1007/s11230-019-09858-8
Reis, R. E., Kullander, S. O., Ferraris, C. J. (2003). Check list of the freshwater fishes of South and Central America, Edipucrs, Porto Alegre.
Rogers, W. A. (1967). Polyclithrum mugilini gen. et sp. n. (Gyrodactylidae: Polyclithrinae subfam. n.) from Mugil cephalus L. The Journal of Parasitology, 53(2), 274–276. https://doi.org/10.2307/3276574
Rogers, W. A. (1969). Swingleus polyclithroides gen. et sp. n. (Monogenea: Gyrodactylidae) from Fundulus grandis Baird and Girard. Tulane Studies in Zoology and Botany, 16, 22–25.
Ronquist, F., Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19(12), 1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Rubio-Godoy, M., Paladini, G., García-Vásquez, A., Shinn, A. P. (2010). Gyrodactylus jarocho sp. nov. and Gyrodactylus xalapensis sp. nov. (Platyhelminthes: Monogenea) from Mexican poeciliids (Teleostei: Cyprinodontiformes), with comments on the known gyrodactylid fauna infecting poeciliid fish. Zootaxa, 2509(1), 1–29. https://doi.org/10.11646/zootaxa.2509.1.1
Schelkle, B., Paladini, G., Shinn, A. P., King, S., Johnson, M., Oosterhout, C., Mohammed, R. S., Cable, J. (2011). Ieredactylus rivuli gen. et sp. nov. (Monogenea, Gyrodactylidae) from Rivulus hartii (Cyprinodontiformes, Rivulidae) in Trinidad. Acta Parasitologica, 56(4), 360–370. https://doi.org/10.2478/s11686-011-0081-3
Tamura, K., Nei, M., Kumar, S. (2004). Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences, 101(30), 11030–11035. https://doi.org/10.1073/pnas.0404206101
Tu, X., Ling, F., Huang, A., Wang, G. (2015). An infection of Gyrodactylus kobayashii Hukuda, 1940 (Monogenea) associated with the mortality of goldfish (Carassius auratus) from central China. Parasitology Research, 114: 737–745. https://doi.org/10.1007/s00436-014-4241-x
Vianna, R. T., Boeger, W. A. (2019). Neotropical Monogenoidea. 60. Two new species of Gyrodactylus (Monogenoidea: Gyrodactylidae) from the armored-catfish, Pareiorhaphis parmula Pereira (Loricariidae) and from the cascarudo, Callichthys callichthys (Linnaeus) (Callichthyidae) from Brazil. Zootaxa, 4551(1), 87–93. https://doi.org/10.11646/zootaxa.4551.1.6
Vianna, R. T., Boeger, W. A., Dove, A. D. M. (2007). Neotropical Monogenoidea. 51. Scutalatus magniancoratus gen. et sp. n. (Gyrodactylidae) from the South American electric eel, Electrophorus electricus (Gymnotidae, Gymnotiformes), and redescription of Mormyrogyrodactylus gemini from the African bulldog, Marcusenius macrolepidotus (Mormyridae, Osteoglossiformes). Acta Zoologica, 88, 89–94. https://doi.org/10.1111/j.1463-6395.2007.00255.x
Vianna, R. T., Boeger, W. A., Silva-Souza, A. T. (2008). Neotropical Monogenoidea. 52. Diechodactylus joaberi n. g., n. sp. from the banded knifefish Gymnotus carapo (Gymnotiformes: Gymnotidae) in southeastern Brazil. Systematic Parasitology, 69, 45–50. https://doi.org/10.1007/s11230-007-9107-5
You, P., King, S. D., Ye, F., Cone, D. K. (2014). Paragyrodactylus variegatus n. sp. (Gyrodactylidae) from Homatula variegate (Dabry De Thiersant, 1874) (Nemacheilidae) in Central China. The Journal of Parasitology, 100, 350–355. https://doi.org/10.1186/1756-3305-7-377
Ziętara, M. S., Huyse, T., Lumme, J. (2003). The crossroads of molecular, typological and biological species concepts: two new species of Gyrodactylus Nordmann, 1832 (Monogenea: Gyrodactylidae). Systematic Parasitology, 55, 39-52. https://doi.org/10.1023/A:1023938415148
Ziętara, M. S., Huyse, T., Lumme, J., Volckaert, F. A. (2002). Deep divergence among subgenera of Gyrodactylus inferred from rDNA ITS region. Parasitology, 124, 39–52. https://doi.org/10.1017/s0031182001008939
Acknowledgments
We would like to thank the São Paulo Research Foundation (FAPESP) for the research project grant of Vanessa D. Abdallah (2012/23655-0) and the National Council for Scientific and Technological Development (306987/2018-0).
Author information
Authors and Affiliations
Contributions
RTV, LSP and DHMDV. wrote the main manuscript text. LSP collected the data. RTV, LSP and DHMDV performed the analysis. RTV prepared the figures. LSP and VDA conceived the original idea. VDA and RKA obtained financial support for the project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Consent to participate
All authors gave their consent to participate in the research.
Consent for publication
All authors gave their consent for publication.
Ethical approval
Compliance with ethical standards (information about ethical committee for animal use in research in Materials and Methods section).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vianna, R.T., Pelegrini, L.S., Vieira, D.H.M.D. et al. Oncoceratium amphidactylum n. gen. n. sp. (Monogenoidea: Gyrodactylidae) from Hoplosternum littorale (Hancock) (Siluriformes, Callichthyidae) from southeastern Brazil. Syst Parasitol 100, 455–471 (2023). https://doi.org/10.1007/s11230-023-10097-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-023-10097-1