Abstract
A new species of capsalid monogenean, Benedenia armata n. sp., is described from Lethrinus haematopterus Temminck & Schlegel (Perciformes: Lethrinidae) from off Danjo Islands, Nagasaki Prefecture, Japan. The new species differs from other species of Benedenia Diesing, 1858 in that the common genital atrium has spines and the vas deferens has two swellings. Lethrinus haematopterus has been considered as the type-host of Benedenia ishikawae (Goto, 1894) based on the local name ‘Kuchibi-dai’. However, this name refers not only to L. haematopterus but also to L. nebulosus (Forsskål), and L. haematopterus examined in this study was not infected by B. ishikawae. Further study is required to determine the type-host of B. ishikawae and to redetermine the species of Benedenia that are parasitic on Lethrinus spp. in the type-locality of B. ishikawae (Hagi, Yamaguchi Prefecture, Japan).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lethrinus haematopterus Temminck & Schlegel (Perciformes: Lethrinidae) is a marine fish that occurs in waters around southern China and southern Japan (Carpenter & Allen, 1989). The monogenean fauna of this fish is poorly known; only one dactylogyrid species, Lethrinitrema lethrini (Yamaguti, 1937), has been described (Yamaguti, 1937; Lim & Justine, 2011). Goto (1894) described Benedenia ishikawae (Goto, 1894) (Monogenea: Capsalidae) from “Lethrinus sp.? (Jap. Kuchibi-dai)” from off Japan, and Lawler (1981) suggested the host is likely L. haematopterus according to Okada (1955). However, the local name ‘Kuchibi-dai’ refers not only to L. haematopterus but also to L. nebulosus (Forsskål) (Ichthyological Society of Japan, 1981), and it is important to clarify the original capsalid fauna of these fishes in Japan.
Benedenia Diesing, 1858 contains 28 species considered valid, ectoparasites of marine fishes (Whittington et al., 2001; Deveney & Wittington, 2010; Whittington & Deveney, 2011). To date, seven species have been recorded from Japan (Whittington et al., 2001; Deveney & Wittington, 2010): B. elongata (Yamaguti, 1968); B. epinepheli (Yamaguti, 1937); B. hoshinai Ogawa, 1984; B. ishikawae; B. ovata (Goto, 1894); B. sekii (Yamaguti, 1937); and B. seriolae (Yamaguti, 1934). Two Benedenia spp. described off Japan from Pagrus major (Temminck & Schlegel) by Ishii & Sawada (1937, 1938), i.e. B. pagrosomi (Ishii & Sawada, 1937) and B. madai (Ishii & Sawada, 1938), were regarded as species inquirenda by Whittington et al. (2001). In addition, B. synagris Yamaguti, 1953 recoded from Arothron mappa (Lesson) off Okinawa, Japan, by Dyer et al. (1989) was likely an undescribed Benedenia species (Whittington et al., 2001). This paper provides morphological and molecular information for a new species of Benedenia from L. haematopterus.
Materials and methods
Four specimens of Lethrinus haematopterus (228.0–305.2 mm in standard length) were captured from the East China Sea off Maehama (31°59.8′N, 128°21.2′E), Meshima Island, the Danjo Islands, Gotō City, Nagasaki Prefecture, Japan on 19 September 2017. The fish were frozen in plastic bags until parasitological examination. The capsalid monogeneans were removed and collected from body surface and branchial cavity by the double-netting method (Madinabeitia & Nagasawa, 2013) and fixed in acetic acid-formalin-alcohol (AFA) or 99 % ethanol. The right lateral part of one specimen fixed in 99 % ethanol was cut using a razor for molecular analysis, and the other part was cleared using xylene and mounted in Canada balsam. Other specimens that were fixed in AFA were stained with Heidenhain’s iron haematoxylin, dehydrated through a graded ethanol series, cleared in xylene and mounted in Canada balsam. Drawings were made with the aid of a drawing tube fitted on an Olympus BX51 and BX60 light microscopes (Olympus, Tokyo, Japan). Photographs of the common genital atrium were taken using a CANON EOS Kiss X2 digital camera (Canon, Tokyo, Japan) fitted on an Olympus BX60 microscope. Focus stacking was made using the Combine ZM (Alan Hadley, UK) automontage software. Parasite measurements are given in micrometres (μm) and expressed as the range followed by the mean and the number (n) of specimens in parentheses. The prevalence and mean intensity of infection were calculated according to Bush et al. (1997). Taxonomic identification of the fish follows Shimada (2013). Specimens were deposited in the Platyhelminthes Collection of the National Museum of Nature and Science, Tsukuba city, Ibaraki Prefecture, Japan (NSMT-Pl).
DNA was extracted from the right lateral part of a paratype (NSMT-Pl 6380) using the DNeasy blood and tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Partial fragments of 28S rRNA gene were amplified using the polymerase chain reaction (PCR) with the primer pair C1 (5′-ACC CGC TGA ATT TAA GCA T-3′) and D2 (5′-TGG TCC GTG TTT CAA GAC-3′) (Le et al., 1993), and partial fragments of the mitochondrial cytochrome c oxidase gene subunit 1 (cox1) were amplified using the primer pair JB3 (5′-TTT TTT GGG CAT CCT GAG GTT TAT-3′) and CO1-R trema (5′-CAA CAA ATC ATG ATG CAA AAG G-3′) (Bowles et al., 1993; Miura et al., 2005). PCR were performed in a total volume of 20 μl, containing 0.1 μl Takara Ex Taq DNA polymerase (TaKaRa, Kusatsu, Shiga Prefecture, Japan), 2.0 μl PCR buffer (TaKaRa), 1.6 μl dNTP mixture (2.5 mM of each dNTP) (TaKaRa), 0.6 μl of each 10 μM primer, 1.0 μl of extracted DNA, and 14.1 μl of distilled water. The cycling conditions included an initial denaturation at 94°C for 5 min followed by 35 cycles at 94°C for 60 s, at 54°C for 60 s, at 72°C for 60 s, and 10 min at 72°C for final extension. Amplified PCR products were purified using the NucleoSpin Gel and PCR Clean-up kit (Macherey-Nagel, Düren, Germany) and sequenced with a 3130X Genetic Analyzer (Applied Biosystems, Waltham, MA, USA) with the PCR primers. The partial 28S rDNA (793 bp) and cox1 with partial t-RNA regions (876 bp) sequences obtained from a paratype (NSMT-Pl 6380) were submitted to the DNA Data Bank of Japan Centre (DDBJ) and were compared with the available sequences for species of Capsalidae in Gen Bank using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) software on 25 June 2018.
Order Capsalidea Lebedev, 1988
Family Capsalidae Baird, 1853
Genus Benedenia Diesing, 1858
Benedenia armata n. sp.
Type-host: Lethrinus haematopterus Temminck & Schlegel (Perciformes: Lethrinidae).
Type-locality: East China Sea off Maehama (31°59.8′N, 128°21.2′E), Meshima Island, the Danjo Islands, Gotō City, Nagasaki Prefecture, Japan.
Type-material: Holotype (NSMT-Pl 6377) and 16 paratypes (NSMT-Pl 6378–6380).
Site in host: Body surface, branchial cavity.
Prevalence and mean intensity: 100% and 5 worms per infected fish (range 1–12).
Representative DNA sequences: DDBJ accession numbers LC408960 (cox1 with partial t-RNA) and LC408961 (28S rRNA gene).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Benedenia armata n. sp. is urn:lsid:zoobank.org:act: D69163BD-1A3B-437E-AB35-4D67C5E483FD.
Etymology: The specific name “armata” (armed in Latin) relates to the characteristic feature of this species: the common genital atrium and its lobe armed by spines.
Description (Figs. 1–2)
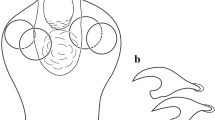
[Based on 10 stained and one unstained specimens.] Total length of body including haptor 1,937–4,072 (2,605, n = 11), maximum width 729–1,634 (960, n = 11) (Fig. 1A). Haptor slightly elongate-oval, 463–746 (578, n = 11) long, 441–728 (517, n = 11) wide (Fig. 1A). Accessory sclerite 88–160 (115, n = 11) long, slightly curved, with pointed distal tip (Fig. 1C). Anterior hamulus 78–138 (96, n = 11) long, shaft straight, strongly curved distal hook (Fig. 1D). Posterior hamulus 60–88 (77, n = 11) long, with hooked distal tip (Fig. 1E). Hooklets 14, at haptor periphery, each 6–8 (7, n = 11) long (Fig. 1F). Marginal valve scalloped with consistent and characteristic number of lobes between hooklets on each side of haptor as follows (10 specimens examined): 1 large lobe between hooklets of pair II on posterior border of haptor; l large lobe between hooklets II and distal position of posterior hamuli; 1 large lobe between distal position of posterior hamuli and hooklets III; 1 large lobe between hooklets III and IV; 1 large lobe between hooklets IV and V; 3–4 smaller lobes between hooklets V and VI; 5–6 small lobes between hooklets VI and VIl; 8–9 small lobes between hooklets VIl and VIII; 9–12 small lobes on anterior border of haptor between hooklets of pair VIII (Fig. 1A).
Benedenia armata n. sp. parasitic on Lethrinus haematopterus Temminck & Schlegel from off Japan. A, B, Holotype (NSMT-Pl 6377); C–H, paratype (NSMT-Pl 6380). A, Whole body, ventral view; B, Genitalia, ventral view; C, Accessory sclerite; D, Anterior hamulus; E, Posterior hamulus; F, Hooklet; G, Sclerotised structure of common genital aperture; H, Screrotised structure of vaginal pore. Abbreviations: a, anterior attachment organ; ah, anterior hamulus; ang, anterior gland; as, accessory sclerite; bag, bulb of accessory gland; cg, common genital atrium; e, eyespots; eb, excretory bladder; g, germarium; gg, gland of Goto; h, hooklet; ha, haptor; i, intestinal caeca; lc, lobe associated with the common genital pore; lv, lobe associated with the vaginal pore; mag, male accessory gland; mg, Mehlisʼ gland; o, oötype; p, penis; pc, penis canal; ph, posterior hamulus; px, pharynx; sr, seminal receptacle; sv, seminal vesicle; v, vitellarium, va, vagina; vd, vas deferens; ve, vasa efferentia; vp, vaginal pore; vr, vitelline reservoir. Scale-bars: A, 500 μm; B, 250 μm; C–E, 50 μm; F, 5 μm; G, H, 10 μm
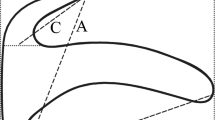
Common genital atrium of Benedenia armata n. sp. (Holotype, NSMT-Pl 6377) parasitic on Lethrinus haematopterus from Japan. Arrows indicate points with spines. Abbreviations: cg, common genital atrium; cga, common genital aperture; cs, line of short conical spines; lc, lobe associated with the common genital pore; pc, penis canal; ss, screrotised structure. Scale-bar: 50 μm
Anterior attachment organs approximately elliptical, 183–323 (234, n = 11) long, 230–371 (288, n = 11) wide with posterior muscular region and anterior glandular region (Fig. lA). Two pairs of anterior gland cells located in anterior part of body. Pharynx 138–345 (192, n = 11) long, 158–368 (199, n = 11) wide. Eyespots 2 pairs, dorsal, anterior to pharynx. Intestinal caeca extending posteriorly to end of body, terminating blindly; each caecum with numerous secondary and tertiary diverticula; first diverticula from right caecum upwardly extending to posterior margin of right anterior attachment organ; diverticula from left caecum upwardly extending to left side of pharynx, respectively. Pair of elongate excretory bladders present.
Glands of Goto paired, in posterior angle between testes, 180–225 (201, n = 11) long, 188–333 (219, n = 11) wide. Testes 2, intercaecal, juxtaposed near middle of body, postovarian, 180–225 (201, n = 11) long, 188–333 (219, n = 11) wide (Fig. 1A, B). Vasa efferentia comprising paired ducts extending laterally from testes, uniting between testes. Vas deferens swelling to form seminal vesicle between testes and germarium. Vas deferens extending anteriorly and sinistral to germarium and vitelline reservoir, winding anterior or dorsal to vitelline reservoir, looping along anterior margin of vitelline reservoir, coursing between oötype and proximal vagina, narrowing before looping dorsal to distal region of oötype and median region of penis canal, entering penis dorsally, slightly expanding, joining with duct of male accessory gland reservoir at middle of penis (Fig. 1A, B). Wall of vas deferens with two swellings; small proximal swelling dorsal of oötype and large distal swelling immediately before entering penis canal. Male accessory gland reservoir rounded, proximal to penis. Weakly muscular bulb of accessory gland occupying posterior two-fifths of penis. Penis muscular, protrusible via common genital aperture and common genital atrium.
Common genital pore opening dorsal, at level of left-posterior margin of left anterior attachment organ. Lobe (Figs. 1B, 2) associated with common genital pore well developed; anterior lobe margin armed with short conical spines in line (Figs. 1B, 2), arranged in three points on right side and at a point on left to common genital atrium. Sclerotised structure (Figs. 1G, 2) present on left side of common genital atrium opening.
Germarium (Fig. 1A, B) globular, anterior to testes, 73–263 (126, n = 11) long, 113–305 (158, n = 11) wide. Oviduct arising from anterior margin of germarium, receives duct from dorsal aspect of vitelline reservoir (Fig. 1A, B). Ovovitelline duct extending anteriorly dorsal to vitelline reservoir, connecting with oötype, followed by uterus which opening into middle of penis canal (Fig. 1A, B). Mehlisʼ gland connected at junction of ovovitelline duct and oötype. Vaginal pore associated with sclerotised structure (Fig. 1H), on left side of body at mid-level of pharynx. Lobe associated with vaginal pore well developed. Vagina narrow, tubular, running to seminal receptacle lying left to anterior margin of vitelline reservoir. Seminal receptacle connecting with vitelline reservoir by short, narrow duct. Vitellarium coexists with caeca from levels of eyespots on right side and posterior margin of pharynx on left side to posterior extremity of body. Egg tetrahedral, side length 78–83 (80, n = 3), with long filament.
Molecular data comparison
Comparing the sequences of B. armata n. sp. with sequences for other species of Capsalidae available on GenBank, did not result in high similarities by BLAST searches. The closest hits were B. hoshinai Ogawa, 1984 (GenBank: EF055880, 82% similarity with 100% coverage) for the cox1 sequence and B. rohdei Whittington, Kearn & Beverley-Burton, 1994 (GenBank: AY033940, 87% similarity with 99% coverage) and B. lutjani Whittington & Kearn, 1993 (GenBank: AY033939, 87% similarity with 99% coverage) for the 28S rDNA sequence.
Discussion
A molecular study of the Capsalidae Baird, 1853 that included six species of Benedenia suggested that the genus is polyphyletic (Perkins et al., 2009). However, a review of Benedenia that reflects molecular data has not yet been proposed, so the Benedenia review according to Whittington et al. (2001) following Deveney & Wittington (2010) and Whittington & Deveney (2011) was used for this study. Benedenia armata n. sp. corresponds with the diagnostic morphological characters of the genus. Comparisons with 28S rDNA sequences for Benedenia spp. on GenBank revealed that B. armata n. sp. shows similarity with Benedenia spp. parasitising on Lutjanus spp. (Perciformes: Lutjanidae), B. lutjani and B. rohdei (see Whittington & Kearn, 1993; Whittington et al., 1994). These three species share a common morphological characteristic: the wall of the vas deferens swells before entering the penis (Whittington & Kearn, 1993; Whittington et al., 1994). However, Benedenia armata n. sp. is distinguished from B. rohdei and B. lutjani by the following characters that are unique to the new species: the presence of the well-developed lobe associated with the common genital pore; the lack of a screrotised structure at the distal tip of the penis; and the vaginal pore being located posterior to the common genital pore. Furthermore, the new species is differentiated from the existing species of Benedenia by the presence of spines on the anterior margin of the lobe and the common genital atrium (Whittington et al., 2001; Deveney & Wittington, 2010; Whittington & Deveney, 2011).
Lawler (1981) suggested that “Lethrinus sp.? (Jap. Kuchibi-dai)”, which was reported as a type-host of Benedenia ishikawae, is likely Lethrinus haematopterus according to Okada (1955). However, the local name ‘Kuchibi-dai’ refers not only to L. haematopterus but also to L. nebulosus (Ichthyological Society of Japan, 1981). Benedenia ishikawae is parasitic on lethrinids and is widely distributed throughout Japan and Australia (Goto, 1894; Deveney & Wittington, 2010); however, the four specimens of L. haematopterus examined in this study were not infected with B. ishikawae, and the type-host of this species requires further consideration. The type-locality of B. ishikawae is Hagi, Yamaguchi Prefecture (according to Goto, 1894), which is along the Sea of Japan, and both fish species (L. haematopterus and L. nebulosus) occur in the area (see Kawano et al., 2011). It is necessary to reinvestigate the species of Benedenia parasitic on Lethrinus spp. in this area.
References
Bowles, J., Hope, M., Tiu, W. U., Liu, X., & McManus, D. P. (1993). Nuclear and mitochondrial genetic markers highly conserved between Chinese and Philippine Schistosoma japonicum. Acta Tropica, 55, 217–229.
Bush, A. O., Lafferty, K. D., Lotz, J. M., Shostak, A. W. (1997). Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology, 83, 575–583.
Carpenter, K. E., & Allen, G. R. (1989). FAO Species Catalogue. vol. 9. Emperor fishes and large-eye breams of the world (family Lethrinidae). An annotated and illustrated catalogue of lethrinid species known to date. FAO Fisheries Synopsis. Rome: FAO, v+118 pp., 8 pls.
Dyer, W. G., Ernest, H., Williams, H., Jr., & Williams, L. B. (1989). Monogeneans from marine fishes of Okinawa. Journal of the Helminthological Society of Washington, 56, 64–68.
Deveney, M. R., & Wittington, I. D. (2010). Three new species of Benedenia Diesing, 1858 from the Great Barrier Reef, Australia with a key to species of the genus. Zootaxa, 2348, 1–22.
Goto, S. (1894). Studies on the ectoparasitic trematodes of Japan. Journal of the College of Sciences, Imperial University Tokyo, 8, 1–173.
ICZN (2012). International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bulletin of Zoological Nomenclature, 69, 161–169.
Ichthyological Society of Japan (Ed.) (1981). Dictionary of Japanese Fish Names and Their Foreign Equivalents. Tokyo: Sanseido Co., Ltd., iii+vii+834 pp (In Japanese with English abstract).
Ishii, N., & Sawada, T. (1937). Studies on the ectoparasitic trematodes. Nihon Kiseichū Gakkai Kiji, 9, 93–97 (In Japanese).
Ishii, N., & Sawada, T. (1938). Studies on the ectoparasitic trematodes III. Japanese Journal of Experimental Medicine, 16, 239–249.
Kawano, M., Doi, H., & Hori, S. (2011). List of the fishes in the southwestern Japan Sea off Yamaguchi Prefecture. Bulletin of Yamaguchi Prefectural Fisheries Research Center, 9, 29–64 (In Japanese with English abstract).
Lawler, A. R. (1981) Zoogeography and Host-Specificity of the Superfamily Capsaloidea Price, 1936 (Monogenea, Monopisthocolylea): An Evaluation of the Host-Parasite Lcality Records of the Superfamily Capsaloidea Price, 1936, and their Utility in Determinations of Host-Specificity and Zoogeography. Special Publications in Marine Science, Number 6. Virginia: Virginia Institute of Marine Science and School of Marine Science, 650 pp.
Le, H. L. V., Lecointre, G., & Perasso, R. (1993). A 28S rRNA based phylogeny of the gnathostomes: First steps in the analysis of conflict and congruence with morphological based cladograms. Molecular Phylogenetics and Evolution, 2, 31–51.
Lim, L. H. S., & Justine, J.-L. (2011). Two new species of ancyrocephalid monogeneans from Lethrinus rubrioperculatus Sato (Perciformes: Lethrinidae) off New Caledonia, with the proposal of Lethrinitrema n. g. Systematic Parasitology, 78, 123–138.
Miura, O., Kuris, A. M., Torchin, M. E., Hechinger, R. F., Dunham, E. J., & Chiba, S. (2005). Molecular-genetic analyses reveal cryptic species of trematodes in the intertidal gastropod, Batillaria cumingi (Crosse). International Journal for Parasitology, 35, 793–801.
Madinabeitia, I., & Nagasawa, K. (2013). Double-netting: An alternative approach for the recovery of parasitic copepods from finfishes. Journal of Natural History, 47, 529–541.
Okada, Y. (1955). Fishes of Japan. Tokyo: Maruzen Co., Ltd., 434+28 pp.
Perkins, E. M., Donnellan, S. C., Bertozzi, T., Chisholm, L. A., & Whittington, I. D. (2009). Looks can deceive: Molecular phylogeny of a family of flatworm ectoparasites (Monogenea: Capsalidae) does not reflect current morphological classification. Molecular Phylogenetics and Evolution, 52, 705–714.
Shimada, K. (2013). Lethrinidae. In: Nakabo, T. (Ed.) Fishes of Japan with pictorial keys to the species, Third edition. Hadano: Tokai University Press, pp. 960–969, 2014–2017 (In Japanese).
Whittington, I. D., & Deveney, M. R. (2011). New Benedenia species (Monogenea: Capsalidae) from Diagramma labiosum (Perciformes: Haemulidae) on the Great Barrier Reef, Australia, with oncomiracidial descriptions and a report of egg attachment to the host. Journal of Parasitology, 97, 1026–1034.
Whittington, I. D., & Kearn, G. C. (1993). A new species of skin-parasitic benedeniine monogenean with a preference for the pelvic fins of its host, Lutjanus carponotatus (Perciformes: Lutjanidae) from the Great Barrier Reef. Journal of Natural History, 27, 1–14.
Whittington, I. D., Deveney, M. R., & Wyborn, S. J. (2001). A revision of Benedenia Diesing, 1858 including a redescription of B. sciaenae (van Beneden, 1856) Odhner, 1905 and recognition of Menziesia Gibson, 1976 (Monogenea: Capsalidae). Journal of Natural History, 35, 663–777.
Whittington, I. D., Kearn, G. C., & Beverley-Burton, M. (1994). Benedenia rohdei n. sp. (Monogenea: Capsalidae) from the gills of Lutjanus carponotatus (Perciformes: Lutjanidae) from the Great Barrier Reef, Queensland, Australia, with a description of the oncomiracidium. Systematic Parasitology, 28, 5–13.
Yamaguti, S. (1937). Studies on the helminth fauna of Japan. Part 19. Fourteen new ectoparasitic trematodes of fishes. Published by the author, 28 pp., 6 pls.
Acknowledgements
I am grateful to Koichiro Kawai, Kazuya Nagasawa (Hiroshima University) and Takuya Sato (Kobe University) for providing laboratory facilities, and Seiji Arakaki and Taku Hotta (Kyushu University) for help collecting fish. Thanks go to an anonymous reviewer for valuable comments to improve the manuscript.
Funding
This study was supported by JSPS KAKENHI grants (no. 18J00466 to MN) and the hosts were collected under the biodiversity research partially supported by 27th Pro Natura Foundation Japan (to S. Arakaki).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as C5BAEDD0-0C70-4E8B-B7DC-FE6A52CD7F9A. This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number. This is the Version of Record.
This article is part of the Topical Collection Monogenea.
Rights and permissions
About this article
Cite this article
Nitta, M. A new species of Benedenia Diesing, 1858 (Monogenea: Capsalidae) parasitic on Lethrinus haematopterus Temminck & Schlegel (Perciformes: Lethrinidae) from Japan. Syst Parasitol 96, 199–205 (2019). https://doi.org/10.1007/s11230-019-09840-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-019-09840-4