Abstract
Penicillium sp. is among fungi that can infect cereals, maize, and other foodstuffs, causing not only important yield losses but also contamination of the crops by toxins production, which make them unsafety for consumption. In this context, the main objective of this study is the development of clay/essential oil formulations against this type of fungus. Double-layer hydroxides (LDHs) and Ghassoul were chosen as clays, and Thyme as an essential oil and its active principle, thymol. The LDHs and Ghassoul materials underwent purification and activation treatments to increase their compatibility with the chosen essential oil. The different materials obtained were characterized by XRD and FTIR. The chemical compositions of the EOs were analyzed by GC–MS chromatography. The main constituents of Thyme oil founded were thymol (67.13%), ρ-cymene (4.85%), Z-Caryophyllene (1.77%) and γ-terpinene (2.74%). The adsorption of thymol on the different materials developed: activated Ghassoul (Gh-A), Hydrotalcite (HT3), and LDH (ZnAl-CO3), showed that the adsorption isotherms can be satisfactorily described by the Langmuir model. Also, the modeling of the adsorption kinetics revealed its conformity to the pseudo-second order model for the different materials. As a result, the adsorption capacity of thymol increases in the following order: ZnAl-CO3, HT3, and Gh-A. The formulations made from the different clays allowed for the preparation of an effective and useful fungicide for wheat storage. The in vitro antifungal activities were tested against the strain Penicillium sp. The results obtained revealed that the formulations based on Gh-A and LDHs retained their antifungal activity even after 5 months of storage. Therefore, in order to evaluate the potential of the formulations on cereals storage (wheat storage case), in vitro antifungal tests with the Gh-A/Thyme formulation were performed on four types of wheat (sterile, non-sterile, dried, and washed then dried wheat). These experiments demonstrated that sterile and washed-dried wheat had a significant inhibition against Penicillium sp. growth even after six months of storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fungal contamination of food was considered one of the major concerns in food storage and preservation [1] and poses a serious threat to human and livestock health. Aspergillus, Penicillium, and Fusarium are the major genera of fungi infecting wheat, rye, and corn grains during various field-related manipulations and storage [2]. Fungal growth on raw and processed foods can lead to several types of spoilage, including textural and sensory changes, development of off-flavors and emission of odors, decay, and formation of pathogenic and allergenic propagules [3].
The conditions and practices developed at the ‘storage’ stage contribute to the development of favorable conditions for the growth of filamentous fungi, mainly (Penicillium and Aspergillus) and other microorganisms on cereals. Fungi may originate from the soil and be transmitted to the grain by air or water, or they may be transported from the field at harvest time or during grain handling operations [4, 5]. Once the development of the fungi takes place, they can cause important losses and an alteration of the food and sanitary quality of cereals. More seriously, they can cause intoxication by the production of mycotoxins [6]. Worldwide, more than 5 to 10% of the economic losses of cereals are generated by the development of filamentous fungi and the release of their secondary metabolites ‘mycotoxins’ [7]. According to Pitt et al., about 25–40% of cereals are contaminated by mycotoxins [8]. The countries concerned are African countries, South Asia and South America, which are known for their hot and humid climatic conditions.
Due to pressure from consumers and government agencies to either eliminate these toxic chemicals or use natural alternatives for the preservation and extension of the product’s shelf life, the food sector tends to employ fewer chemical preservatives with antifungal activity [9]. Essential oils (EOs) or their active compounds represent one of these natural additives and have powerful biological activities. These EOs are used as protective agents against phytopathogenic fungi [10] and microorganisms that invade foodstuffs [11]. They have no negative effects on the environment and the consumer [12, 13]. Unlike chemicals such as pesticides, insecticides, and fertilizers.
For the protection of foods that have been preserved, EOs are currently an alternative control method [14, 15]. Due to the short period of their actions, their volatility creates a serious challenge for their use [15]. Some techniques can reduce the impact of these concerns that determine the industrial application of EOs. Two techniques are commonly used: Encapsulation and adsorption on support like clays or what was called formulation [14,15,16]. The formulations allow to immobilize the volatile compounds of the EO, protect against the light and the temperature of the EO constituents, and also modulate their releases in time. Furthermore, formulations can decrease introduced concentrations without compromising the efficacy of the finished product, which will assist the company cut costs. Due to the actions of the clay and the EO when used together, the clay/EO mixture delivers a double benefit. Applications as an insecticide against corn weevil have been made [14, 16, 17]. In order to investigate the in vitro and in situ antifungal activity against the rice-affecting Aspergillus niger, Aspergillus flavus, Aspergillus parasiticus, and Penicillium chrysogenum, Hossian et al. developed formulations based on EO and "chitosan/cellulose" nanocomposite [18].
In the context of preserving the quality of cereals, which are one of the vital pillars of the Moroccan diet, the use of formulations appears to be an advantageous approach for the conservation of cereals. Indeed, this sector requires more effort for the implementation and improvement in storage techniques which remains, in most cases, traditional.
The objective of this work was initially the regulation of the volatilization of terpene compounds of the EO to increase their activity duration. To do this, formulations based on Thyme EO and its active ingredient ‘thymol’ with a natural clay Ghassoul activated by acid and a synthetic clay double lamellar Hydroxides ‘LDHs’ have been developed. It was also a question of evaluating the adsorption capacity of the constituents of Thyme EO by these various clays. The chosen materials were Ghassoul, because of its availability and its low cost, and LDHs in particular Hydrotalcite (MgAl-CO3) for their easy synthesis and their availability in a wide variety of compositions. Secondly, the study of the antifungal activity of the formulations prepared in vitro was carried out. Finally, tests were conducted to explore the potential of the prepared formulations by evaluating their application in wheat preservation during storage.
Material and methods
Reagents and products
NaOH 98%, Na2CO3 99.9%, metal salts (MgCl2, 6H2O), and (AlCl3, 6H2O) 99%, NaNO3, MgSO4; 7H2O, KCl, FeSO4; 7H2O, K2HPO4, sucrose, agar, hexane (C6H14) > 85% and thymol > 99% used in this research were provided by LOBA Chemie and Sigma-Aldrich. Ghassoul is a natural clay. It is identical to the natural product without any processing and is dried at 100 °C to remove any water that is only weakly linked to the material. After drying, the product is crushed and filtered using standard sieves in accordance with AFNOR. Only the particles with a diameter inferior to 63 µm were retained. The essential oil on which our study focused is the EO of Thyme of commercial origin and its active ingredient thymol. It is in principle extracted by the method of training with water vapor.
The thymol used in this work came from the commercial manufacturing company chemicals at 100% purity. The physicochemical properties of thymol are reported in Table 1. The stock solution was prepared in distilled water.
Microorganism tested
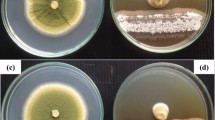
The performance and biological activity of the prepared formulations were tested against the growth inhibition of Penicillium sp. This species was chosen due to its frequent involvement in food product contamination and its pathological implication in both humans and animals. The fungus isolated from cereals was provided by the laboratory of Biotechnology and Bioresources Valorization at the Faculty of Sciences of Meknes, Moulay Ismail University (Morocco). The microorganism was maintained at 4 °C on Czapek Agar medium (Fig. 1) [19].
Characterization techniques
Thyme EO was detected using the GC–MS technique, and the materials were analyzed using XRD and FTIR. X-ray diffraction (XRD) patterns were obtained using a BRUKER-AXS type D8 diffractometer. X-rays were produced from the Kα radiation of the Cu source, having a wavelength equal to 1.54183 Å, by applying an accelerating voltage of 40 kV and a current of 40 mA on the cathode. The Fourier-Transform Infrared (FTIR) analysis was performed on a Shimadzu IR Affinity-1S instrument equipped with a TriGlycine Sulfate (TGS) detector, having an absorbance between 400 cm−1 and 4000 cm−1 and a resolution of 2 cm−1. The EO was analyzed by gas chromatography coupled to mass spectrometry GC–MS THERMO, equipped with a DB1 fused silica capillary column [60 m × 0.25 mm ID, 0.25 µm film thickness coated with phenyl (5%) and dimethylpolysiloxane (95%) as stationary phase (Agilent HP-5MS)] and a split injector at 250 °C.
Methods
Synthesis of MgAl-CO3 and ZnAl-CO3 by co-precipitation (Mg/Al = 3 and Zn/Al = 3)
The different LDHs were prepared by the co-precipitation method with Mg/Al and Zn/Al molar ratios equal to 3. A first aqueous solution (A) of magnesium (or zinc) and aluminum chloride salts was obtained by dissolving 60.99 g of MgCl2, 6H2O (or 40.88 g of ZnCl2, 6H2O) and 13.34 g of AlCl3 in 300 ml of distilled water. A second solution (B) was prepared by dissolving 2.12 g of sodium carbonate (Na2CO3) and 32 g of sodium hydroxide (NaOH) in 300 ml of distilled water. These two solutions were added dropwise, under magnetic stirring, maintaining the pH at a basic value (pH = 10). The temperature was kept between 60 °C and 65 °C during the whole addition. The mixture was then refluxed at 65 °C for 17 h to allow crystal growth. The precipitate was filtered and the solid obtained was washed several times with warm distilled water until complete elimination of excess ions on the solid and then dried in the oven for one night. The materials obtained were named MgAl-CO3 (or HT3) and ZnAl-CO3.
Preparation of the Ghassoul-activated (Gh-A)
The acid activation of Ghassoul was done by the following method, a mass m of Ghassoul of size less than 63 µm was introduced into a flask with a volume of sulfuric acid solution (2 M) [20]. The mixture was homogenized at a temperature of 90 °C for 4 h. At the end of this period, the mixture was filtered, the solid obtained was washed with distilled water several times. The activated clay obtained was then dried in an oven for one night. The Ghassoul obtained was crushed, and named Gh-A.
Thymol adsorption experiments
The adsorption experiments were performed in closed bottles. For this purpose, a mass of 100 mg of each clay sample is put in contact with 20 ml of a thymol solution of concentration C = 80 mg/L, then stirred at room temperature for times t (15 min, 30 min, 1 h, 1 h30 min, 2 h and 3 h) at pH = 9. After the adsorption time t, the solution was filtered and the filtrate was analyzed by UV–Visible spectrophotometry in the wavelength range 200–400 nm and the residual concentration was determined by measuring the absorbance at the wavelength of 273 nm. The amount of adsorbed thymol (mg g−1) was calculated using the equation:
where: C0 and Ce were the initial and equilibrium concentrations of adsorbate (mg L−1); m is the mass of the adsorbent (g); V is the volume of the adsorbate (mL), and qe is the amount adsorbed per gram of adsorbent (mg g−1).
Adsorption isotherm experiments were performed under the same conditions as the adsorption kinetics using increasing concentrations of thymol (0 to 300 mg L−1) for the three solids at room temperature. After three hours of shaking to attain equilibrium, the contents of the flasks were centrifuged. The equation provided the residual concentrations and adsorbed quantities (Eq. 1).
Kinetic and isothermal modeling
In order to determine the adsorption mechanism of thymol adsorption on three solids (Gh-A, HT3, and ZnAl-CO3), the experimental data were adjusted using linear models. Indeed, the modeling of the adsorption kinetics was carried out by the pseudo-first order (Eq. 2) [21] and pseudo-second order models (Eq. 3) [22]:
While those of the isotherms were carried by the nonlinear models of Langmuir (Eq. 4) and Freundlich (Eq. 5) in order to identify the parameters involved in these isotherms [23, 24]:
Langmuir:
Freundlich:
where KL and KF were the Langmuir and Freundlich constants, respectively, and n relates to adsorption intensity, qe (mg g−1) is the adsorption capacity at equilibrium, qm (mg g−1) is the maximum amount which needed to form a monolayer coverage on the adsorbent surface (mg g−1), and Ce is the equilibrium concentration (mol L−1). k1 (min−1) and k2 (g mg−1 min−1) were rate constant for a kinetic of the pseudo-first order and pseudo-second order, respectively; qt (mg g−1) adsorption capacity at time t; qe (mg g−1) adsorption capacity at equilibrium; t is the contact time (min).
Fungicidal formulation preparation and antifungal activities
The objective was to prepare a powder formulation based on Thyme EO and its active ingredient thymol, and acid-activated Ghassoul (Gh-A), or based on a synthesized clay which was LDH with different compositions. An adequate quantity of Thyme EO, diluted in 10 mL of hexane, was added to a mass of clay powder to create these formulations, maintaining the following ratio [25, 26] (Eq. 6):
With: mEO: mass of essential oil and mclay: mass of clay.
The mixture was placed in a water bath at 30 °C after mixing for approximately 3 h to allow the solvent to completely evaporate. At the end, a powder flavored with Thyme EO was obtained; by the same process, the formulation based on thymol was prepared. The formulation was left in the open air for one month, three months, and finally five months, and antifungal tests were carried out each time to evaluate the stability of the formulation;
Study of the stability of formulations
Preparation of the Czapek culture medium
Czapek Agar medium which was suitable and commonly used for fungi cultivation was prepared as following: For one liter of distilled water, 2 g of NaNO3; 0.5 g of MgSO4, 7H2O; 0.5 g of KCl; 0.01 g of FeSO4, 7H2O; 1 g of K2HPO4; 30 g of sucrose; 15 g of agar were mixed. The pH was adjusted to 7.3. The medium was boiled until completely dissolved and then autoclaved at 121 °C for 20 min. Finally, it was mixed with the prepared formulations and distributed to sterile Petri dishes until it solidifies.
Study of the formulation’s remanence
The aim was to evaluate the performance of the EO antifungal activity in the formulation by recording the percentage of inhibitory power against fungal growth. For one month, three months, and eventually five months, the created formulations were kept in open bottles at room temperature. Antifungal tests were done for each period to verify the stability of the formulation. 0.06 g of each formulation was added to 20 mL of Czapek Agar growth medium before the mixture was placed into Petri dishes with a 90 mm diameter. Each of the Petri dishes was inoculated with Penicillium sp. The fungus was prepared as a suspension of spores in distilled water. A volume of this suspension was placed in the center of the Petri dish, which was then sealed with the para film. Finally, incubation was performed at 25 °C for 15 days. Each experiment was performed three times to reduce experimental error. Colony diameter was tracked over time in order to evaluate each formulation's ability by limiting fungal growth, which were determined using the formula below [27]:
Evaluation of the formulation performance in wheat storage
Protocol 1:
To evaluate the antifungal activity of the powders after adsorption of essential oils, formulations were prepared from Ghassoul activated by sulfuric acid (Gh-A). It was selected based on its best capacity for adsorption of terpene compounds. For this purpose, the clay/EO formulation used were Gh-A-Thyme in powder form, this formulation was added to the different wheat samples with the percentages of 5%, 1%, and 0.1%. The tests were done on two types of wheat: sterile wheat and non-sterile wheat to study the growth of Penicillium sp. on this substrate. The storage was done in glass bottles at room temperature (Fig. 2). After 7 days, 1 month, 2 months, 3 months, 4 months, etc.; wheat grains were taken from each flask and put in a Petri dish in the presence of a Czapek Agar medium. Then, after 7–10 days of incubation at 25 °C, the growth of the fungus was examined.
Protocol 2:
Wheat storage conditions were tested by the following methods: Non-sterile wheat was taken and dried in the oven at different temperatures (80 °C, 60 °C, and 40 °C) at different time intervals (1 h or 30 min), then a mass of the prepared formulation (Gh-A-Thyme) with the mass ratio 5% was added to each sample. Then, each sample is put in small glass bottles, and they were kept for 7 days, 1 month, 2 months, 3 months, 4 months, 5 months, and 6 months at room temperature. After each period, antifungal tests were performed in Petri dishes. The last test was devoted to non-sterile wheat wash then dried and put in a bottle where a mass of the prepared formulation (Gh-A-Thyme) was also added, by always keeping the mass ratio of 5%. The antifungal test was carried out in the same way mentioned before. All experiments were performed 3 times to evaluate the reproducibility.
Results and discussion
Characterization of solids
XRD analysis
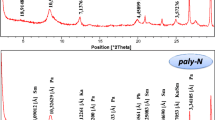
Figure 3 shows the XRD patterns of HT3 and ZnAl-CO3 materials. The general appearance of the patterns is typical of LDHs type compounds and characteristic of a hexagonal network with rhombohedral symmetry (space group: R-3 m) [28,29,30]. In fact, this characteristic might be seen in the presence of the family lines (00 l), which were intense and symmetrical to the low values of two theta encountered in the LDHs compounds. According to Bragg's law, the first peak, which was positioned at 2θ≈ 11°, has the index (003) and measures d003≈7.8 Å. This basic reflection corresponds to the interleaf space and was used to calculate the mesh parameter ‘c’ (c = 3d003). The intense reflection at enivrons of 2θ≈60° has been indexed as (110) and corresponds to d110≈1.54 Å. The stacking mechanism of the layers making up the lamellar hydroxide had no effect on this reflection. In fact, the inter-lattice distance of the (110) line is half the metal-to-metal distance in the sheet (a = 2d110). The mesh parameters of the LDHs materials are summarized in Table 2. The literature and these findings were in agreement [31, 32]. We note a larger value of the mesh parameter ‘c’ for Hydrotalcite HT3 compared to ZnAl-CO3. This variation would reflect more electrostatic attraction between the anionic layer and the zinc-based matrix. The mesh parameter ‘a’ remains almost invariant.
Figure 4 shows the XRD patterns of Ghassoul and Ghassoul activated by sulfuric acid (Gh-A). The attack of the material by sulfuric acid clearly causes a decrease in the intensity of some lines. This occurs in particular for the stevensite phase, which causes a reduction in its content. The increase in the intensity of the quartz line around 2θ = 20.69° and the appearance of new fine and intense lines means that the acid attack affects the stevensite structure of Ghassoul and leads to the formation of new unidentified phases as shown in XRD patterns. In previous literature [33, 34], similar results have been discussed. The structural cations were then released as a result of the disintegration of the tetrahedral and octahedral sheets, which left some gaps in the octahedral structure [33].
FTIR analysis
The FTIR spectra of HT3 and ZnAl-CO3 are plotted in Fig. 5. The appearance of the spectra is similar to that of the double lamellar hydroxides containing intercalated carbonate anions. These spectra demonstrate a large band at 3400 cm−1, which was related to the elongation vibration of the lamellar hydroxide groups attached to the various metals (M–OH, M = Al, Mg, or Zn) [29]. A vibrational band observed in the proximity of 1630 cm−1 can also be attributed to the deformation of intercalated water molecules [30]. A vibrational band observed in the proximity of 1360 cm−1 which corresponds to the antisymmetric elongation of interlamellar carbonates ν(CO3−2) [30]. The series of bands appearing between 400 cm−1 and 900 cm−1 corresponds to the elongation frequency of ν(M–OH) bonds and to the ν(O–M–O) valence vibrations forming the layers of LDHs [29, 35].
The infrared spectra of the Gh-A under investigation are shown in Fig. 6. Typically, the absorption bands in the 3600–3500 cm−1 range correspond to the vibrations of the structural hydroxyl groups that were unique to Ghassoul M–OH (M = Al, Fe, Mg). Depending on the type of the molecule’s bonds, these bands exact positions and intensities vary. The vibrations of elongation and deformation of the OH group of the adsorbed water were represented by the bands that appear approximately toward 3415 cm−1 and 1620 cm−1, respectively. While the distinctive carbonate band is found at a spectrum of 1387 cm−1. The Si–O bond of quartz exhibits the characteristic deformation vibration band at 487 cm−1 [36]. Additionally, the Si–O–Si group of quartz elongation vibration bands at 1090 cm−1 were visible in the infrared spectra of Gh-A. Bentahar et al. obtained similar results [37]. The results of the FTIR analysis and those of the X-ray diffraction analyses were in good agreement.
GC–MS analysis
The chemical composition of tested Thyme EO is depicted in Table 3. A total of 11 compounds were identified by CG-MS mainly thymol (67.13%), ρ- cymene (4.85%), γ-terpinene (2.74%), Z-caryophyllene (1.77%), and caryophyllene oxide (1.26%), which were the major components of the EO. The presence of other compounds with antifungal activities (e.g., carvacrol) was also detected but in lower proportions (0.12%). According to Baranauskiené et al. [38], phenolic compounds were the major constituents and the aroma principles of Thyme EO. However, in this study monoterpenic alcohols such as thymol and ρ-cymene were found in great abundance in the tested essential oil. Barros et al. discovered similar results for the essential oil of Thymus vulgaris, which is primarily composed of thymol [39].
Study of the thymol adsorption
Adsorption kinetics
Figure 7 shows the adsorbed amounts of thymol on the different materials at room temperature. The adsorption pH was maintained at pH = 9. From these results, a rapid increase in the amount of thymol adsorbed at t < 30 min can be seen for all solids considered. Two phases can be highlighted by the design of these graphs, which depict the adsorption kinetics of thymol: firstly; fast one taking place during the first 30 min where the adsorption rate is the most important for the three solids. The curves of Gh-A, HT3, and ZnAl-CO3 (Fig. 7) show that the quantity of thymol adsorbed increases with time. The rapid rise, observed initially, is due to the availability of adsorption sites. It can be noticed that after 1 h of adsorption, the adsorbed amount does not vary with contact time for all three solids. The equilibrium adsorbed amounts of thymol were 15.93 mg g−1, 12.2 mg g−1, and 5.2 mg g−1 for Gh-A, HT3, and ZnAl-CO3, respectively. The treatment of the adsorption kinetics results shows that the amount of thymol adsorbed is higher in the case of Gh-A compared to HT3 and ZnAl-CO3. Therefore, it can be concluded that adsorption is preferentially carried out via hydrogen bonds. The graphical representations of ln(qe − qt) and of \(\frac{t}{{q_{t} }}\) versus t are given in Figs. 8 and 9. The values of the constants of the two kinetic models are grouped in Table 4. From these results (Fig. 8 and Table 4), we can see that for the pseudo-first order model, the experimental points were not perfectly linear and that the experimentally determined adsorbed amounts of thymol, qexp, are very far from the calculated ones, qe, and the R2 coefficients deviate a lot from 1. So, we deduce that this model cannot be applied to the adsorption process of thymol on the different materials. From the results of the modeling (Fig. 9) we notice that the values of the adsorbed quantity found in this model (qe) are very close to those obtained experimentally (qexp) with correlation coefficients R2 close to 1 for the different adsorbents. Therefore, it can be concluded that the pseudo-second order model accurately depicts the process of thymol adsorption on the various materials. Thymol adsorption using clay-based adsorbents has shown similar results [40,41,42].
Adsorption isotherms
The results of the adsorption isotherm study of thymol on HT3, ZnAl-CO3 and Gh-A were represented by the adsorbed amount as a function of residual concentration. Figure 10 depicts the experimental isotherms that were obtained. The curves from these models were superimposed on the experimental points (Fig. 10), it follows that the models considered can be used to describe the adsorption isotherms of thymol on the three solids at room temperature. The parameters calculated from these models are collected in Table 5. From the results in Table 5, it can be seen that the R2 correlation coefficients of both Langmuir and Freundlich models correlate well with the experimental data for the different solids (R2 ≥ 0.90). On the other hand, it can be seen that the Langmuir model is the best fit to describe the adsorption isotherms of thymol on the different solids in the range of initial concentrations studied from the analysis of the results presented in Table 5. The regression coefficients obtained are all very close to 1. These results indicate that the thymol molecules adsorb onto energetically homogeneous sites covering a monolayer on the surface of the different solids. We could conclude that the Langmuir model is the best one for accurately expressing the experimental results on the three materials ability to adsorb thymol. This finding is in-line with other studies that investigate the adsorption of thymol on pure bentonite [43].
Antifungal activity of formulations prepared on the basis of clays
The results of the antifungal activity of several LDHs and activated Ghassoul-based formulations are described in Table 6 and those of the antifungal tests are represented in Fig. 11. The results showed that after one month of conservation, the formulations displayed a very significant activity against Penicillium sp. They completely inhibit the growth of this fungus, except ZnAl-Thymol, where the microorganism started to grow but with a low percentage of 20%. After 3 months, the strain still showed high sensitivity to HT3-Thyme and Gh-A-Thyme with 100% inhibition. This capacity was decreased for the other formulations especially for ZnAl-Thymol and HT3-Thymol, with an inhibition power of 66% and 43%, respectively. However, the formulations ZnAl-Thyme and Gh-A-Thyme have a slightly higher antifungal activity than the other formulations with an inhibition percentage of about 94%. Also, after 5 months, the formulations ZnAl-Thyme, HT3-Thyme, and Gh-A-Thyme exerted significant inhibitory activity against Penicillium sp. with very significant percentages greater than or equal to 83% (Table 6). However, the fungus showed a resistance to the three formulations ZnAl-Thymol, HT3-Thymol, and Gh-A-Thymol with a percentage of inhibition of 59.52%, 40%, and 76%, respectively. These results can be explained by the synergistic effects of the constituents of the essential oil used (Thyme), which is not the case for formulations based on thymol (the active ingredient of Thyme) [44, 45]. Therefore, it appeared from the results of the antifungal potential evaluation of the formulations, that Thyme EO has the best effect on the Penicillium sp. strain (Fig. 11). This conclusion was confirmed by the study conducted by Hossain et al., who reported that oregano and Thyme EOs with high antifungal activity, alone and in combination, showed increased efficacy against the growth of the following fungi: Aspergillus flavus, Aspergillus parasiticus and Penicillium chrysogenum [46]. Similar results were cited by Abbaszadeh et al., who studied the antifungal activity of thymol, Carvacrol, Eugenol, and Menthol in controlling the growth of food spoilage fungi [47]. In our previous study, tested in vitro antifungal activity of formulations based on oregano and Thyme essential oils with a natural clay (Ghassoul), we reported that the clay/EO formulations show an inhibitory effect against the fungus Penicillium sp. [26]. Similarly, Hossain et al. conducted studies in similar situation which demonstrated that Thyme-Oregano, Thyme-tea tree, and Thyme-peppermint EO blends have considerable antifungal efficacy against Aspergillus niger, Aspergillus flavus, Aspergillus parasiticus, and Penicillium chrysogenum, lowering their growth by 51–77% [18]. Therefore, using plant-derived ingredients with clays to prevent fungal growth during grain storage could be seen of as a good alternative to synthetic fungicides. According to these results, it can be seen that the Gh-A formulation preserves better the constituents of the studied essential oils, hence this Gh-A-Thyme formulation is more stable than other formulations based on LDHs materials. Accordingly, it can be concluded that oxygenated terpenic compounds would be subject to a variety of specific and non-specific interactions, including hydrogen bonds on the one hand, electrostatic and Van Der Waals on the other. Acid clays, as well as hand, have a much less positive surface charge than basic clays. While the hydrocarbon compound would be limited simply to interactions of Van Der Waals type. This result can be also associated to the study of thymol adsorption, which showed that Gh-A present the high quantity of adsorbed thymol.
Formulation performance in wheat storage
Effect of formulation mass on wheat storage
The antifungal activity of the Gh-A-Thyme formulation is presented in Table 7. The product was chosen for its good inhibition performance, on the Penicillium sp. strain by varying the % of the formulation in two types of wheat, sterile wheat and non-sterile. The experiment was conducted in glass vials, during a period of 7 days, one month and 7 months of storage. The objective was to define the lowest concentration giving the best result, and their application in the preservation of wheat during its storage.
The antifungal activity of Thyme essential oil against Penicillium sp. was evaluated with the powder formulation after 7 days, 1 month and 7 months of storage using 0.1%, 1% and 5% of Gh-A formulation to the total sample mass each time. In the case of sterile wheat inoculated with Penicillium sp. strain, a total inhibition for the three masses (5%, 1%, and 0.1%) of Gh-A-Thyme was detected, while for the tests with only the EO, no inhibition of Penicillium sp. was noted. Under the same conditions, but in the absence of Gh-A-Thyme formulation (Wheat + Penicillium sp.), it was observed that there is no inhibition. Concerning the tests of non-sterile wheat with the different mass ratios of the formulation used (5%, 1%, and 0.1% of Gh-A-Thyme), it was noticed that there is no inhibition of Penicillium sp. and the same remark for the test with control wheat and wheat with 1% of Thyme EO, where they showed that the use of Gh-A-Thyme formulations is beneficial in the conservation of wheat (Fig. 12). From these results, it is evident that the formulations prepared with clays and essential oils inhibit well the growth of the fungus Penicillium sp. up to 7 months of storage. The finding gives a positive sign that these formulations can be used in cereal storage against the fungi growth. These formulations could be an alternative fungicide for cereal storage protection, because they have no harmful effects on the environment compared to synthetic fungicides. Since this study was devoted to tests on the Penicillium sp. strain and gave good results, it is possible to extend the use of this type of formulation to other existing fungi in cereals, like Aspergillus, and Fusarium.
Effect of drying and washing treatment on wheat storage in the presence of the formulation
The results of the antifungal tests for non-sterile wheat with the effect of two treatments during storage is shown in Table 8. It can be seen that, for drying treatment, there was strong growth of Penicillium sp. for all samples at different temperatures studied (40 °C, 60 °C, and 80 °C) from 7 days of incubation. While for the wheat washed with warm water and dried, total inhibition was observed until the fifth month. After this period, a weak growth of the fungus appeared. These results show that washing wheat with warm water followed by drying can be an effective treatment to decrease the existence load of fungi in wheat, while drying alone does not contribute to any growth inhibition of the tested strain (Figs. 13 and 14). Pre-cleaning before storing the cereals, and the addition of formulations, allow good preservation of the seeds for long periods up to 6 months. In general, it can be concluded that the formulations based on clays and essential oils constitute a beneficial technique for the storage of cereals for a long time because of the remanence of essential oils on clays. On the one hand, the technique of preparation is economic by the small quantity used of the essential oil or of one of these its components, and on the other hand by their aptitude to stabilize the volatility of the terpene compounds of essential oils. Similar studies were done by, Ahmed Reda Chami, who used clay/EO formulations as feed additives for the purpose of promoting growth and lowering the risk of infection and mycotoxin poisoning in poultry and ruminants [48]. Also, another study was done by Adnan Sabehat who showed that microporous active solid materials based on essential oils (material/EO) offer several applications, including antimicrobial properties and shelf life extension of packaged perishable products [49].
Conclusions
The elaboration of clay/EO formulations was the target in this work, considering the advantages of this type of compounds in the food supply of certain animal species and the protection of wheat grains or other foods against fungi. All the materials obtained were characterized by XRD and FTIR. The results showed that the synthesized LDHs present a good crystallinity and good purity, and the activation of the Ghassoul by the acid allowed the improvement in the structural properties of this material. Analyses performed by GC–MS chromatography confirmed that thymol is the majority terpene compound in Thyme essential oil (67.13%) followed by ρ-cymene (4.85%). The adsorption of thymol by the different materials (LDHs and activated Ghassoul) is consistent with pseudo-second order kinetics. The adsorption isotherms of thymol on the different materials were satisfactorily described by the Langmuir model. The adsorption capacity of thymol increases in the following order: ZnAl-CO3, HT3, and Gh-A. This adsorption capacity was attributed to the specific interaction between the OH hydroxide groups on the clay surface and terpene compounds. Therefore, the antifungal activities of the clay/Thyme and clay/Thymol formulations were evaluated against Penicillium sp. The antifungal tests were followed during three different periods (1 month, 3 months, and 5 months). The Gh-A/Thyme formulation was found to maintain its antifungal activity even after 5 months of conservation in the open air, compared to the other formulations which present a lower antifungal profile under the same conditions. Ultimately, since the stored seeds are subject to several factors that influence their keeping properties, the formulations were added to wheat subjected to different treatments before storage during the period used by professionals in this sector. The performance of the products was observed on washed-dried wheat where the growth of Penicillium sp. was prevented even after six months of storage. Accordingly, these formulations can be recommended as an effective means of preserving wheat quality during storage. Thus, other studies can be carried out to evaluate the effect of this kind of products on other cereal grains and to analyze whether they can present modification on the organoleptic characteristics of the preserved product.
Data availability
No data supporting the findings of this study are available in the supporting information of this article.
References
S. Zhaveh, A. Mohsenifar, M. Beiki, S.T. Khalili, A. Abdollahi, T. Rahmani-Cherati, M. Tabatabaei, Ind. Crops Prod. 69, 251 (2015)
J. Krisch, R. Tserennadmid, and C. Vágvölgyi, Sci. Microb. Pathog. Commun. Curr. Res. Technol. Adv. 1135 (2011).
P.D. Dellavalle, A. Cabrera, D. Alem, P. Larrañaga, F. Ferreira, M.D. Rizza, Chil. J. Agric. Res. 71, 231 (2011)
S. Marín, V. Sanchis, A.J. Ramos, I. Vinas, N. Magan, Mycol. Res. 102, 831 (1998)
A. Molinié, V. Faucet, M. Castegnaro, A. Pfohl-Leszkowicz, Food Chem. 92, 391 (2005)
A. El Khoury, Champignons mycotoxinogenes et ochratoxine A (OTA) et aflatoxine B1 (AFB1) dans les vignobles libanais: occurrence et origine/mycotoxigenic fungi and ochratoxin a (OTA) and Aflatoxin B1 (AFB1) in Lebanese Vineyards: Occurrence and origin. (2007).
Pfohl-leszkowicz A., Lavoisier, Tec Doc (Paris, 1999), pp. 17
J.I. Pitt, S. Ail, B.F. Hocking, M.C. Le, R.K. Kapti, S. Endang, S.A. Rahayu, J. Food Mycol. 1(1), 41 (1998)
M. Beyki, S. Zhaveh, S.T. Khalili, T. Rahmani-Cherati, A. Abollahi, M. Bayat, M. Tabatabaei, A. Mohsenifar, Ind. Crops Prod. 54, 310 (2014)
A. Zambonelli, A.Z. D’Aulerio, A. Severi, S. Benvenuti, L. Maggi, A. Bianchi, J. Essent. Oil Res. 16, 69 (2004)
T. Mangena, N. Muyima, Lett. Appl. Microbiol. 28, 291 (1999)
M.B. Isman, Annu. Rev. Entomol. 51, 45 (2006)
B. Conti, A. Canale, P.L. Cioni, G. Flamini, Bull. Insectol. 63, 197 (2010)
M.M.G. Nguemtchouin, M.B. Ngassoum, L.S.T. Ngamo, X. Gaudu, M. Cretin, Crop Prot. 29, 985 (2010)
M. Nguemtchouin Goletti, Formulation d’insecticides en poudre par adsorption des huiles essentielles de Xylopia Aethiopica et de Ocimum Gratissimum sur des argiles camerounaises modifiees (2012).
M.M.G. Nguemtchouin, M.B. Ngassoum, L.S.T. Ngamo, P.M. Mapongmetsem, J. Sieliechi, F. Malaisse, G.C. Lognay, E. Haubruge, T. Hance, Appl. Clay Sci. 44, 1 (2009)
K.S. Moussa, V. Charles, S.J. Pierre, A.J. Thor, A. Bélanger, J. Stored Prod. Res. 37, 339 (2001)
F. Hossain, P. Follett, S. Salmieri, K.D. Vu, C. Fraschini, M. Lacroix, Int. J. Food Microbiol. 295, 33 (2019)
H.N. Marek, B. Taidi, T. Smaoui, M. BenAziz, A. Mansouri, H. Hajjaj, Biotechnol. Agron. Soc. Environ. 23, 207 (2019)
H. Babaki, A. Salem, A. Jafarizad, Mater. Chem. Phys. 108, 263 (2008)
Y.S. Ho, Scientometrics 59, 171 (2004)
Y.S. Ho, G. McKay, Chem. Eng. J. 70, 115 (1998)
I. Langmuir, J. Am. Chem. Soc. 40, 1361 (1918)
H. Freundlich, J. Phys. Chem. 57, 385 (1906)
M.G.M. Nguemtchouin, M.B. Ngassoum, P. Chalier, R. Kamga, L.S.T. Ngamo, M. Cretin, J. Stored Prod. Res. 52, 57 (2013)
H. Ziyat, M.N. Bennani, S. Allaoui, J. Houssaini, H.N. M’Barek, S. Arif, H. Hajjaj, J. Chem. 2021, 1–8 (2021)
N. Ahmad, S. Sultana, G. Kumar, M. Zuhaib, S. Sabir, M.Z. Khan, J. Environ. Chem. Eng. 7, 102804 (2019)
F. Cavani, F. Trifiro, A. Vaccari, Catal. Today 11, 173 (1991)
H. Ziyat, S. Elmzioui, M. Naciri Bennani, J. Houssaini, S. Allaoui, S. Arhzaf, Res. Chem. Intermed. 47, 2605 (2021)
H. Ziyat, M. Naciri Bennani, H. Hajjaj, S. Mekdad, O. Qabaqous, Res. Chem. Intermed. 44, 4163 (2018)
Y. Lin, Q. Fang, B. Chen, J. Environ. Sci. China 26, 493 (2014)
M. Naciri Bennani, D. Tichit, F. Figueras, S. Abouarnadasse, J. Chim. Phys. Phys. Chim. Biol. 96, 498 (1999)
K. Al-Essa, J. Chem. 2018, 10 (2018)
J.P. Nguetnkam, R. Kamga, F. Villiéras, G.E. Ekodeck, A. Razafitianamaharavo, J. Yvon, J. Colloid Interface Sci. 289, 104 (2005)
H. Ziyat, M. Naciri Bennani, Y. Dehmani, J. Houssaini, S. Allaoui, R. Kacimi and H. Hajjaj, Int. J. Environ. Anal. Chem. 1–20 (2022) https://doi.org/10.1080/03067319.2022.2032006
N.I.A. Acevedo, M.C.G. Rocha, L.C. Bertolino, Ceramica 63, 253 (2017)
Y. Bentahar, C. Hurel, K. Draoui, S. Khairoun, N. Marmier, Appl. Clay Sci. 119, 385 (2016)
R. Baranauskiene, P.R. Venskutonis, P. Viškelis, E. Dambrauskiene, J. Agric. Food Chem. 51, 7751 (2003)
F.A.P. Barros, M. Radünz, M.A. Scariot, T.M. Camargo, C.F.P. Nunes, R.R. de Souza, I.K. Gilson, H.C.S. Hackbart, L.L. Radünz, J.V. Oliveira, M.A. Tramontin, A.L. Radünz, J.D. Magro, Crop Prot. 153, 105885 (2022)
M.G.M. Nguemtchouin, M. Benoît, R. Kamga, S. Deabate, S. Lagerge, E. Gastaldi, P. Chalier, M. Cretin, Appl. Clay Sci. 104, 110 (2015)
H. Ziyat, M.N. Bennani, H. Hajjaj, O. Qabaqous, S. Arhzaf, S. Mekdad, S. Allaoui, J. Chem. 2020, 1 (2020)
H. Ziyat, M.N. Bennani, H. Hajjaj, S. Mekdad, O. Qabaqous, Res. Chem. Intermed. 44, 4163 (2018)
M. el Miz, S. Salhi, A. El Bachiri, M. Fauconnier, A. Tahani, J. Environ. Solut. 2, 31 (2013)
E. Eagleshham, M. Lis-Balchin, S.G. Deans, Flavour. Fragr. J. 13, 98 (1998)
E. Farrag, A.E. Edris, Food 47, 117 (2003)
F. Hossain, P. Follett, K. Dang Vu, M. Harich, S. Salmieri, and M. Lacroix, Food Microbiol. 53, 24 (2016).
S. Abbaszadeh, A. Sharifzadeh, H. Shokri, A. R. Khosravi, and A. Abbaszadeh, J. Mycol. Med. 24, (2014).
A.R. Chami, Patent, Advanced Scientific Developments (2014)
Sabehat Adnan, Patent, WO-2008149232-A2 (2008)
Acknowledgements
This work was supported by MESRSI and CNRST–Rabat-Morocco, within the framework of the PPR2- project.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by H.Z. et al. The first draft of the manuscript was written by H.Z. and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ziyat, H., Naciri Bennani, M., Arif, S. et al. Fungicide formulation based on Thyme essential oil and clay for wheat protection. Res Chem Intermed 49, 2769–2792 (2023). https://doi.org/10.1007/s11164-023-05013-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-05013-7