Abstract
Three monogenean species, Anacanthorus luquei n. sp., A. scholzi n. sp. and A. cohenae n. sp. are described from the gills of the tetra fish Markiana nigripinnis (Perugia) (Characidae), collected in the Pantanal wetlands, State of Mato Grosso do Sul, Brazil. Among other differences, Anacanthorus luquei n. sp. differs from the most morphologically similar species, based on the structure of the accessory piece as follows: branches with smooth margins (vs with irregular margins in A. cuticulovaginus), without pointed projections at distal end (vs with projections in A. dipelecinus) and with 2 branches (vs 3 in A. quinqueramus). Anacanthorus scholzi n. sp. is most morphologically similar to A. luquei n. sp., differing from it because one of the branches of the accessory piece is bifurcated at the distal portion. Anacanthorus cohenae n. sp. can be differentiated from the congeners based on the combination of the following features: MCO cylindrical and robust with sclerotised flanges on the extremities, accessory piece V-shaped, bearing two branches similar in length and with blunt distal ends, and hooks with a proximal bulb. This is the first parasitological study on M. nigripinnis and, currently, Anacanthorus allocates 88 species infesting characiform fishes in the Neotropical region, including the three new species described here.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pantanal is one of the richest biomes within the Neotropical region regarding animal species (Polaz et al., 2014). Its marked seasonal variations, characterised by distinct periods of flood and drainage, support a rich ichthyofauna (Corrêa et al., 2009; Britski et al., 2007). Regarding these fishes as hosts, it is possible to assume that they support communities of parasites also rich and abundant (Hechinger & Lafferty, 2005; Poulin, 2014). However, considering the high biodiversity potential of the Neotropics (and consequently of the Pantanal wetlands), our current knowledge of these organisms is incipient since less than 25% of the species of fish have been investigated for parasites (Luque & Poulin, 2007; Luque et al., 2017).
Among the helminth parasites of fishes, dactylogyrid monogeneans (Monogenea: Dactylogyridae) represent a large and specious group (Boeger & Vianna, 2006; Cohen et al., 2013). Similarly, Anacanthorus Mizelle & Price, 1965 is a highly diverse genus of dactylogyrids, allocating a large number of host-specific species, which infect the gills of freshwater characiforms (Actinopterygii: Characiformes) in the Neotropical region (Cohen et al., 2013). Currently, Anacanthorus includes 85 species, of which 37 were originally described from fishes belonging to the Serrasalmidae, 21 to the Triportheidae, 15 to the Bryconidae, 11 to the Erythrinidae and one to the Characidae (Cohen et al., 2013; Leão et al., 2015; Monteiro et al., 2015; Moreira et al., 2019; Santos-Neto et al., 2019).
During the first parasitological study of Markiana nigripinnis (Perugia) (Characidae), collected in the Pantanal wetlands, State of Mato Grosso do Sul, Brazil, several helminths were recovered. Morphological observations of the monogeneans revealed that they belong to Anacanthorus and represent three new species described herein.
Materials and methods
Fifty-seven specimens of M. nigripinnis were acquired from local fishermen from December 2013 to September 2014, at the municipality of Corumbá, Pantanal wetlands, State of Mato Grosso do Sul, Brazil. Fish were kept in water tanks with oxygen pumps prior to necropsy. Host identification follows Britski et al. (2007); nomenclature and classification were updated according to the FishBase (Froese & Pauly, 2019). Monogeneans were removed from the gills of fresh killed hosts with aid of a dissecting microscope and fixed in hot 4% formalin. Some specimens were mounted unstained in Grey & Wess medium for observation of the sclerotised structures; others were stained with Gomori’s trichrome and mounted in Canada balsam in order to study internal anatomy (Humason, 1979; Boeger & Viana, 2006). Light microscopic observations, drawings and measurements (obtained according to the procedures of Mizele & Klucka, 1953) were made with the aid of a Leica DM 5500B equipped with Nomarski interference contrast, a camera Leica DFC495 and the software Leica LAS (version 4.0) with multifocus imaging capture system, as well as a Olympus BX51 with phase contrast and a drawing tube attached. Classification of hooks is according to Mizelle & Price (1963), the description of male copulatory organ (MCO) and morphological terminology follow Kritsky & Mizelle (1968), Mizelle et al. (1968) and Boeger & Vianna (2006). The filamentous hook is indicated as FH throughout the text. All measurements are in micrometres and are expressed as the range, followed by the mean and the number of measured structures (n) in parentheses.
The type-specimens were deposited in the “Coleção Zoologica da Universidade Federal de Mato Grosso do Sul” (acronym ZUFMS).
To comply with the regulations set out in Article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of all new taxa have been submitted to ZooBank. For each new taxon, the Life Science Identifier (LSID) is reported in the taxonomic summary.
Family Dactylogyridae Bychowsky, 1933
Genus Anacanthorus Mizelle & Price, 1965
Anacanthorus luquei n. sp.
Type-host: Markiana nigripinnis (Perugia) (Characiformes: Characidae), tetra fish.
Type-locality: Marginal lake to the road MS184 (19°34.576′S, 57°00.823′W), Corumbá, State of
Mato Grosso do Sul, Brazil.
Type-material: Holotype (ZUFMS-PLA00017) and 3 paratypes (ZUFMS-PLA00018–20).
Site on host: Gill filaments.
Prevalence: 12.3% (7 fish infected of 57 examined).
ZooBank registration: The Life Science Identifier (LSID) for Anacanthorus luquei n. sp. is urn:lsid:zoobank.org:act:38C6D732-DE8A-4C76-9C78-7BDD63A02402.
Etymology: The specific name honours Dr José Luis Luque for his contributions to the study of Neotropical parasites of fish.
Description
[Based on 21 specimens: 15 mounted in Gray & Wess medium and 6 stained with Gomori’s trichrome; Figs. 1–4.] Body fusiform, 315–1,340 (545; n = 21) long, with greatest width 87–155 (131; n = 21) at midlength (Fig. 1). Tegument smooth. Cephalic lobes 3, well defined, with Y-shaped cephalic organs: 1 medial bearing a pair of organs and 2 lateral bearing a single pair. Eyes 4, equidistant, with irregular shape, posterior pair larger than anterior; accessory granules absent or rarely present. Pharynx spherical to subspherical, 18–47 (34; n = 18) in diameter. Intestinal caeca 2, encircling internal organs, bifurcating slightly posterior to pharynx and joining slightly posterior to testis.
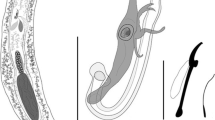
Anacanthorus spp. ex Markiana nigripinnis, from Pantanal wetlands, State of Mato Grosso do Sul, Brazil. 1–4, Anacanthorus luquei n. sp. 1, Whole mount, ventral view; 2, Copulatory complex, ventral view; 3, Hook 4A; 4, Hook. 5–8, Anacanthorus scholzi n. sp. 5, Whole mount, ventral view; 6, Copulatory complex, ventral view; 7, Hook 4A; 8, Hook
Haptor 38–85 (54; n = 19) long, 46–114 (80; n = 19) wide, bearing 7 pairs of hooks and 2 pairs (1 dorsal and 1 ventral) of 4A hooks with anacanthorine distribution; anchors and bars absent (Fig. 1). Hooks similar in shape and size, 23–26 (25; n = 17) long, with truncate broad thumb, slightly curved shaft and point, and thin, straight shank with expanded proximal bulbous portion (Fig. 4). Length of FH loop representing c.40% of shank length. Hooks 4A small, similar in shape and size, proximal end rounded, broader than distal thinner end, 8–12 (10; n = 8) long (Fig. 3).
MCO forming conspicuous thick-walled and slightly L-shaped tube, 58–107 (74; n = 21) long, 12–20 (15; n = 21) wide; its proximal opening with flap-like projection (sclerotised flange). Accessory piece 47–107 (65; n = 21) long, slightly V-shaped, articulated with MCO base through proximal process; consisting of 2 unequal branches bifurcating near base, each branch with single tapering distally. Longer branch bearing 1 submedial reduced flat branch. Shorter branch sigmoid, with blunt button-like projection on its distal third (Fig. 2). Gonads slightly overlapping, intercaecal; germarium sub-ovate with irregular edges, 60–142 (92; n = 7) long, 22–56 (37; n = 7) wide. Testis postgermarial, elongate-oval, 55–77 (67; n = 3) long, 32–45 (40; n = 3) wide (Fig. 1). Vitellarium composed of small follicles, evenly extending from pharyngeal level to slightly posterior to caeca loop (Fig. 1). Eggs, oviduct and oötype not observed.
Remarks
The present specimens belong to Anacanthorus mainly by the absence of bars and anchors, as well as in the presence of 7 pairs of similar hooks and 2 pairs of distinct hooks denominated 4A (see Mizelle & Price, 1965; Kritsky et al., 1992).
The accessory piece of A. luquei n. sp. composed of two long branches in a somewhat V-shaped pattern, resembles the following congeners: A. bicuspidatus Cohen, Kohn & Boeger, 2012; A. carinatus Kritsky, Boeger & Van Every, 1992; A. chaunophallus Kritsky, Boeger & Van Every, 1992; A. cuticulovaginus Kritsky & Thatcher, 1974; A. daulometrus Cohen, Kohn & Boeger, 2012; A. dipelecinus Kritsky, Boeger & Van Every, 1992; A. douradensis Cohen, Kohn & Boeger, 2012; A. furculus Kritsky, Boeger & Van Every, 1992; A. myleusi Oliveira, Carneiro, Ruz & Luque, 2019; A. neotropicalis Mizelle & Price, 1965; A. paradouradensis Monteiro, Cohen & Brasil-Sato, 2015; A. quinqueramus Kritsky, Boeger & Van Every, 1992 and A. tricornis Kritsky, Boeger & Van Every, 1992. Besides several differences, e.g. in the morphology of MCO and hooks, none of these species have the longer branch of the accessory piece with an arising submedial, short and flat branch, referred to as “hatchet-like” by Kristsky et al. (1992), except for A. cuticulovaginus, A. dipelecinus and A. quinqueramus. However, the accessory piece of A. cuticulovaginus has one branch with irregular margins (Kritsky & Thatcher, 1974), that of A. dipelecinus has conspicuous pointed projections on the distal end of the branches, which are similar in size and each has a short branch, and that of A. quinqueramus has an additional small and median branch (Kritsky et al., 1992), features absent in the new species.
Anacanthorus scholzi n. sp.
Type-host: Markiana nigripinnis (Perugia) (Characiformes: Characidae), tetra fish.
Type-locality: Marginal lake to the road MS184 (19°34.576′S, 57°00.823′W), Corumbá, State of Mato Grosso do Sul, Brazil.
Type-material: Holotype (ZUFMS-PLA00021) and 2 paratypes (ZUFMS-PLA00022, 23).
Site on host: Gill filaments.
Prevalence: 7% (4 fish infected of 57 examined).
ZooBank registration: The Life Science Identifier (LSID) for Anacanthorus scholzi n. sp. is urn:lsid:zoobank.org:act:D4A553F6-342D-48E0-BB47-037082FB5721.
Etymology: The specific name honours Dr Tomaš Scholz for his contributions to the taxonomy of helminth parasites of fish.
Description
[Based on 4 specimens: 3 mounted in Gray & Wess medium and 1 stained with Gomori’s trichrome; Figs. 5–8.] Body fusiform, 397–485 (471; n = 4) long, with greatest width 131–178 (163; n = 4) at midlength (Fig. 5). Tegument smooth. Cephalic lobes 3, well defined, with Y-shaped cephalic organs: 1 medial bearing a pair of organs and 2 lateral bearing a single pair. Eyes 4, equidistant, with irregular shape, posterior pair larger than anterior; accessory granules absent or rarely present. Pharynx spherical to subspherical, 38–43 (41; n = 4) in diameter. Intestinal caeca 2, encircling internal organs, bifurcating slightly posterior to pharynx and joining slightly posterior to testis.
Haptor 43–100 (64; n = 4) long, 72–105 (89; n = 4) wide, bearing 7 pairs of hooks and 2 pairs (1 dorsal and 1 ventral) of 4A hooks, with anacanthorine distribution; anchors and bars absent (Fig. 5). Hooks similar in shape and size, 22–31 (28; n = 4) long, with truncate broad thumb, slightly curved shaft, straight point and straight shank with expanded proximal bulbous portion (Fig. 8). Length of FH loop representing c.70% of shank length. Hooks 4A small, similar in shape and size, thin and rod-shaped, 9–15 (11; n = 4) long (Fig. 7).
MCO forming sclerotised tube with slightly curved ends, 63–73 (70; n = 4) long, 10–20 (16; n = 4) wide (Fig. 6). Proximal opening of MCO bearing short flap-like projections (sclerotised flanges) (Fig. 6). Accessory piece 60–70 (65; n = 4) long, articulated with MCO base through proximal process; consisting of 2 main branches bifurcating near base: 1 evenly curved and 1 sigmoid (Fig. 6). Evenly curved branch bearing 1 submedial reduced flat branch, ending in single blunt tip; sigmoid branch bearing submedial blunt button-like projection and distal bifurcation with blunt ends (Fig. 6). Gonads slightly overlapping, intercaecal; germarium sub-ovate with irregular edges, 60–108 (85; n = 3) long, 36–55 (46; n = 3) wide. Testis postgermarial elongate-oval, 45–70 (53; n = 3) long, 18–40 (31; n = 3) wide. Vitellarium composed of small follicles, evenly extending from pharyngeal level to slightly posterior to caeca loop (Fig. 5). Eggs, oviduct and oötype not observed.
Remarks
The general morphology of A. scholzi n. sp. agrees with the generic diagnosis of Anacanthorus (see previous remarks). Anacanthorus scholzi n. sp. is distinct from A. luquei n. sp. in having one branch of the accessory piece bifurcated at the distal portion (vs absent in A. luquei n. sp.).
The following species are morphologically similar to A. scholzi n. sp. based on the shape of the accessory piece, i.e. with one of the two branches bifurcated at the distal end: A. acuminatus Kritsky, Boeger & Van Every, 1992; A. bellus Kritsky, Boeger & Van Every, 1992; A. bicuspidatus; A. daulometrus; A. euryphallus Kritsky, Boeger & Van Every, 1992 and A. tricornis. However, none of these species possess a “hatchet-like” or a button-like projection on the accessory piece branches as in the new species (see Kritsky et al., 1992; Cohen et al., 2012). The copulatory complex of A. quiqueramus resembles that of A. scholzi n. sp. but differs in having an accessory piece with an additional small, median branch and the MCO with a pointed projection near the distal end (vs absence of additional branch and pointed projection in the accessory piece and MCO, respectively) (Kritsky et al., 1992).
Anacanthorus cohenae n. sp.
Type-host: Markiana nigripinnis (Perugia) (Characiformes: Characidae), tetra fish.
Type-locality: Marginal lake to the road MS184 (19°34.576′S, 57°00.823′W), Corumbá, State of Mato Grosso do Sul, Brazil.
Type-material: Holotype (ZUFMS-PLA00024) and 2 paratypes (ZUFMS-PLA00025, 26).
Site on host: Gill filaments.
Prevalence: 3.5% (2 fish infected of 57 examined).
ZooBank registration: The Life Science Identifier (LSID) for Anacanthorus cohenae n. sp. is urn:lsid:zoobank.org:act:0C843D36-8BE3-44D0-BD8E-5652534B064C.
Etymology: The specific name honours Dr Simone Chinicz Cohen for her contributions to the knowledge of the Neotropical fauna of monogeneans.
Description
[Based on 3 specimens: 2 mounted in Gray & Wess medium and 1 stained with Gomori’s trichrome; Figs. 9–12.] Body fusiform, 206–611 (367; n = 3) long, with greatest width 69–104 (90; n = 3) at midlength (Fig. 9). Tegument smooth. Cephalic lobes 3, well defined, with Y-shaped cephalic organs:1 medial bearing a pair of organs and 2 lateral bearing a single pair. Eyes 4, equidistant, with irregular shape, posterior pair larger than anterior; accessory granules absent. Pharynx spherical, 27–39 (34; n = 2) in diameter. Intestinal caeca 2, encircling internal organs, bifurcating slightly posterior to pharynx and joining slightly posterior to testis.
Haptor 38–72 (50; n = 3) long, 45–84 (68; n = 3) wide, bearing 7 pairs of hooks and 2 pairs (1 dorsal and 1 ventral) of 4A hooks, with anacanthorine distribution; anchors and bars absent (Fig. 9). Hooks similar in shape and size, 26–27 (26; n = 3) long, with truncate broad thumb, curved shaft and straight point, thin shank with expanded proximal bulbous portion (Fig. 12). Length of FH loop representing c.60% of shank length. Hooks 4A small, similar in shape and size, proximal end slightly rounded, broader than distal thinner end, 9–12 (11; n = 3) long (Fig. 11).
MCO forming evenly curved sclerotised tube, thicker at proximal portion, 41–67 (55; n = 3) long, 10–13 (12; n = 3) wide (Fig. 10), with basal flap-like structures (sclerotised flanges); distal flange pronouncedly expanded (Fig. 10). Accessory piece 37–72 (50 long; n = 3), slightly V-shaped, articulated with MCO base through proximal well-developed process; consisting of 2 long branches bifurcating near base, with blunt and folded distal ends (Fig. 8). Gonads slightly overlapping, intercaecal; germarium sub-ovate with irregular edges, 34–115 (61; n = 3) long, 21–47 (34; n = 3) wide. Testis postgermarial, elongate-oval, 43 (n = 1) long, 20 (n = 1) wide. Vitellarium composed of small follicles, evenly extending from pharyngeal level to slightly posterior to caeca loop (Fig. 9). Eggs, oviduct and oötype not observed.
Remarks
As for A. cohenae n. sp., the following species have the accessory piece composed of two not bifurcated branches resembling a V-shaped pattern: A. carinatus; A. cuticulovaginus; A. dipelecinus; A. douradensis; A. furculus; A. luquei n. sp., A. neotropicalis; A. paradouradensis and A. parakruidenieri Cohen, Kohn & Boeger, 2012, but they differ from the new species as follows. Anacanthorus douradensis, A. furculus and A. paradouradensis have the MCO with other shapes than an evenly curved sclerotised tube as in A. cohenae n. sp. (see Kritsky et al., 1992; Cohen et al., 2012; Monteiro et al., 2015). Anacanthorus carinatus has a secondary accessory piece, which is absent in the new species (see Kritsky et al., 1992). Anacanthorus cuticulovaginus has one branch of the accessory piece with irregular edge, A. luquei n. sp. has a “hatchet-like” structure on one of the main branches (see previous remarks for details) and A. dipelecinus has “hatchet-like” structures on both branches, which end in pointed projections (vs smooth-edged branches, without “hatchet-like” structures and with blunt distal ends in A. cohenae n. sp.) (Kritsky & Thatcher, 1974; Kritsly et al., 1992). The accessory piece of A. parakruidenieri is thin, bifid near its mid-length and the MCO lacks terminal sclerotised flanges (vs robust accessory piece, bifid near its base and MCO with conspicuous terminal flanges in A. cohenae n. sp.) (Cohen et al., 2012). Anacanthorus neotropicalis has the accessory piece formed by two branches evidently different in length, with pointed ends and hooks with shank expanded from the mid-portion to proximal end (vs accessory piece with two branches of similar length and blunt ends, and hook shank with uniform width and a proximal bulb in the new species) (Mizelle & Price, 1965).
References
Boeger, W. A., & Vianna, R. T. (2006). Monogenoidea. In: Thatcher, V. E. (Ed.), Aquatic Biodiversity in Latin America. Amazon fish parasites. Sofia-Moscow: Pensoft Publishers, pp. 42–116.
Britski, H. A., Silimon, K. Z. S., & Lopes, B. S. (2007). Peixes do Pantanal. Manual de Identificação. Corumbá, MS: Embrapa Informação Tecnológica.
Cohen, S. C., Justo, M. C. N., & Kohn, A. (2013). South American Monogenoidea parasites of fishes, amphibians and reptiles. Rio de Janeiro, RJ: Oficina de Livros.
Cohen, S. C., Kohn, A., & Boeger, W. A. (2012). Neotropical Monogenoidea. 57. Nine new species of Dactylogyridae (Monogenoidea) from the gill of Salminus brasiliensis (Characidae, Characiformes) from the Paraná River, State of Paraná. Brazil. Zootaxa, 3049, 57–68.
Corrêa, C. E., Petry, A. C., & Hahn, N. S. (2009). Influência do ciclo hidrológico na dieta e estrutura trófica da ictiofauna do rio Cuiabá, Pantanal Matogrossense. Iheringia Série Zoologia, 99, 456–463.
Froese R., & Pauly, D. (Eds) (2019). FishBase. World Wide Web electronic publication. http://www.fishbase.org, version 08/2019.
Hechinger, R. F., & Lafferty, K. D. (2005). Host diversity begets parasite diversity: bird final hosts and trematodes in snail intermediate hosts. Proceedings of the Royal Society of Biology, 272, 1059–1066.
Humason, G. L. (1979). Animal tissue techniques. San Francisco, CA: Freeman and Company.
Kritsky, D. C., & Thatcher, V. E. (1974). Monogenetic trematodes (Monopisthocotylea: Dactylogyridae) from freshwater fishes of Colombia, South America. Journal of Helminthology, 48, 59–66.
Kritsky, D. C., Boeger, W. A., & Van Every, L. R. (1992). Neotropical Monogenoidea. 17. Anacanthorus Mizelle & Price, 1965 (Dactylogyridae, Anacanthorinae) from characoid fishes of the Central Amazon. Journal of the Helminthological Society of Washington, 59, 25–51.
Kritsky, D. C., & Mizelle, J. D. (1968). Studies on monogenetic trematodes. XXXV. Some new and previously described North American species of Gyrodactylus. American Midland Naturalist, 79, 205–215.
Leão, M. S. L., São Clemente, S. C., & Cohen, S. C. (2015). Acanathorus toledoensis n. sp. and Mymarothecium ianwhittingtoni n. sp. (Dactylogyridae: Monogenoidea) parasitizing cage-reared Piaractus mesopotamicus (Characiformes, Characidae) in the State of Paraná, Brazil. Comparative Parasitology, 82, 269–274.
Luque, J. L., Pereira, F. B., Alves, P. V., Oliva, M. E., & Timi, J. T. (2017). Helminth parasites of South American fishes: current status and characterization as a model for studies of biodiversity. Journal of Helminthology, 91, 150–164.
Luque, J. L., & Poulin, R. (2007). Metazoan parasite richness in Neotropical fishes: hotspots and the geography of biodiversity. Parasitology, 134, 865–878.
Mizelle, J. D., & Klucka, A. R. (1953). Studies on monogenetic trematodes. XVI. Dactylogyridae from Wisconsin fishes. American Midland Naturalist, 49, 720–733.
Mizelle, J. D., Kritsky, D. C., & Crane, J. W. (1968). Studies on monogenetic trematodes. XXXVIII. Ancyrocephalinae from South America with the proposal of Jainus gen. n. American Midland Naturalist, 80, 186–198.
Mizelle, J. D., & Price, C. E. (1963). Additional haptoral hooks in the genus Dactylogyrus. Journal of Parasitology, 49, 1028–1029.
Mizelle, J. D., & Price, C. E. (1965). Studies on monogenetic trematodes. XXVIII. Gill parasites of the piranha with the proposal of Anacanthorus gen. n. Journal of Parasitology, 51, 30–36.
Moreira, J., Carneiro, J. S., Ruz, E. J. H., & Luque, J. L. (2019). New species and records of Anacanthorus (Monogenea: Dactylogyridae) parasitizing serrasalmid fish (Characiformes) from Brazil, including molecular data. Acta Parasitologica, 64, 449–455.
Monteiro, C. M., Cohen, S. C., & Brasil-Sato, M. C. (2015). New species and reports of dactylogirids (Monogenoidea) from Salminus franciscanus (Actinopterygii: Bryconidae) from the upper São Francisco River, Brazil. Zootaxa, 3941, 137–143.
Polaz, C. N. M., Melo, B. F., Britske, R., Resende, E. K., Machado, F. A., Lima, J. A. F., et al. (2014). Fishes from the Parque Nacional do Pantanal Matogrossense, upper Paraguay River basin, Brazil. Check List, 10, 122–130.
Poulin, R. (2014). Parasite biodiversity revisited: frontiers and constraints. International Journal for Parasitology, 44, 581–589.
Santos-Neto, J. F., Muriel-Cunha, J., & Domingues, M. V. (2019). A new species of Anacanthorus (Dactylogyridae: Anacanthorinae) from the gills of Hoplerythrinus unitaeniatus and Erythrinus erythrinus (Characiformes: Erythrinidae) of the coastal drainage in the Eastern Amazon, Brazil. Zootaxa, 4615, 3030–3320.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance code 001. L.E.R.T. was supported by a Research fellowship from CNPq (Conselho Nacional de Desenvolvimento Cientifico e Tecnológico do Brasil) (313292/2018-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures involving animals were according to the rules of the Sistema de Autorização e Informação em Biodiversidade (SISBIO 22119-2) and the Comissão de ética no uso de animais / Universidade Federal de Mato Grosso do Sul (CEUA/UFMS 572/2913).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as urn:lsid:zoobank.org:pub:8EE3D1B6-CB37-4AF9-9672-342254E32BE9. This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number. This is the Version of Record.
This article is part of the Topical Collection Monogenea.
Rights and permissions
About this article
Cite this article
Pereira, F.B., Mota, M.E.B.P., Paiva, F. et al. Three new species of Anacanthorus Mizelle & Price, 1965 (Monogenea: Dactylogyridae) from Markiana nigripinnis Perugia (Actinopterygii: Characidae) in Pantanal wetlands, Brazil. Syst Parasitol 97, 661–667 (2020). https://doi.org/10.1007/s11230-020-09935-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-020-09935-3