Abstract
By doping π-π systems with Li atom, a series of Li@sandwich configuration and Li@T-shaped configuration compounds have been theoretically designed and investigated using density functional theory. It is revealed that energy gaps (E gap) between highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of all compounds are in a range of 0.4–0.9 ev. When Li atom is introduced into different sandwich configuration π-π systems (C60-toluene, C60-fluorobenzene, C60-phenol, C60-benzonitrile), Li@C60-benzonitrile exhibits considerable first hyperpolarizability as large as 19,759 au, which is larger by about 18,372–18,664 au than those of other compounds. When Li atom is introduced into different T-shaped configuration π-π systems (C60-pyridine, C60-pyrazine, C60-1, 3, 5-triazine, C60-pyridazine), Li@C60-pyridazine is found to present largest first hyperpolarizability up to 67,945 au in all compounds. All compounds are transparency in the deep ultraviolet spectrum range. We hope that this study could provide a new idea for designing nonlinear optical materials using π-π systems as building blocks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past 2 decades, the π-π interactions in aromatic systems have aroused great interest of scientists [1–6], in view of an important role in biology, chemistry, and materials [7–10]. For example, supramolecular organization and recognition processes can be achieved through π-π interactions in biomolecules [11, 12]. The interaction of some drugs with DNA depends on π-π interactions [13, 14]. Crystal packing is influenced by the π-π interactions [15]. Despite a lot of experimental and theoretical studies indicating the important of π-π interactions [16–18], describing the strength and geometrical parameters of π-π interactions has been a challenge for scientists due to the weakness of π-π interactions. Heteroatoms and substituents can tune π-π interactions across the energy landscape, which is helpful in crystal engineering. The effect of substituents on the binding of π-π interactions was studied by Sherrill et al. It is found that all substituted sandwich dimers bind more strongly than benzene dimer [19]. The effect of heteroatoms on the π-π interactions was investigated by using quantum chemical computations. The results show that the bind energies of these systems are larger in contrast to that of benzene dimer [20].
Design and synthesis of novel materials with large first hyperpolarizability is always a hot issue in the past several decades due to a wide range of applications [21–24]. Recently, it was found that alkali metal atom doped system can exhibit a large nonlinear optical response because electrides or alkalides with excess electrons are formed [25, 26]. Doping Li atom into different systems (organic molecules, graphene, carbon nanotube, supermolecules, etc.) has been designed in theory, and the results indicate that these compounds become candidates for exhibiting excellent nonlinear optical response [25–30]. For example, a new multilithium salt, which was obtained by substituting H of the [5] cyclacene with Li atom, was obtained in theory and investigated by using quantum chemistry method, and the results indicate that the first hyperpolarizability of Li5-[5] cyclacene is greatly increased compared to that of [5] cyclacene on account of the lithiation effect [25]. In order to research the Li atom effect on the nonlinear optical response of graphene, graphene multilithium salts were designed and investigated. The results indicate that the first hyperpolarizability obviously increases with an increase in the number of Li atoms [26].
In this work, doping Li atom into π-π interaction systems, including sandwich configurations or T-shaped configurations, was investigated by using quantum chemistry method. We mainly address the following questions: (1) whether the π-π interaction systems can be used as an effective structural unit to obtain the compounds with large first hyperpolarizability, (2) if the compounds exhibit excellent nonlinear optical response, can they have a wide transparency in ultraviolet region?, and (3) what are some of the characteristics of electronic properties and optical spectrum for Li@sandwich configuration and Li@T-shaped configuration compounds? In order to clarify these questions, we study the electronic properties, absorption spectrum, and nonlinear optical responses of these compounds and expect that such a theoretical study can provide helpful information for further experimental studies of nonlinear optical materials of Li@sandwich configuration and Li@T-shaped configuration compounds.
Computational details
When a system is in the weak and homogeneous electric field, its energy can be written as [31–33]
Here, E 0 is the molecular total energy without the electric field, and F α is the electric field component along α direction; μ α , α αβ , and β αβγ are the dipole, the polarizability, and the first hyperpolarizability, respectively. The dipole moment (μ 0) and polarizability (α 0) are defined as follows:
The first hyperpolarizability is obtained as
in which
The geometric structures of Li@sandwich configuration and Li@T-shaped configuration compounds were optimized using the B3LYP method with the 6-31G(d) basis set. It is well known that B3LYP method can predict geometric structures of nanostructure, which have been proved by a lot of scientists [34–38]. In order to confirm whether geometric structures are minima in global scope, frequency analysis was carried out at the same level.
For the calculation of the hyperpolarizability of system, selecting appropriate computational methods is particularly important. As is known to all, MP2 method is more reliable compared with B3LYP. When we compute hyperpolarizability of large molecular system, it consumes a lot of time. Recently, Nakano et al. proposed BHandHLYP method, which achieves a good balance between quality and efficiency [39]. Such as, hyperpolarizabilities of Li@n-Acenes salt were investigating using BHandHLYP method [40]. In our work, the BHandHLYP method and the 6-31+G(d) basis set were chosen to explore the first hyperpolarizabilities of the compounds. The crucial transition energies were calculated by time-dependent density functional theory (TD-DFT). All of the calculations were carried out by using the GAUSSIAN 09 program package [41]. The molecular orbitals of the compounds were plotted with the GaussView program [42]. The UV-Vis spectra of the molecules are obtained by Multiwfn procedure [43].
Results and discussion
Equilibrium geometries and natural bond orbital charges
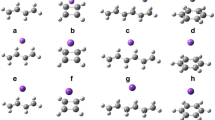
The systems are classified into two groups by the differently π-π configurations (see Fig. 1), in which benzene is substituted or doped, respectively. Li doped sandwich π-π interaction systems are named as Li@C60-toluene (1a), Li@C60-fluorobenzene (1b), Li@C60-phenol (1c), and Li@C60-benzonitrile (1d), respectively, in which H atom in benzene is substituted with CH3, F, OH, and CN. Li doped T-shaped π-π interaction systems are named as Li@C60-pyridine (2a), Li@C60-pyrazine (2b), Li@C60-1, 3, 5-triazine (2c), and Li@C60-pyridazine (2d), respectively, in which N was doped into the different positions of benzene. The optimized geometries of the compounds at the B3LYP/6-31G(d) level are presented in Fig. 1.
The natural bond orbital (NBO) charges of the compounds are displayed in Table 1. When Li atom is introduced to sandwich configuration systems (C60-toluene, C60-fluorobenzene, C60-phenol, C60-benzonitrile), q(Li+) values of Li@C60-toluene (1a), Li@C60-fluorobenzene (1b), Li@C60-phenol (1c), and Li@C60-benzonitrile (1d) are 0.683, 0.681, 0.680, and 0.685, respectively. When Li atom is introduced to T-shaped configuration systems (C60-pyridine, C60- pyrazine, C60-1, 3, 5-triazine, C60-pyridazine), q(Li+) values of Li@C60-pyridine (2a), Li@C60-pyrazine (2b), Li@C60-1, 3, 5-triazine (2c), and Li@C60-pyridazine (2d) are 0.739, 0.735, 0.727, and 0.717, respectively. The results indicate that doping Li atom can cause the occurrence of charge transfer from Li atom to π-π systems, which are beneficial to the obtained large first hyperpolarizability.
Electronic structures and absorption spectra
The electronic structures of the compounds were calculated at B3LYP/6-31G(d) level, and the results are given in Table 1. From Table 1, when Li atom is doped into sandwich π-π systems, the energy gaps E gap between HOMO and LUMO of Li@sandwich configuration compounds change in the order of Li@C60-toluene (1a) >Li@C60-fluorobenzene (1b) = Li@C60-phenol (1c) >Li@C60-benzonitrile (1d), which show that different substitution is an effective strategy to regulate electronic properties of the compounds. When Li atom is doped into T-shaped π-π systems, the energy gaps E gap between HOMO and LUMO of Li@T-shaped configuration compounds change in the order of Li@C60-pyridine (2a) >Li@C60- pyrazine (2b) >Li@C60-1, 3, 5-triazine (2c) >Li@C60-pyridazine (2d), which show that heteroatom benzene is a new factor for regulating electronic properties of the compounds.
The absorption spectra were calculated using time-dependent density functional theory (TD-DFT) at the BHandHLYP/6-31G level of theory. The absorption spectra of the compounds have been plotted in Fig. 2 and Fig. 3. It is shown that the maximum absorption wavelengths of compounds 1a, 1b, and 1c are in the infrared spectral region, at 837.66, 833.14, and 837.91 nm, respectively. From Fig. 2 and Table 2, the maximum absorption wavelengths of 1d are also in the infrared spectral region, which are 989.5 for 1d. Among structures, the maximum absorption wavelength of 1d is the largest, which may make the compound exhibiting large first hyperpolarizability due to small transition energy. From Fig. 3, for compounds 2a, 2b, 2c, and 2d, the maximum absorption wavelengths of these compounds are in the infrared spectral region, which change in the order of 2c <2b <2a <2d. Among structures, the maximum absorption wavelength of 2d is the largest in contrast to other three compounds (2a, 2b, and 2c). Especially, the main absorption spectrum ranges from 350 to 1800 nm, which exhibits a wide absorption spectrum. In other words, all compounds are transparent in the deep ultraviolet spectrum range, which is of great importance in practice.
Linear and nonlinear optical properties
The polarizabilities of Li@sandwich configuration and Li@T-shaped configuration compounds calculated at BHandHLYP/6-31+G(d) level are given in Table 2. When Li atom is introduced to sandwich configuration systems (C60-toluene, C60-fluorobenzene, C60-phenol, C60-benzonitrile), the polarizabilities of the compounds (1a–1d) are 659, 647, 652, and 681 au, respectively. The results indicate that the polarizabilities change in the order of 1b <1c <1a <1d. When Li atom is introduced to T-shaped configuration systems (C60-pyridine, C60- pyrazine, C60-1, 3, 5-triazine, C60-pyridazine), the polarizabilities of the compounds (2a–2d) are 650, 646, 640, and 679 au, respectively. The results indicate that the polarizabilities change in the order of 2c <2b <2a <2d.
In this work, we focus on how to build excellent nonlinear optical material using π-π systems (sandwich and T-shaped configuration). It is previously reported that doping the alkali atom into a compounds may lead to larger first hyperpolarizability of the system due to loosely excess electron. In this work, Li@sandwich configuration and Li@T-shaped configuration compounds are designed for the first time. We look forward to these compounds possessing large NLO response.
The static first hyperpolarizabilities of these compounds were calculated at the BHandHLYP/6-31+G(d) level. The dependence of the first hyperpolarizabilities on different sandwich configurations is illustrated in Fig. 4. First of all, Li atom is introduced to sandwich configuration π-π systems. The interesting results are obtained from Table 2. When the H atom of benzene is substituted with CH3, F, and OH, respectively, these compounds exhibit almost the same value, which were 1096 au for 1a, 1146 au for 1b, and 1387 au for 1c. However, when the H atom of benzene is substituted with CN, the compound exhibits considerable first hyperpolarizability as large as 19,759 au, which is larger by about 18,372–18,664 au than that of compounds (1a–1c). The first hyperpolarizabilities of Li@sandwich configuration compounds are sensitive to different substituted benzene. The results indicate that the π-π systems can be considered as building blocks for designing new nonlinear optical materials.
The dependence of the first hyperpolarizabilities on different T-shaped configurations is illustrated in Fig. 4. When Li atom is doped into T-shaped π-π interaction systems, these compounds (2a, 2b, 2c) exhibit considerable first hyperpolarizabilities, which are 1687 au for 2a, 1042 au for 2b, and 1524 au for 2c. However, Li@C60-pyridazine (2d) is found to present larger first hyperpolarizability in contrast to that of these compounds (2a, 2b, 2c). The first hyperpolarizability is increased to 67,945 au. The results indicate that these compounds can be considered as building blocks for designing new nonlinear optical materials. More important, the first hyperpolarizability of Li@C60-pyridazine (2d) is about two times larger than that of Li+(calix[4]pyrrole) K− [44], which is famous alkalide compound with the cuplike complexant and is close to that of Li-doped fluorocarbon chain [27], which is an excellent electride.
To understand the origin of the large first hyperpolarizabilities of these compounds, two-level model is widely employed [45–47].
where ΔE, f 0, and Δμ are, respectively, the transition energy, oscillator strength, and the difference in dipole moment between the ground state and the most relevant excited state. The third power of the transition energy is inversely proportional to the β 0 value. Therefore, the transition energy is the decisive factor in calculation of the β 0 value.
The transition energies (ΔE) of these compounds were calculated by the TDDFT method, and the corresponding results are listed in Table 2. From Table 2 and Fig. 4, the ∆E values of the systems are 1.4801 (1a), 1.4882 (1b), 1.4797 (1c), and 1.253 (1d) ev, respectively. It is obvious that transition energies of 1a, 1b, and 1c are almost the same values, which are larger than that of 1d. Among these structures, oscillator strength of Li@C60-benzonitrile (1d) is the largest value compared to other three compounds (1a, 1b, and 1c). For 2a–2d, transition energies of 2a, 2b, and 2c are larger than that of 2d. Oscillator strength of Li@C60-pyridazine (2d) is the largest value compared to other three compounds (2a, 2b, and 2c). Thus, transition energy and oscillator strength play an important role in increasing the first hyperpolarizability. In other words, two-level model can well explain the variation in β 0 values.
Furthermore, in order to understand the origin of the first hyperpolarizabilities, we focused on the molecular orbit of the crucial transition states of the compounds. The molecular orbitals involved in dominant transitions are given in Fig. 5 and Fig. 6. Figure 5 gives the crucial transitions for Li@sandwich compounds. For Li@C60-toluene (1a), Li@C60-fluorobenzene (1b), and Li@C60-phenol (1c), it can be found that the electron cloud in their related occupied orbitals (HOMO) can be distributed on C60 cage; the electron cloud in their related unoccupied orbitals (LUMO+2) can be distributed on substituted benzene. The crucial transition of Li@C60-benzonitrile (1d) is from HOMO to LUMO+2. The HOMO is distributed on C60 cage and substituted benzene, and the LUMO+2 is distributed on C60 cage. Why would Li@C60-benzonitrile (1d) has the largest first hyperpolarizability compared to other compounds (1a, 1b, and 1c)? It is closely related to HOMO of 1d, which is dispersed. However, HOMOs of 1a, 1b, and 1c are centralized. Figure 6 gives the crucial transitions for Li@T-shaped compounds. For Li@C60-pyridine (2a) and Li@C60-pyrazine (2b), the occupied molecular orbitals (HUMO) are mainly contributed by the C60 cage; the unoccupied molecular orbitals (LUMO+3) are mainly contributed by nitrogen-doped benzene. For Li@C60-1, 3, 5-triazine (2c), the crucial transition is from HOMO to LUMO+4. The HUMO is distributed on C60 cage, and the LUMO+4 is also distributed on C60 cage. For Li@C60-pyridazine (2d), the crucial transition is from HOMO to LUMO+4. The HOMO is distributed on C60 cage and nitrogen-doped benzene, and the LUMO+4 is distributed on C60, which is different from than that of compounds (2a, 2b, and 2c). The largest first hyperpolarizability of Li@C60-pyridazine is related to crucial transition.
Conclusions
In this work, we studied the electronic structures, absorption spectrums, and nonlinear optical properties of Li@sandwich configuration and Li@T-shaped configuration compounds by means of density functional theory methods. Computational results show that substitutes and heteroatoms effect on electronic structures of the compounds are obvious. Different sandwich configuration π-π systems (C60-toluene, C60-fluorobenzene, C60-phenol, C60-benzonitrile) interacted with Li atom exhibit large first hyperpolarizability. It is proved that the Li@C60-benzonitrile compound exhibits the largest first hyperpolarizability in Li@sandwich configuration compounds; after doping Li atom into T-shaped π-π systems (C60-pyridine, C60-pyrazine, C60-1,3,5-triazine, C60-pyridazine), it is found that the first hyperpolarizability of Li@C60-pyridazine is the largest up to 67,945 au among these structures. More important, the ultraviolet-visible-infrared absorption spectrum of all compounds has been analyzed. The calculation results indicate Li@C60-benzonitrile and Li@C60-pyridazine not only presented the large first hyperpolarizabilities but also a wide transparent region in deep ultraviolet spectrum range. We hope that this work could provide valuable knowledge for scientists to design nonlinear optical material by introducing Li atom into π-π systems.
References
Sherrill CD (2013) Acc Chem Res 46:1020–1028
Mignon P, Loverix S, Geerlings P (2005) Chem Phys Lett 401:40–46
Ercolani G, Mencarelli P (2003) J Organomet Chem 68:6470–6473
Mishra BK, Sathyamurthy N (2005) J Phys Chem A 109:6–8
Grimme S (2008) Angew Chem Int Ed 47:3430–3434
Sinnokrot MO, Sherrill CD (2004) J Phys Chem A 108:10200–10207
Meyer EA, Castellano RK, Diederich F (2003) Angew Chem Int Ed 42:210–1250
Burley SK, Petsko GA (1985) Science 23:229
Mulliken RS (1952) J Am Chem Soc 74:811–824
Mcneil AJ, Muller P, Whitten JE, Swager TM (2006) J Am Chem Soc 128:12426–12427
Hunter CA, Meah MN, Sanders JKM (1990) J Am Chem Soc 112:5773–5780
Philp D, Stoddart JF (1996) Angew Chem, Int Ed Engl 35:1154–1196
Lerman LS (1961) J Mol Biol 3(1):18IN13–30IN14
Saenger W (1984) Principles of nucleic acid structure. Springer, New York
Hunter CA, Sanders JKM (1990) J Am Chem Soc 112:5525–5534
Arunan E, Gutowsky HS (1993) J Chem Phys 98:4294–4296
Sinnokrot MO, Valeev EF, Sherrill CDI (2002) J Am Chem Soc 124:10887–10893
Tsuzuki S, Honda K, Uchimaru T, Mikami M, Tanabe K (2002) J Am Chem Soc 124:104–112
Sinnokrot MO, Sherrill CD (2004) J Am Chem Soc 126:7690–7697
Hohenstein EG, Sherrill CD (2009) J Phys Chem A 113:878–886
Nakano M, Fujita H, Takahata M, Yamaguchi K (2002) J Am Chem Soc 124:9648–9655
Geskin VM, Lambert C, Brédas JL (2003) J Am Chem Soc 125:15651–15658
Ostroverkhova O, Moemer WE (2004) Chem Rev 104:3267–3314
Coe BJ (2006) Acc Chem Res 39:383–393
Xu HL, Li ZR, Wu D, Ma F, Li ZJ, Gu FL (2009) J Phys Chem C 113:4984–4986
Hu YY, Sun SL, Muhammad S, Xu HL, Su ZM (2010) J Phys Chem C 114:19792–19798
Xu HL, Li ZR, Wu D, Wang BQ, Li Y, Gu FL, Aoki Y (2007) J Am Chem Soc 129:2967–2970
Wu HQ, Zhong RL, Sun SL, Xu HL, Su ZM (2014) J Phys Chem C 118:6952–6958
Wang SJ, Li Y, Wang YF, Wu D, Li ZR (2013) Phys Chem Chem Phys 15:12903–12910
Xu HL, Zhang CC, Sun SL, Su ZM (2012) Organometallics 31:4409–4414
Shelton DP, Rice JE (1994) Chem Rev 94:3–29
Willets A, Rice JE, Burland DM, Shelton DP (1992) J Chem Phys 97:7590–7599
Kanis DR, Ratner MA, Marks TJ (1994) Chem Rev 94:195–242
Huang W, Sergeeva AP, Zhai HJ, Averkiev BB, Wang LS, Boldyrev AI (2010) Nat Chem 2:202–206
Jimenez-Halla JOC, Islas R, Heine T, Merino G (2010) Angew Chem Int Ed 49:5668–5671
Uchino T, Kurumoto N, Natsuko S (2006) Phys Rev B 73:233203
Fazio G, Ferrighi L, Valentin CD (2014) J Catal 318:203–210
Zaboli M, Raissi H (2010) Struct Chem 26:1059–1075
Champagne B, Botek E, Nakano M, Nitta T, Yamaguchi K (2005) J Chem Phys 122:114315
Zhang CC, Xu HL, Hu YY, Sun SL, Su ZM (2011) J Phys Chem A 115:2035–2040
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09. Gaussian Inc., Wallingford
Dennington R, Keith T, Millam JGV (2009) GaussView, version 5. Semichem, Shawnee Mission, KS
Lu T, Chen FW (2012) J Comput Chem 33:580–592
Chen W, Li ZR, Wu D, Li Y, Sun CC, Gu FL, Aoki Y (2006) J Am Chem Soc 128:1072–1073
Oudar JL, Chemla DS (1977) J Chem Phys 66:2664–2668
Oudar JL (1977) J Chem Phys 67:446–457
Datta A, Pati SK (2006) Chem Soc Rev 35:1305–1323
Acknowledgements
The authors gratefully acknowledge financial support from the Fujian University of Technology (GY-Z13109), the Education Department of Fujian Province (JB14075), and the Development Fund of Fujian University of Technology (GY-Z160127).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Song, YD., Wang, L. & Wu, LM. Constructing a novel nonlinear optical materials: substituents and heteroatoms in π-π systems effect on the first hyperpolarizability. Struct Chem 28, 1623–1630 (2017). https://doi.org/10.1007/s11224-017-0918-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-017-0918-y