Abstract
A new microcotylid, Tinrovia mamaevi n. sp. (Monogenea: Polyopisthocotylea), is described from the gills of Notacanthus bonaparte Risso (Notacanthiformes: Notacanthidae), sampled in the Western Mediterranean and North East Atlantic. This species is allocated to the subfamily Syncoelicotylinae Mamaev & Zubchenko, 1978 due to the possession of a symmetrical haptor with two separate frills. The clamps in T. mamaevi n. sp. are of the “microcotylid” type, arranged in two distinct lateral haptoral frills; the genital atrium and the copulatory organ are armed and the vaginal pore is unarmed. The new species differs from the type- and only species of the genus, T. papiliocauda Mamaev, 1987, in having a shorter and narrower haptor with a smaller number of clamps. Clamps are also smaller in the new species, testes are more numerous, the genital atrium is smaller, divided into two lateral lobes (instead of five) with a smaller number of spines and the eggs have a short and a long filament (instead of two short filaments). The host species and locality of T. mamaevi n. sp. also differ as T. papiliocauda which was recorded in Notacanthus sexspinis Richardson from the South Pacific.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fish family Notacanthidae Rafinesque (Notacanthiformes: Notacanthidae) has a global distribution and includes species inhabiting the deep-sea between depths of 200–3,500 m (Nelson, 2006). These fishes, commonly known as deep-sea spiny eels, are benthopelagic, feeding mainly on different small benthic invertebrates and nektonic crustaceans (Macpherson, 1981; Coggan et al., 1998; Carrassón & Matallanas, 2002). One of the nominal species belonging to this family is the shortfin spiny eel Notacanthus bonaparte Risso exhibiting a distribution restricted to the North East Atlantic off Faroe Islands to Mauritania and Western Mediterranean Sea (Froese & Pauly, 2016). The poorly studied parasite fauna of this fish comprises three species only: the cestode Steringovermes notacanthi Bray, 2004, the trematode Bathycestus brayi Kuchta & Scholz, 2004 and the nematode Dichelyne (Cucullanellus) romani Isbert, Montero, Carrassón & González-Solís, 2015.
No monogenean species have been recorded to date in N. bonaparte; however other species of Notacanthus Bloch were reported to harbour monogeneans in gills: Atlanticotyle notacanthi Mamaev & Zubchenko, 1978 (Diclidophoridae) ex N. chemnitzii Bloch; and two representatives of the Syncoelicotylinae Mamaev & Zubchenko, 1978 (Microcotylidae), Syncoelicotyle polyorchis Mamaev & Zubchenko, 1978 ex N. chemnitzii and Tinrovia papiliocauda Mamaev, 1987 ex N. sexspinis Richardson.
During studies on parasitic helminths of marine deep-sea fishes from the Western Mediterranean and the North East Atlantic, specimens of a microcotylid monogenean were recovered from the gills of N. bonaparte. Detailed morphological study of these specimens by light and confocal laser scanning microscopy revealed that they represent a species new to science, Tinrovia mamaevi n. sp. which is described here.
Materials and methods
A total of 150 specimens of N. bonaparte [total length (TL) 13.5–29.0 cm] was sampled during two projects (BIOMARE, ANTROMARE) carried out in the Balearic Sea in the Western Mediterranean during 2007/2008 and 2011. Additionally, we examined 15 specimens (TL 22.4–38.0 cm) caught in the Galicia Bank (North East Atlantic) during 2010 within the framework of the INDEMARES EU-Life+project. All fish specimens were measured and weighed, and in case of the surveys in the Mediterranean Sea, the gills were removed from some specimens and preserved in 10% formal saline. All other specimens were frozen at -25°C; examination for the presence of parasites was later conducted in the laboratory. Monogeneans isolated from gills preserved in 10% formal saline or from thawed fish, were washed in physiological saline and preserved in 70% ethanol. Partially bent specimens were gently flattened in saline under a coverslip with a 2 g scale weight overnight in a refrigerator.
For morphological examination, monogeneans were stained with iron acetocarmine (Georgiev et al., 1986), dehydrated in an ethanol series, cleared in dimethyl phthalate and mounted in Canada balsam. Specimens were examined morphologically under a light microscope equipped with differential interference contrast (DIC). Drawings were made with the aid of a drawing tube. When available, measurements of all body parts were taken from specimens with relaxed bodies which had been removed from frozen fish. However, in order to increase the sample size, some additional measurements were made from those traits which were not affected by body contraction in specimens formerly preserved in formal saline. The type-material was deposited in the British Museum (Natural History) Collection of the Natural History Museum, London, UK (NHMUK) and in the Helminthological Collection of the Institute of Parasitology, Biology Centre of the Czech Academy of Sciences (IPCAS), České Budějovice, Czech Republic. Voucher material was deposited in the Marine Zoology Collection of the Cavanilles Institute of Biodiversity and Evolutionary Biology (ICBiBE) of the University of Valencia, Spain.
All measurements are presented as the range followed by the mean in parentheses. The mean length and width of some characters were calculated, when possible, from a maximum of ten measurements (testes, clamps and spines of the genital atrium) and from both buccal suckers of each specimen. One specimen was dissected in order to extract the egg and measure and illustrate its filaments. The terminology follows Rubec & Dronen (1994) and terms for the description of the microcotylid clamps follow Boeger & Kritsky (1993).
Additionally, images of some body parts from selected specimens were taken by means of a confocal laser scanning microscopy (CLSM) to obtain a better insight into details which were not well/completely visible under light microscopy. For CLSM, specimens stained in iron acetocarmine and mounted in Canada balsam were used. Samples were examined with an Olympus FV1000 (inverted IX81) confocal microscope using the following objectives: Super Apochromat UPLSAPO 10× 2 (numerical aperture 0.40) and UPLFLN 40× (oil) (numerical aperture 1.30). Laser emission wave length was 603 nm, BF position 570 nm, BF range 100 nm. Images were processed with the software FLUOVIEW Ver. 4.2a Viewer (FV10-ASW Version 04.02.02.09).
Family Microcotylidae Taschenberg, 1879
Subfamily Syncoelicotylinae Mamaev & Zubchenko, 1978
Genus Tinrovia Mamaev, 1987
Tinrovia mamaevi n. sp.
Type-host: Notacanthus bonaparte Risso (Notacanthiformes: Notacanthidae).
Type-locality: Balearic Sea, Western Mediterranean; 40°10’N, 01°30′E – 41°12′N, 02°26′E; depth 620–1,009 m (mean 741 m).
Other localities: Galicia Bank, North East Atlantic; 42°43′N, 11°40′W – 42°47′N, 11°47′W; depth 771–837 m (mean 828 m).
Type-material: Holotype and 3 paratypes (NHMUK 2017.4.13.1 – NHMUK 2017.4.13.4), 2 paratypes (Cat. No. IPCAS M-579) and 8 vouchers (ICBiBE UV/ZOOMAR/N. bonaparte/ 12734–12741).
Site on host: Gill filaments.
Prevalence and intensity: Balearic Sea: 8.7% (13 infected out of 150 examined); 1–4 (1.5) monogeneans per infected fish (fish total length: 19.4–29.0 cm); Galicia Bank (North East Atlantic): 40.0 % (6 infected out of 15 examined); 1–3 (1.8) monogeneans per infected fish (TL 23.9–36.4 cm).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Tinrovia mamaevi n. sp. is urn:lsid:zoobank.org:act: E5B52C99-0731-4625-927F-CD88778E11CE.
Etymology: The new species is named after the late Dr Yuri L. Mamaev, of the Institute of Biology and Pedology of the Russian Academy of Sciences, Vladivostok, Russia in recognition to his invaluable contribution to the knowledge of the monogeneans.
Description (Figs. 1–3)
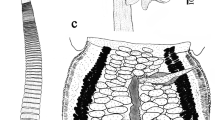
[Based on 12 adult whole-mounted specimens from the Balearic Sea; see Table 1 for measurements.] Body variably elongated, digitiform, dorsoventrally flattened, with rounded anterior extremity (Fig. 1A). Posterior extremity with relatively short, well-defined haptor; mean haptor length/ total body length ratio 22%. Haptor differentiated from body, symmetrical, winged formed by 2 slightly expanded lateral frills, bearing clamps (Figs. 1A, B, 2A); anterior ends of each frill forming 1 lobe; posterior extremities of frills not joining posteriorly, forming broad median groove. Larval hooks absent. Clamp number equal on both sides (32–40). Clamps of “microcotylid” type, with very short peduncles, wider than long; smallest clamps located on anterior and posterior extremities of haptoral frills. Clamp sclerites: lateral sclerites (c, d) slender, middle sclerite (a) thick, with longitudinal grooves (Figs. 1C, 2B–D, Online Resource 1); accessory sclerite (e) single-pointed on apical part of middle sclerite, deeply grooved under DIC light microscopy (Figs. 1C, 2C, D). Clamp musculature slender, extended at basis of jaws.
Tinrovia mamaevi n. sp. type-material ex Notacanthus bonaparte. A, Whole-mount (composite, ventral view); B, Haptor with frills bent at medial level (paratype, dorsal view); C, Clamp (anterior view: a, anterior mid-sclerite; c, antero-lateral sclerite; d, postero-lateral sclerite; e, accessory sclerite); D, Genital atrium with copulatory organ (paratype, ventral view); E, Proximal female genitalia, ventral view; F, Egg with detail of egg capsule (ex voucher specimen). Drawings from the holotype except otherwise indicated. Scale-bars: A, 500 µm; B, E 100 µm; C, D, F, 50 µm
Tinrovia mamaevi n. sp. type-material ex Notacanthus bonaparte, confocal laser scanning micrographs of haptoral and mouth regions. A, Haptor with frills bent at medial level (dorsal view, paratype); B, Row of clamps on lateral frill (posterior view); C, D, Details of clamps, ventral and lateral view, respectively (a, anterior mid-sclerite; c, antero-lateral sclerite; d, postero-lateral sclerite; e, accessory sclerite); E, Section of buccal suckers and mouth vestibule (paratype); F, Section of buccal sucker (paratype). α and β represent diagrammatic figures of the section plain of buccal suckers in Figs. E and F, respectively. Scale-bars: A, 50 µm; B, 30 µm; C, 15 µm; D, 20 µm; E, F 10 µm
Mouth ventral, subterminal; vestibule cup-like, with 2 oval, septate buccal suckers with mid-ventral, ellipsoidal aperture (Figs. 1A, 2E, F, Online Resource 2). Pharynx as long as wide (Fig. 1A); mean ratio buccal sucker to pharynx length 1.3. Oesophagus short. Intestinal bifurcation just anterior to or at level of genital atrium. Caeca with lateral internal and external ramifications overlapped by vitelline follicles (caeca not distinguished at haptor level).
Testes numerous, sub-ellipsoidal, flattened, post-germarial, intercaecal, pre-haptoral or slightly extending into haptoral region; arranged in 2 overlapped dorso-ventral levels. Vas deferens submedial, sinuous, ascending dorsal to uterus, terminating in small seminal vesicle (Fig. 3A–C, Online Resource 3, 4). Common genital pore midventral. Genital atrium posterior to pharynx, muscular, armed with numerous spines (Figs. 1A, D, 3B, C, Online Resource 3); divided into 2 lateral semi-circular lobes with armed muscular pads, each often apparently separated in 2 regions: proximal thick and distal thin (Figs, 1D, 3A–C, Online Resource 3); spines numerous, slender, often contorted and/or slightly hooked. Copulatory organ formed by medial tongue-shaped muscular pad with thick, rose-thorn-shaped spines.
Tinrovia mamaevi n. sp. ex Notacanthus bonaparte. Paratype, confocal laser scanning micrographs of genital atrium, ventral views. A, Ventralmost section, close to genital pore; B, Mid-level section; C, 3D micrograph with diagrammatic reconstruction (c). Arrows indicate seminal vesicle; arrowheads indicate copulatory organ with spines. Scale-bars: 10 µm
Germarium in anterior half of body, elongated, question-mark shaped, with globular posterior germinal area, with distal part directed posteriorly (Fig. 1E). Oviduct oriented sinistro-posteriorly, straight or slightly coiled ending in oötype. Oötype sinistral to germarium; Mehlis’ gland well developed. Genito-intestinal duct connected to oötype, dextrally oriented. Uterus originating from oötype, directed to right, looping anteriorly, continuing in slightly sinuous ventromedial duct terminating in common genital pore (Fig. 1A, E). Vagina posterior to level of copulatory organ (Fig. 1A); vaginal pore single, dorsal, unarmed, sub-elliptical, surrounded by tegumentary wrinkles, leading to wide vaginal atrium with 2 anterolateral chambers; vaginal duct connection wide, duct not observed. Vitelline follicles extending from level of copulatory organ into haptor, anterolaterally in variably irregular intercaecal fields; follicles scarce in haptor, reaching approximately to its midlevel. Vitelline ducts wide Y-shaped, variably coiled, joining in common duct at level of germarium. Intrauterine eggs not numerous, elliptical, with very long opercular filament and shorter needle-shaped abopercular filament (Fig. 1F); filament ends pointed.
Remarks
Apart from Tinrovia, the subfamily Syncoelicotylinae includes three species only within two genera, all having symmetrical haptor with two separate lobes (Mamaev, 1986): Syncoelicotyle polyorchis Mamaev & Zubchenko, 1978 in Notacanthus chemnitzii Bloch from Reykjanes Ridge Seamount (North Atlantic Ocean); and two species of Syncoelicotyloides, S. macruri Mamaev & Brashovian, 1989 in Macrourus holotrachys Günther [most probably M. carinatus (Günther) according to Rubec et al., 1995] from the Walvis Ridge (South East Atlantic) and S. zaniophori in Coryphaenoides zaniophorus (Vaillant) in the DeSoto Canyon in the Gulf of Mexico (Western Central Atlantic).
The morphological traits of Tinrovia mamaevi n. sp. clearly justify the inclusion of the specimens from the Western Mediterranean and North East Atlantic within the genus Tinrovia Mamaev, 1987: two-lobed differentiated haptor (“butterfly-shaped” sensu Mamaev, 1987; see also discussion), complex genital atrium and a single unarmed vagina (Mamaev, 1987; Mamaev & Brashovian, 1989; Rubec et al., 1995). The new species differs from the type- and only other species in this genus, T. papiliocauda, in the lower number of clamps (36 vs 45; see also Table 1). Clamps are also smaller in the new species (64–110 vs 120–140 µm); and as a consequence its haptor is always shorter (1,051 vs 1,100 µm) even in worms with similar length (4,885 vs 4,700 µm); additionally, the haptor of T. mamaevi n. sp. is narrower (797 vs 1,400 µm) and the lateral frills appear to be relatively smaller. Testes of the new species are notably more numerous than those of the type-species. Tinrovia mamaevi n. sp. exhibits a smaller genital atrium despite the total body length of the holotype of T. papiliocauda lies within the length range of the new species. The number of spines in T. mamaevi n. sp. is distinctly smaller in both, the genital atrium (23 vs 81) and copulatory organ (9 vs 14). The musculature of the genital atrium in T. mamaevi n. sp. is divided into two lateral semi-circular lobes with armed muscular pads forming an incomplete circle anterior to the medial, armed, tongue-shaped copulatory organ. In contrast, Mamaev (1987) described the genital atrium of T. papiliocauda as a complex organ consisting of five lobes with muscular spined pads surrounding one central armed circular pad. The eggs of both species are two-filamented, but in T. mamaevi n. sp. one filament is short and the other is very long whereas T. papiliocauda has short filaments on both poles of the egg. Furthermore, T. mamaevi n. sp. differs in the type-host species and locality; T. papiliocauda was described from Notacanthus sexspinis Richardson collected close to New Zealand. Notacanthus sexspinis and N. bonaparte exhibit different and non-overlapping geographical distributions (South Atlantic, to the Indian and the South Pacific Ocean vs North East Atlantic and Western Mediterranean; Mamaev, 1987; Mundy et al., 2011 and references therein; Froese & Pauly, 2016).
Discussion
The previous studies and descriptions of species of the Syncoelicotylinae were based on few parasite individuals collected from very few host specimens. Parasites are often lost during the sampling procedures from deep waters (Bray et al., 1999). Commonly monogeneans are considered as particularly scarce in deep waters (Campbell et al., 1980; Rohde, 2005); in a review of studies on marine parasites in deep-sea fishes and invertebrates, de Buron & Morand (2004) reported that monogeneans have been recorded only in shallower waters at depths up to 1,000 m, while copepods showed a distinctly higher diversity and were also recorded at depths up to 6,000 m. The scarcity of some parasites such as monogeneans in deep waters has been related mostly to the lower density of their host species (Campbell et al., 1980; de Buron & Morand, 2004) and, to a lesser extent, to environmental conditions (Bray et al., 1999). Notwithstanding the difficulties complicating the collection of parasite samples from deep-sea fish, future studies on Syncoelicotylinae, and in particular on Tinrovia, obtaining more specimens would be extremely useful to clarify questions concerning taxonomic issues.
The diagnoses of the species of Syncoelicotylinae refer to some characters which can be controversial in view of the species described to date (Mamaev & Zubchenko, 1978; Mamaev, 1987; Mamaev & Brashovian, 1989; Rubec et al., 1995; present study). In the generic diagnoses of Syncoelicotyle and Tinrovia the haptor is described as “butterfly-shaped”, meaning wide, separated haptor frills (Mamaev & Zubchenko, 1978; Mamaev, 1987) whereas in contrast, the genus Syncoelicotyloides was partly differed by having a “haptor undifferentiated as a separate organ” and not “butterfly-like” shaped (Mamaev & Brashovian, 1989; Rubec et al., 1995). The lateral frills of the haptor in Tinrovia mamaevi n. sp. are narrower than observed in the type-species T. papiliocauda (see Table 1); therefore this character cannot be longer diagnostic for the genus. Moreover, morphological characters are often affected by sampling and fixation conditions as already observed in studies on other platyhelminths (e.g. CCME, 2011; Ahuir-Baraja et al., 2015). Formalin is considered an appropriate fixative for monogeneans with respect to their morphological preservation (e.g. Snyder & Clopton, 2005; Strona et al., 2009). Parasites of the present study were preserved in formalin or frozen, subsequently fixed in ethanol. Comparisons of specimens fixed by both procedures did not reveal morphometric differences; however, the haptors bent laterally at medial level could be observed in two T. mamaevi n. sp. specimens only preserved frozen and subsequently fixed in ethanol. Due to the medial folding each frill appeared to be two-lobed in dorsal view, which could be described at first glance as “butterfly-like shape” (Figs. 1B, 2A). We suggest using the more generic term “winged” to refer to the wide haptor frills in species of Tinrovia and Syncoelicotyle as descriptions referring to peculiar shapes can be misinterpreted depending on the observer.
Clamps in species of the Syncoelicotylinae described to date were considered “massive” (Mamaev & Zubchenko, 1978; Mamaev, 1987; Mamaev & Brashovian, 1989). This description could be ambiguous as it could be referred to the total size of clamps in relation to body size, or to the relative size of the sclerites. Clamps in T. mamaevi n. sp. were not only relatively smaller than those in T. papiliocauda, but their sclerites were also apparently slender and delicate as well. This fact might also reflect the overall smaller host species N. bonaparte, which in part shows a generally lower body size (present study max. 38 cm) compared to the type-host of T. papiliocauda (maximum body size of N. sexspinis, 60 cm; Froese & Pauly, 2016). Hayward (2005) indicated that host size, and consequently the size of the gill lamellae, can determine the maximum clamp size.
Caeca of the species of Syncoelicotylinae have been described to date as profusely branched and anastomosed, reaching the posterior part of the haptor; Mamaev (1987) also included this feature to characterise the subfamily. The caeca could not be observed at haptor level in the 14 specimens analysed in the present study. Traits referred to the arrangement and extension of the caeca are often difficult to be distinguished in polyopisthocotyleans, as the dense vitelline follicles often obscure the caeca impeding the observations of the anastomosis.
The genital atrium of T. papiliocauda was described with five lobes whereas T. mamaevi n. sp. possesses an atrium divided into two lobes. The different genital atrium lobulation should be interpreted with caution as the two lateral muscular pads of T. mamaevi n. sp. seem to be divided into distal and proximal regions which, together with the copulatory organ, may give the appearance of five slightly notched lobes in some specimens. Confocal techniques applied in the present study were very useful for the interpretation of the 3D-structure of the genital atrium and especially the arrangement and dimensions of the copulatory organ within the atrium. In general, this technique provides great support for the three dimensional interpretation of chambers or empty spaces which could hardly be described by conventional light microscopy alone, such as those of the mouth vestibule (Fig. 2E, Online Resource 2) or the genital atrium with the copulatory organ (Fig. 3A–C, Online Resource 3, 4).
The six eggs observed in the specimens of T. mamaevi n. sp. (see Table 1) were found at different degrees of development, showing an extended range of length and width. The short abopercular and the long opercular filaments could be distinguished in one mature egg only dissected out of the genital atrium. This observation differs from the diagnosis of the genus, as eggs of the type-species were described as bearing two short filaments (Mamaev, 1987). The presence and length of filaments has also been used to characterise the genus Syncoelicotyloides (see Mamaev & Brashovian, 1989) while no eggs were found in Syncoelicotyle polyorchis (see Mamaev & Zubchenko, 1978). This trait often seems to be insufficiently reliable and unavailable in these monogeneans, consequently the use of egg morphology in generic diagnoses should be treated with caution.
In view of some morphological traits of Tinrovia mamaevi n. sp. differing from those of the type-species of the genus, T. papiliocauda, some aspects on the generic diagnosis should be emended.
Emended diagnosis of the genus Tinrovia: as in Mamaev (1987) except for: Haptor with two lateral frills not joining posteriorly, markedly winged when frills wide; eggs with two filaments (short or long).
References
Ahuir-Baraja, A. E., Padrós, F., Palacios-Abella, J. F., Raga, J. A., & Montero, F. E. (2015). Accacoelium contortum (Trematoda: Accacoeliidae) a trematode living as a monogenean: morphological and pathological implications. Parasites & Vectors, 8, 1–11.
Boeger, W. A., & Kritsky, D. C. (1993). Phylogeny and a revised classification of the Monogenoidea Bychowsky, 1937 (Platyhelminthes). Systematic Parasitology, 26, 1–32.
Bray, R. A., Littlewood, D. T. J., Herniou, E. A., Williams, B., & Henderson, R. E. (1999). Digenean parasites of deep-sea teleosts: a review and case studies of intrageneric phylogenies. Parasitology, 119, 125–144.
Campbell, R. A., Haedrich, R. L., & Munroe, T. A. (1980). Parasitism and ecological relationships among deep-sea benthic species. Marine Biology, 57, 301–313.
Carrassón, M., & Matallanas, J. (2002). Feeding strategies of Polyacanthonotus rissoanus (Pisces: Notacanthidae) in the deep western Mediterranean. Journal of the Marine Biological Association of the United Kingdom, 82, 665–671.
CCME - Canadian Council of Ministers of the Environment (2011). Protocols Manual for Water Quality Sampling in Canada. 2011. pp. 180. Available at: http://www.ccme.ca/files/Resources/water/water_quality/protocols_document_e_final_101.pdf. Accessed on 21st February 2017.
Coggan, R. A., Gordon, J. D. M., & Merrett, N. R. (1998). Abundance, distribution, reproduction and diet of notacanthid fishes from the north-east Atlantic. Journal of Fish Biology, 52, 1038–1057.
De Buron, I., & Morand, S. (2004). Deep-sea hydrothermal vent parasites: why do we not find more? Parasitology, 128, 1–6.
Froese, R., & Pauly, D. (Eds) (2016). FishBase. World Wide Web electronic publication. www.fishbase.org, version (10/2016).
Georgiev, B., Biserkov, V., & Genov, T. (1986). In toto staining method for cestodes with iron acetocarmine. Helminthologia, 23, 279–281.
Hayward, C. (2005). Chapter 3: Helminth parasites. In: Rohde, K. (Ed.), Marine Parasitology. Collingwood, Victoria: CSIRO Publishing, 592 pp.
ICZN (2012). International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Zootaxa, 3450, 1–7.
Macpherson, E. (1981). Resource partitioning in a Mediterranean demersal fish community. Marine Ecology Progress Series, 4, 183–193.
Mamaev, Y. L. (1986). The taxonomical composition of the family Microcotylidae. Taschenberg, 1879 (Monogenea). Folia Parasitologica, 33, 199–206.
Mamaev, Y. L. (1987). [Some new and insufficiently known monogeneans from the family Microcotylidae. In: Mamaev, Y. L. (Ed.) Helminths and diseases caused by them.] Vladivostok: Biologo-Pochvennyi Institut, Akademiya Nauk USSR, pp. 13–25 (In Russian).
Mamaev, Y. L., & Brashovian P. P. (1989). [Syncoelicotyloides macruri gen. et sp. n. - the first representative of the subfamily Syncoelidotylinae (Microcotylidae, Monogenea) from macruriform fishes.] Parazitologiya, 23, 532–536 (In Russian).
Mamaev, Y. L., & Zubchenko, A. V. (1978). [Two new genera of higher monogeneans from the North Atlantic.] Zoologicheskii Zhurnal, 57, 1131–1139 (In Russian).
Mundy, B. C., Cole, K., Chave, E. H., & Moffitt, R. B. (2011). Two deep-sea spiny eels, Notacanthus abbotti and Lipogenys gillii (Albuliformes: Notacanthidae), from the Hawaiian Archipelago and Emperor Seamounts with notes on their identification and biogeography. Ichthyological Research, 58, 263–271.
Nelson, J. S. (2006). Fishes of the World. 4th edition. Hoboken, New Jersey: Wiley, 601 pp.
Rohde, K. (2005). Chapter 9: Zoogeography. In: Rohde, K. (Ed.) Marine Parasitology. Collingwood, Victoria: CSIRO Publishing, pp. 348–351..
Rubec, L., & Dronen, N. (1994). Revision of the genus Diclidophora Krøyer, 1838 (Monogenea: Diclidophoridae), with the proposal of Macrouridophora n. g. Systematic Parasitology, 28, 159–185.
Rubec, L. A., Blend, C. K., & Dronen, N. O. (1995). Syncoelicotyloides zaniophori n. sp. (Monogenea: Microcotylidae) from the gills of Coryphaenoides zaniophorus (Macrouridae) from the Gulf of Mexico. Journal of Parasitology, 81, 957–960.
Snyder, S. D., & Clopton, R. E. (2005). New methods for the collection and preservation of spirorchiid trematodes and polystomatid monogeneans from turtles. Comparative Parasitology, 72, 102–107.
Strona, G., Stefani, F., & Galli, P. (2009). Field preservation of monogenean parasites for molecular and morphological analyses. Parasitology International, 58, 51–54.
Acknowledgements
The authors thank the participants and staff on board during the BIOMARE and ANTROMARE oceanographic campaigns for their assistance. We thank the staff of the Central Service for Experimental Research (SCSIE) of the University of Valencia for technical assistance, in particular to Enrique Navarro Raga for his support. We are grateful to Aneta Kostadinova (Institute of Parasitology, Czech Academy of Sciences) for the translation of the Russian publications. We thank two anonymous reviewers and the editor for their constructive criticisms and helpful corrections improving the manuscript.
Funding
This study was supported by the Spanish Ministry of Science and Innovation (MICINN) Projects BIOMARE (CTM2006-13508-C02-01MAR), ANTROMARE (CTM2009-12214-C02-02-MAR). Part of the samples was collected within the frame of the EU LIFE+Project ‘INDEMARES: Inventory and designation of marine Natura 2000 areas in the Spanish sea’ (07/NAT/E/000732).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Additional information
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as 2833829D-9339-44DF-BC2E-D5BF2E5761A2. This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number. This is the Version of Record.
This article is part of the Topical Collection Monogenea.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1 Tinrovia mamaevi n. sp. type-material ex Notacanthus bonaparte, confocal laser scanning three-dimensional image of a row of clamps on lateral frill (posterior view). Supplementary material 1 (WMV 3786 kb)
Online Resource 2 Tinrovia mamaevi n. sp. paratype ex Notacanthus bonaparte, consecutive sections of confocal laser scanning micrographs from mouth region. Supplementary material 2 (WMV 3442 kb)
Online Resource 3 Tinrovia mamaevi n. sp. paratype ex Notacanthus bonaparte, confocal laser scanning three-dimensional image of the genital atrium. Supplementary material 3 (WMV 2997 kb)
Online Resource 4 Tinrovia mamaevi n. sp. paratype material ex Notacanthus bonaparte, consecutive sections of confocal laser scanning micrographs from the genital atrium. Supplementary material 4 (WMV 9044 kb)
Rights and permissions
About this article
Cite this article
Isbert, W., Carrassón, M., Pérez-del-Olmo, A. et al. A new species of Tinrovia Mamaev, 1987 (Monogenea: Microcotylidae) from the deep-sea fish Notacanthus bonaparte Risso (Notacanthiformes: Notacanthidae) in the Western Mediterranean and the North East Atlantic. Syst Parasitol 94, 609–619 (2017). https://doi.org/10.1007/s11230-017-9727-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-017-9727-3