Abstract
Novel strong superacids HMF6 (M=Au, Ir, Os, Re, Ta, W) are proposed and are investigated with the help of DFT/B3LYP method and SDD basis set for 5d transition metals as well as 6-311++G (d) basis set for H and F atoms. These HMF6 superacids are composed with Brønsted/Lewis (MF5/HF). The stabilities of HMF6 are discussed with the help of structure, dissociation energy through HF channel, and normal mode analysis. The ΔEdisso>0 shows that all HMF6 superacids are energetically stable through HF dissociation channel. The gas phase acidity of HMF6 has been calculated by the Gibbs free deprotonation energy. All species of HMF6 belong to superacids having smaller deprotonation energy; 100% concentrated H2SO4 acids however predicted ΔGdep of HAuF6, is nearly equal to ΔGdep of HSbF6. The strength of acidity of HMF6 is closely related to vertical detachment energy (VDE) of their corresponding superhalogen anions \( \kern0.5em {\mathrm{MF}}_6^{-} \). This study provide appropriate path to design new class of superacids which is more acidic than HSbF6. We have also modelled and discussed supersalt by the interaction of Li with MF6 superhalogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Superhalogens with electron affinity (EA) and vertical detachment energy (VDE) values exceed than halogen, which got much attention from last four decades. These abnormal complexes are presented by a central atom decorated with electronegative legends which lead to increase their EA greater than Cl. The superhalogens play an important role in chemical and health industry as they purify air [1, 2]. In the last decade, some useful application of superhalogen, e.g., safe electrolytic salts for lithium ion batteries [3, 4] and efficient materials for hydrogen storage area, came into light [5]. In recent years, this is noticed that protonation of superhalogen guides formation of superacids [6]. The concept of superacids was introduced by Hall and Conant in 1927 [7]. The chemistry of superacids was developed by Olah and Hogeveen [8, 9] and in 1970, Gillespie [10, 11] gave the scientific definition of superacids. According to him, superacids are those species which are more acidic than 100% H2SO4 or with Hammett acidity function smaller than −12. Olah et al. reported the first magic acids (HSO3F:SbF5) with mixture of 1:1 which goes under the criteria of superacid [12]. Superacids are mainly classified as Lewis/Brønsted and conjugate Brønsted/Lewis. Recently, most of the strong superacids are reported by combination of conjugate Brønsted/Lewis [13, 14]. Currently, HSbF6 is considered to be the strongest superacid as it is 109 times more acidic than 100% H2SO4 [15]. This HSbF6 superacid can also be expressed as a combination of (SbF5/HF) SbF5 Lewis acid and HF Brønsted acid. Koppel et al. theoretically predicted that F(SO3)4H, FSO3SbF5H, and HAlCl4 are stronger acids on basis of Gibbs free energies of deprotonation (ΔGdep) which were similar to one of the strongest superacid HSbF6 (255.5 kcal/mol) [6]. At this place, it is necessary to point out that ΔGdep is the most important factor for describing the gas phase acidity of any species. The protonation of anion of superhalogen AlnF3n+1 (n=1–4) easily relied as HF/AlnF3n [16] and their gas phase acidity exceed to HSbF6 for n=4. In similar fashion, protonation of anion of superhalogen InnF3n+1, SnnF4n+1, and SbnF5n+1 (n=1–3) easily relied as HF/InnF3n, HF/SnnF4n, and HF/SbnF5n respectively and shows more acidity than HSbF6 [17]. Ambrish et al. have reported that protonation of B(BF4)4−, B(AlF4)4−, and Al(BF4)4− as well as Al(AlF4)4− superhalogens shows more acidic behavior than HSbF6 in the gas phase [18]. In the above example, it is noticed that these superhalogens contain F as ligands and rely to HF (Brønsted acid) by protonation. The role of HF (Brønsted acid) in increasing acidity of superacids is reported [19]. This was also reported that successive attachments of HF to Lewis acid gradually increase gas phase acidity [20]. So it is difficult to say that the presence of HF enhances protonation of superhalogen or this is an intrinsic property of superhalogen. Recently, it is reported that protonation of halogen-free anions superhalogen BnH3n+1(n=1–5) gives new class of superacids which have no role of HF (Brønsted acid) so superacidic behavior of complex is an intrinsic property of superhalogen [21]. Indeed, acidity of any superacidic should relate to electronic stability of their anionic superhalogen part. The VDE is an important parameter to determine electronic stability of anionic part of superhalogen. This is also reported that VDE of anions of superhalogen as well as gradually increases the number of ligands that are directly related to gas phase acidity. To verify this, we fix a number of ligand of six in present communication and we choose some hydrogenated complex of AuF6, IrF6, OsF6, ReF6, TaF6, and WF6 series noticed that they really belong to superacid family. In this study, we again find a correlation in between VDE and interaction energy of HF-MF5 with acidity of superacids. We reveals that the acidity of these HMF6 (M=Au, Ir, Os) species are comparable to the strongest superacids HSbF6. We also hope that our study provides new path for designing new superacids in the future. At last, we have also modelled and discussed supersalt by the interaction of Li with MF6 superhalogen.
Computational details
All anionic hexafluorides and their corresponding protonated structure are fully optimized with the help of DFT/B3LYP method using G09 program package [22]. In this study, we have employed SDD basis set for 5d transition metals and 6-311++G (d,p) basis set for H and F atoms. All structures are optimized without any symmetry constrains. The above method and basis sets are also employed in previous studies.
The vibrational analysis is performed to ensure that all structures belong to true minima. The vertical detachment energy of \( {\mathrm{MF}}_6^{-} \) is calculated by energy difference between optimized \( {\mathrm{MF}}_6^{-} \) structure to single point energy calculation of corresponding neutral MF6:
VDE= E (\( {\mathrm{MF}}_6^{-}\Big) \)optimized − E (MF6) single point
The acidity of HMF6 in gas phase is defined by change in Gibbs free energy of deprotonation reaction.
\( {\mathrm{HMF}}_6\to \mathrm{H}+{\mathrm{MF}}_6^{-} \) at T=298.15K is calculated by the following reaction:
where ΔG [H+] = −6.28 kcal/mol is taken from literature [23].
The HOMO and LUMO are plotted with the help of Gauss View 5.0 [24]. Gauss sum 3.0 is used for PDOS plot of LiMF6 supersalts.
Result and discussion
In this paper, we have discussed the formation of superacids by protonation of anions of transition metal hexafluoride \( {\mathrm{MF}}_6^{-} \) . So, we have performed our calculation on \( {\mathrm{MF}}_6^{-} \)/ HMF6 containing representative 5d transition metal such as Au, Ir, Os, Re, Ta, and W. Our study is based on the following criteria:
-
(1)
Both \( {\mathrm{MF}}_6^{-} \)/HMF6 are chemically and thermodynamically stable.
-
(2)
To establish superacidic behavior of HMF6, Gibbs free energy should not exceed 300kcal/mol.
-
(3)
The structure HMF6 is intact with two noticeable parts of HF/MF5.
-
(4)
Finally, see the relation between VDE of \( {\mathrm{MF}}_6^{-} \) with deprotonation energy of HMF6.
Let us discuss these criteria one by one for all six entities:
\( {\mathrm{MF}}_6^{-} \)
First of all, we discuss the structure of conjugate base anions ( \( {\mathrm{MF}}_6^{-} \) ) in HMF6 superacids. For this, we have considered all possible geometry of\( \kern0.5em {\mathrm{MF}}_6^{-} \) and after geometry optimization, most stable geometries of \( {\mathrm{MF}}_6^{-} \) are displayed in Figure 1. We have also optimized most stable geometry of \( {\mathrm{MF}}_6^{-} \) at various multiplicities by using combination DFT/B3LYP method and SDD/6-311++G (d, p) basis set. We have calculated lowest energy conformers of\( {\mathrm{HMF}}_6^{-}/{\mathrm{MF}}_6^{-} \) at various multiplicity and relative energies presented in supplementary Table 1. In all\( {\mathrm{HMF}}_6^{-}/{\mathrm{MF}}_6^{-} \), Au, Ir, Re, and Ta show odd multiplicity; however, the rest Os and W show even multiplicity. The lowest energy occurs at a singlet electronic state for Au and Ta; however, Ir and Re occur at a triplet state. In even multiplicity state, Os shows the lowest energy at doublet however W at quartet. So we have performed calculations on these states. The\( {\mathrm{AuF}}_6^{-}, \) \( {\mathrm{TaF}}_6^{-},{\mathrm{WF}}_6^{-} \) shows octahedral symmetry Oh; however, \( {\mathrm{IrF}}_6^{-},\kern0.5em {\mathrm{ReF}}_6^{-},{\mathrm{OsF}}_6^{-} \) shows D4h symmetry. The calculated bond lengths of M-F in \( {\mathrm{MF}}_6^{-} \) are well matched with previous calculation [25,26,27,28,29]. The calculated ADE of \( {\mathrm{MF}}_6^{-} \) are also well matched with experimental values [30,31,32,33]. The calculated VDE and ADE of \( {\mathrm{MF}}_6^{-} \) (Table 1) are nearly matched with previously calculated VDE of corresponding species [33] and ADE values respectively [34,35,36,37]. The calculated VDE and ADE of \( {\mathrm{MF}}_6^{-} \) re-established their superhalogenic behavior [38,39,40,41,42]. The dissociation of \( {\mathrm{MF}}_6^{-} \) through F− channel are calculated by:
The \( {\mathrm{OsF}}_6^{-} \) shows the highest dissociation energy (5.02eV) among all species; however, \( {\mathrm{ReF}}_6^{-} \) has the lowest dissociation energy (2.32eV). The calculated dissociation energy ∆Edisso>0 of \( {\mathrm{MF}}_6^{-} \) shows the stability of hexafluoride anion dissociation through F− channel. The numbers of theoretical studies have predicted the dissociation energy through F channel having higher value as compared with dissociation through F− channel [26,27,28,29,30] which shows that the extra charge has been carried by \( {\mathrm{MF}}_5^{-} \) rather than F−.
HMF6
To study HMF6 (M=Au, Ir, Os, Re, Ta, W), we have modelled all possible initial structures. However after optimization, lowest energy of HMF6 corresponds to those structures in which one of F atoms interact with H atom and displayed in Fig. 2. Again to find the most stable geometry, we have run these structures at a different multiplicity employed DFT method and SDD basis set for 5d transition metals and 6-311++G (d,p) basis set for H and F atoms and their relative energy are listed in supplementary Table 1. All calculations are performed at the lowest energy structure. The optimized HMF6 structures have no symmetry. From Fig. 2, it is obvious that there exist HF moieties in HMF6. This HF moieties weakly interact with central metal (Au, Ir, Os, Re, Ta, W) in HMF6. So HMF6 can be expressed as AuF5/HF, IrF5/HF, OsF5/HF, ReF5/HF, TaF5/HF, and WF5/HF. In order to explore this, we further optimized these components of HMF6 and displayed in Fig. 3. This can be also verified by using NBO charge analysis. The calculated Mullikan atomic charges on both (MF5/HF) subunits are collected in Table 3. The atomic charges on both subunits are nearly equal means HF subunit transfer charge to MF5 subunits. The magnitude of charge transfer from HF to MF5 subunits is in order of 0.0001e so they bind with weak electrovalent bond and hence HMF6 can be written as combination MF5/HF subunits. The calculated bond lengths of M-F in MF5 are well matched with previous studied [38,39,40,41,42]. The calculated bond length of H-F by DFT/6-311++G (d, p) method is well matched with experimental result. The H-F bond length calculated by using combination of DFT/B3LYP method SDD/6-311++G (d, p) and basis set in HMF6 lies in between 0.94A0 and 0.93A0 and is matched with H-F bond length in free H-F molecule. However, the distance of HF species from central metal are in this order: HAuF6(2.19A0) <HIrF6(2.24A0)=HOsF6(2.24A0) <HReF6(2.32A0) <HWF6(2.38A0)<HTaF6(2.40A0).
To check stability of HMF6 against dissociation through HF channel, we also calculate dissociation energy using the following formula:
The calculated dissociation energies by using DFT/6-311++G and SDD/6-311++G (d) basis set are listed in Table 2. One can easily see that ΔEdiss>0 for all species shows that these HMF6 are stable against dissociation through HF channel. The calculated dissociation energy is HWF6 followed by HAuF6 through HF dissociation channel having a larger value than other species.
Deprotonation energy of HMF6
In order to analyze gas phase acidity of HMF6, we have calculated deprotonation energy (ΔGdep) of HMF6 species by using DFT/B3LYP method and SDD/6-311++G (d) basis set. The calculated deprotonation energies of HMF6 are listed in Table 2. The calculated ΔGdep values of all HMF6 are smaller than ΔGdep value of H2SO4 (303kcal/mol) which suggests that these HMF6 species are superacids. The calculated ΔGdep (260.48kcal/mol) of HAuF6 is nearly matched with the corresponding value reported by Marcin Czapla (259.5kcal/mol) [43] and also comparable to ΔGdep of HSbF6 (261kcal/mol). All HMF6 except HWF6 are more acidic than HTaF6 (274.6kcal/mol) as reported by Koppel et al. [6] and calculated deprotonation energy of HTaF6 is well matched with ΔGdep value as reported by Koppel et al. To check reliability of our methods, we have calculated ΔGdep of well-known superacid HSbF6 by DFT/B3LYP method and SDD/6-311++G (d) basis set. The calculated value of ΔGdep of HSbF6 by DFT/B3LYP method and SDD/6-311++G (d) basis set (262.76kcal/mol) shows only 1–2 kcal/mol difference which prove the reliability of our method. The variation of deprotonation energy according to HAuF6<HIrF6 <HOsF6 <HReF6 <HTaF6<HWF6 and acidity of HMF6 varies inversely with deprotonation energy of HMF6.
Let us see a closer insight of mechanism of deprotonation by using NBO charge analysis of HMF6 species. The calculated charge on H atom (0.290611e) is the same but opposite as F atom (−0.290611e) in HF by using combination of DFT/B3LYP method and 6-311++G (d, p) basis set. In similar fashion, charges on MF6 unit and H atom are the same but with higher magnitude in HMF6 (Table 3) as compared with HF. In HMF6 species, H atom faces more deficiency of charges as compared with F atom in HF. In this way, HMF6 can be written as HF/MF5 of which again HF relies to H+ easily as compared with free HF species which shows more electrovalent character. In this way, the strength of acidity of any HMF6 species is directly related with electronic stability of their corresponding superhalogen anions. In Fig. 4, we have plotted a correlation graph in between VDE of \( {\mathrm{MF}}_6^{-} \) and acidity (Gas Phase) of HMF6. The correlation follows a linear equation y=0.0498x+3.44 with correlation factor R2=0.93 showing a fair correlation in between that VDE of \( {\mathrm{MF}}_6^{-} \) with acidity of their protonated species as reported by Ambrish et al. [21]. Some deviation occurs in this linear correlation due to unusable behavior of heavy transition metal hexafluoride.
Electronic properties of HMF6
Some electronic parameters of HMF6 are also calculated by DFT/B3LYP method and SDD/6-311++G (d) basis set which are listed in Table 4. The HOMO is known as the highest occupied molecular orbitals and primarily acts as a donor; however, LUMO is the lowest unoccupied molecular orbital and primarily acts as an acceptor. The energy difference in between HOMO and LUMO plays an important role for examining chemical reactivity of any species. The HOMO and LUMO plots of HMF6 species are shown in Fig. 5. The LUMO covered over whole species; however; HOMO covered over whole species except HF Bronsted acid. Note that HMF6 superacids can be expressed as MF5/HF. The calculated Egap for HMF6, calculated by DFT/B3LYP method and SDD/6-311++G (d) basis set, are listed in Table 4. The Egap values for HMF6 are much lower than Egap of HF (11.40eV); however, Egap value of HTaF6 is comparable with Egap of HF. The Egap of HMF6 varies according to HReF6<HIrF6<HWF6<HOsF6<HAuF6<HTaF6. In this way, HReF6 is most reactive than other species; however, HTaF6 is less reactive than all species. The dipole moments of any species are closely related to their geometry and electronic structure.
Salt formation
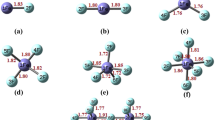
Strong acids and strong bases from salt, superacid, and superbases also form supersalt. In this section, we have modelled supersalt by using HMF6 superacids. For this, we have considered several possible geometries of LiMF6 supersalts and after geometry optimization, one can note that the most stable conformer is displayed in Fig. 6. The geometry of MF6 structure is distorted and Li binds with its two F atoms in LiMF6 supersalt. The calculated bond lengths are displayed with optimized geometry of LiMF6 (Fig. 6).The bond lengths of Li-F lie in between 1.83 A0 and 1.89A0. The calculated value of Ir-F (1.89–2.04A0) is well matched with the experimental value (1.879 A0/1.875 A0) [28, 44]. The vibrational frequencies of LiMF6 are positive to ensure that LiMF6 are stable. The calculated dissociation energy of LiMF6 through LiF channel is calculated:
The calculated dissociation energy shows that these salts are stable against LiF dissociation.
The calculated HOMO-LUMO gaps of LiMF6 are also presented in Fig. 6. The LiTaF6 supersalt has the highest Egap; however, LiIrF6 has the lowest Egap value. The percentage contribution of M, F, and Li atoms in LUMO and HOMO is calculated and listed in Table 5 by Gauss Sum 2.2 [45] program. The PDOS calculation shows that Li atom does not show any contribution of HOMO-LUMO plot. To know about nature of interaction, we have performed QTAIM analysis [46] of LiMF6. Some topological parameters are collected in supplementary Table 5. According to Koch and Popelier criteria [44], no nonbonding interaction appeared in LiMF6. According to Koch and Popelier criteria [44], bonding in LiMF6 salts is electrovalent in nature [26, 47, 48] because for all interactions ∇2ρ>0, H>0 at BCP as in LiCl salt. The electrovalent bonding in LiMF6 can also verify with NBO charge on Li in LiAuF6 (0.8774e), LiIrF6 (0.8443e), LiOsF6 (0.8415e), LiReF6 (0.8483e), LiTaF6 (0.8486e), and LiWF6 (0.8296e) which are nearly equal to one electron charge clear of its electrovalent character.
Conclusion
Our DFT/B3LYP method and SDD/6-311++G (d, p) basis set calculation represent the following conclusions:
1. All HMX6 show potential candidates for superacids, as the MX5 and HX subunits might be noted as Lewis/Brønsted superacids and the value of the gas phase Gibbs free energies of the deprotonation process for HMF6 species are smaller than the gas phase Gibbs free energies of the deprotonation process of H2SO4.
2. The dissociation energy ∆Ediss>0 for HMF6 through HF channel shows that all species are thermodynamically stable against dissociation through HF channel.
3. The acidity of HAuF6 and HOsF6 is nearly the same most acidic species HSbF6.
4. The correlation factor R2=0.93 suggests that a good correlation occurs in between acidity of HMF6 and VDE of their conjugate superhalogen anions\( \Big(\ {\mathrm{MF}}_6^{-} \)).
5.The Egap of HMF6 are lower than Egap of HF which shows that HMF6 are chemically reactive.
6.These superacids have a strong tendency to form new class supersalts as they interact with strong base; however, PDOS calculations plots of LiMF6 verify its covalent charter.
We hope that these new class of superacids may attract those synthetic chemists which are concerned with inorganic reactions. Note that our calculation on these species HMF6 are based on their theoretical deprotonation reactions in which we are neglect various effects observed in gas phase.
Data availability
Data and materials are real. First time any study is done on these aforesaid materials.
References
Nishikawa K, Nojima H (2001) Japan J. Appl. Phys. 40:835–837
Miller NJ (1984). J. Ment. Health Adm 11:36–37
Giri S, Bahera S, Jena P (2014) Angew Chem. Int. Ed 53:13916–13919
Srivastava AK, Misra N (2016). Polyhedron 117:422–426
Srivastava AK, Misra N (2016) Electrochem. Commun 68:99–103
Koppel IA, Burk P, Leito I, Sonoda T, Mishima M (2000). J. Am. Chem. Soc. 122:5114–5124
Hall NF, Conant JB (1927). J. Am. Chem. Soc. 49:3047–3061
Hogeveen H, Bickel AF (1969) Recl. Trav. Chim. Pays-Bas 88:371–378
Hogeveen H, Bickel AF (1967) J. Chem. Soc. Chem. Commun. 13:635–636
Gillespie RJ, Peel TE (1971) Adv. Phys. Org. Chem. 9:1–24
Gillespie RJ, Peel TE (1973) J. Am. Chem. Soc. 95:5173–5178
Olah GA, Schlosberg RH (1968) J. Am. Chem. Soc. 90:2726–2727
Bickel AF, Gaasbeek CJ, Hogeveen H, Oelderik JM, Platteeuw JC (1967) Chem Commun 634–635
Olah GA, Lukas J (1967). J. Am. Chem. Soc. 89:2227–2228
Olah GA, Prakash GK, Sommer J (1979) Superacids. Science 206:13–20
Czapla M, Skurski P (2015). Chem. Phys. Lett. 630:1–5
Czapla M, Skurski P (2015). J. Phys. Chem. A 119:12868–12875
Srivastava AK, Misra N (2015). Polyhedron 3:277–283
Shukla DV, Srivastava AK, Misra N (2017). Main group chemistry 16(2):141–150
Czapla M, Skurski P, Anusiewicz I (2016). RSC Adv. 6:29314–29325
Srivastava A K, Misra N Kumar A (2017) New J Chem 41:5445–5449
Frisch, MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision E.01, Gaussian, Inc., Wallingford CT, 2009
Topol IA, Tawa GJ, Burt SK, Rashin AA (1997). J. Phys. Chem. A 101:10075–10081
Dennington R, Keith T, Millam J (2009) GaussView Version 5, Semichem Inc. Shawnee Mission KS
Macgregor SA, Moock KH (1998). Inorg. Chem. 37:3284
Graudejus O, Wilkinson A P, Chacon L C, Bartlett N (2000) Inorg Chem 39(13):2794–2800
Hoskins BF, Linden A, Mulvaney PC, O’Donnell TA (1984). Inorg.Chim. Acta 88:217
Fitz H, Muller BG, Graudejus O, Bartlett NZ (2002). Z. Anorg. Allg. Chem. 628:133
Graudejus O, Muller BGZ (1996). Z. Anorg. Allg. Chem. 622:1076
George PM, Beauchamp JL (1979). Chem. Phys. 36:345
Viggiano AA, Paulson JF, Dale F, Henchman M, Adams NG, Smith DJ (1985). Phys. Chem. 89:2264
Korobov MV, Kuznetsov SV, Sidorov LN, Shipachev VA, Mit’kin VN (1989). Int. J. Mass Spectrom. Ion Process. 87:13
Friedman JF, Stevens AE, Miller TM, Viggiano AA (2006). J. Chem. Phys. 124:224306
Craciun R, Picone D, Long RT, Li S, Dixon DA (2010). Inorg. Chem. 49:1056–1070
Gutsev GL, Boldyrev AI (1983). Chem. Phys. Lett. 101:441
Miyoshi E, Sakai Y, Murakami A, Iwaki H, Terashima H, Shoda T, Kawaguchi T (1988). J. Chem. Phys. 89:4193
Miyoshi E, Sakai Y (1988). J. Chem. Phys. 89:7363
Koirala P, Willis M, Kiran B, Kandalam AK, Jena P (2010). J. Phys. Chem. C 114:16018–16024
Srivastava AK, Misra N, Pandey SK (2015). Chem. Phys. Lett. 624:15–18
Siddiqui SA, Rasheed T (2012) Int J Quantum Chem 113(7):959–965
Srivastava AK, Misra N (2014). J. Fluor. Chem. 158:65–68
Srivastava AK, Misra N, Pandey AK (2015). J. Chem. Sci. 127:1853–1858
Czapla M, Skurski P (2017). Int. J. Quantum Chem. 25:494
Koch U, Popelier P (1995). J. Phys. Chem. A 99:9747–9754
O’boyle NM, Tenderholt AL, Langner KM (2008). J. Comput. Chem. 29:839–845
Keith T A, Gristmill T K Software overland park KS USA 2019.
Rasheed T, Siddiqui SA, Pandey AK, Bouarissa N, AliAl-Hajry (2017). J. Fluor. Chem. 195:85–92
Rasheed T, Siddiqui SA, Pandey AK, Mishra M (2012). J. Fluor. Chem. 135:285–291
Code availability
Licenced codes are used.
Author information
Authors and Affiliations
Contributions
Anoop Kumar Pandey: Most calculations and writing.
D. V. Shukla: Modelling and writing.
Vijay Narayan: Literature survey and writing.
Vijay Singh: Methods and some calculations.
Apoorva Dwivedi: Whole paper final writing and submission process (corresponding author).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOC 312 kb)
Rights and permissions
About this article
Cite this article
Pandey, A.K., Shukla, D.V., Narayan, V. et al. Protonated MF– (M=Au, Ir, Os, Re, Ta, W) behave as superacids and are building blocks of new class of salt. Struct Chem 33, 91–100 (2022). https://doi.org/10.1007/s11224-021-01809-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-021-01809-8