Abstract
Photocatalysis is considered to be one of the possible routes to solve energy crises at a low cost. Graphitic-C3N4 combined with other semiconductors at the nanoscale has demonstrated a promising potential in photocatalysis application owing to the heterojunction configuration. In this study, Ag/g-C3N4/CuNb2O6-4 (mass ratio of CuNb2O6 to g-C3N4 = 1:4) composites were prepared by photo-deposition of Ag particles on the hydrothermally synthesized g-C3N4/CuNb2O6-4 composites. The microstructure, morphology, and light absorption property of the photocatalysts were characterized by X-ray diffractometry, scanning electron microscopy, transmission electron microscopy, X-ray photoelectron spectroscopy, and UV–Vis diffuse reflectance spectroscopy. The photocatalytic properties of photocatalysts and active species responsible for the degradation of RhB dye under visible light were investigated. After loading Ag particles on the g-C3N4/CuNb2O6-4 composites, the resulting Ag/g-C3N4/CuNb2O6-4 composites show a bandgap of ~ 2.31 eV and a significant increase in photocatalytic efficiency. In particular, the photocatalytic activity of 11% Ag/g-C3N4/CuNb2O6-4 composite (Ag mass fraction = 11%) is nearly seven times that of orthorhombic CuNb2O6 and 3.8 times that of g-C3N4/CNO-4 composite. The Z-scheme heterojunction has been successfully formed in the Ag/g-C3N4/CuNb2O6-4 composites and it enhances the photocatalytic performance significantly with the best degradation efficiency of 88% after 2 h.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, environmental and energy crises have become more severe than ever before [1, 2]. The widespread use of synthetic dyes has resulted in large amounts of wastewater [3,4,5] containing various synthetic dyes [6, 7], such as azo, anthraquinone, sulfur, indigo and triphenylmethyl. Amidst the rising cost of pollutant disposal [8], utilizing solar energy via photocatalysts is unambiguously an effective strategy to solve the crises [9]. The photocatalysis technique not only converts sunlight into a valuable source of energy [10] but also directly uses sustainable solar energy to solve the environmental crises.
Photocatalytic degradation is an effective advanced oxidation process (AOP) to dispose of a small amount of industrial wastewater. AOP is based on hydroxyl radical, an active oxidant that can rapidly and non-selectively oxidize many organic contaminants [11,12,13]. The photocatalytic reaction requires absorption of photons of sufficient energy and a favorable redox potential of the photocatalytic semiconductors [14, 15] because the common photocatalysts are activated only by ultraviolet light. Herein, the use of photocatalysts with better photocatalytic performance and higher efficiency under visible light may have broader prospects.
Niobate is a widely used ferroelectric, piezoelectric, and luminescent material owing to its unique crystal structure and physicochemical properties. In catalytic reactions, niobates show extraordinary catalytic activity and stability under acidic conditions [16]. Niobate photocatalysts were usually prepared using solution combustion synthesis, liquid-phase synthesis, and solid-state reaction [17,18,19]. A low-cost and environmentally friendly photocatalyst, CuNb2O6 shows a favorable band gap of 1.90 eV [20] and can also provide a superior light absorption region after combining with other semiconductors [21]. However, due to the fast recombination of photogenerated carriers in CuNb2O6, the photocatalytic performance of CuNb2O6 needs to be improved further.
g-C3N4 is another attractive photocatalyst owing to its favorable bandgap (2.70 eV) [22]. With a two-dimensional structure, g-C3N4 was reported to have an excellent photocatalytic performance [23,24,25]. The photocatalytic activity of g-C3N4 is still limited by the high recombination rate of photogenerated carriers and the low specific surface area of the catalyst [26]. Qi et al. found that by loading Ag on g-C3N4 nanosheets, the photocatalytic activity of the porous g-C3N4 nanosheets improved effectively [27]. In addition, the study [28] also showed that the g-C3N4/CuNb2O6 composites could effectively enhance the absorption of UV–visible light in photocatalysis, but due to their low carrier separation efficiency and weak REDOX capacity the photocatalytic activity becomes limited.
In this study, we report a heterojunction to improve the photocatalytic activity of the g-C3N4-based catalyst by recombining three phases: CuNb2O6, g-C3N4, and Ag. The orthorhombic CuNb2O6 phase and g-C3N4/CuNb2O6 composites were synthesized using the hydrothermal method. A series of Ag/g-C3N4/CuNb2O6 composites with different mass fractions of Ag was prepared by the photo-deposition of Ag on the surface of g-C3N4/CuNb2O6 composites. The microstructure and photocatalytic properties of the composites responsible for degradation of RhB dye were investigated. The effect of mass fractions of Ag on photocatalytic activity of the composites and the synergistic photocatalytic mechanism was discussed further. The addition of Ag was expected to effectively improve the carrier mobility, separation efficiency of photogenerated electron–hole pairs and promote the formation of an all-solid Z-scheme heterojunction. This further helped to achieve a significantly higher photocatalytic activity under the conditions of low catalyst concentration and lesser light power.
Preparation of g-C3N4-based photocatalysts
The g-C3N4, Nb2O5, RhB, and AgNO3 were purchased from Shanghai Aladdin Biochemical Technology Co., LTD. Citric acid, cupric acetate, and ammonia were purchased from China National Pharmaceutical Group Corporation. All chemical reagents were of analytical grade and used without further purification.
Synthesis of orthorhombic CuNb2O6
The orthorhombic CuNb2O6 phase was synthesized by hydrothermal method using a precursor. The specific steps involved in the synthesis are shown in Fig. 1. In order to prepare the precursor, Nb2O5 was dissolved in hydrofluoric acid at 130 °C for 6 h. Furthermore, all Nb5+ ions were composited into the sediment prepared by adding ammonia dropwise to the solution, and subsequently, Nb-containing powder was obtained by drying the sediment. In addition, the Nb-containing powder, citric acid, and cupric acetate with a molar ratio n(Nb5+): n(citric acid): n(Cu2+) of 1:3:1 were added to deionized water, and the suspension was continuously stirred until all the mixture dissolved. Finally, the solution mixture was heated in a water bath at 80 °C until a blue colloidal solution was obtained, which was the precursor. After calcining the precursor at 900 °C for 12 h, the orthorhombic CuNb2O6 (labeled as o-CNO) powder was obtained.
Preparation of Ag/g-C3N4/CNO composites
The g-C3N4/CNO-4 composite (mass ratio of CNO: g-C3N4 is 1:4) was hydrothermally synthesized at 130 °C for 12 h. Prior to the synthesis, the salts with a required mass ratio were mixed and stirred in the beaker for 30 min to produce a suspension. This suspension was ultrasonically dispersed for 10 min to ensure the good dispersion of salts. The Ag/g-C3N4/CNO-4 composites were prepared by the photo-deposition method. The g-C3N4/CNO-4 composite with a fixed mass of 100 mg was dispersed in deionized water and the solution mixture was continuously stirred using a magnetic stirrer. Then the desired volume of AgNO3 solution (1 mmol/L), corresponding to AgNO3 mass of 1.58, 7.87, 17.32, 26.77, and 47.24 mg, respectively, was added to the suspension in dark and stirred for 1 h. Subsequently, the suspension was irradiated with a Xe lamp for 30 min with continuous stirring. The final sample was obtained by removing the water via filtration followed by drying at 60 °C for 12 h. The complete decomposition of AgNO3 was confirmed by the detection of Ag cation content. The obtained composites were labeled as 1% Ag/g-C3N4/CNO-4, 5% Ag/g-C3N4/CNO-4, 11% Ag/g-C3N4/CNO-4, 17% Ag/g-C3N4/CNO-4, and 30% Ag/g-C3N4/CNO-4 in terms of Ag content.
Characterization
The crystal structure of the composites was characterized by an X-ray diffractometer (XRD, Thermo X’TRA). The X-ray diffraction (XRD) patterns were recorded with a Cu Kα radiation operating at a tube voltage of 40 kV, tube current of 40 mA, scanning speed of 2°/min, and 2θ range of 10°–80°. The surface morphology and elemental distribution of the synthesized CNO and Ag/g-C3N4/CNO composites were observed by scanning electron microscope (SEM, Hitachi-S4700) and built-in energy-dispersive spectrometer (EDS). The fine microstructure of the composites was observed by a high-resolution transmission electron microscope (HRTEM, Tecnai G2 F30 S-Twin) with an accelerating voltage of 300 kV. X-ray photoelectron spectroscopy (XPS, Kratos AXIS Ultra DLD) was performed with a monochromatic Al Kα ray (1486.7 eV) and pass energy of 20 eV. The Shirley baseline subtraction was used in the fitting. The light absorption property and band gap of the composites were determined by an American PE Lambd A750 UV–visible (UV/Vis) near-infrared spectrophotometer equipped with an integrating sphere attachment. The migration, transfer, and recombination of photogenerated electron–hole pairs were characterized by fluorescence spectrophotometer (Hitachi F-4600) at an excitation wavelength of 245 nm. The specific surface areas of the composites were determined by Brunauer–Emmett–Teller (BET) surface area test (ASAP 2010) based on nitrogen gas adsorption and desorption. The electrochemical tests were performed by a CHI660E electrochemical workstation with a three-electrode cell. The potential values were relative to the Ag/AgCl electrode. The active free radicals involved in the photocatalytic process were characterized by electron spin resonance spectroscopy (ESR, Bruker E500). 5,5-dimethyl-1-pyrroline N-oxide (DMPO) was used as the trapping agent. The assays of hydroxyl radical (·OH) and superoxide radical (·O2−) were conducted after irradiating the catalyst for 5 min in H2O and CH3OH, respectively. The holes generated during the photocatalytic process were tested directly with the catalyst.
Photocatalytic reaction
Rhodamine B (RhB) dye was chosen as the model pollutant and photocatalytic activity of the composites was tested through degradation of RhB solution under a 200-W Xe lamp in open air and at room temperature. The catalyst with a mass of 10 mg was added to the RhB solution (10 mg/L, 50 mL) in a beaker. Prior to irradiation, the solution was stirred on the magnetic stirrer for 30 min in dark to achieve the adsorption–desorption equilibrium. The absorbance of RhB solution was measured by ultraviolet spectrophotometer at 554 nm where the strongest peak of RhB was seen and the concentration of RhB in solution was calculated from a working curve. The degradation efficiency was calculated according to formula (1):
where c0 is the original concentration of RhB and c is the RhB concentration after degradation.
In addition, cyclic experiments were carried out four times to test the stability of the catalysts. The catalysts were separated by centrifuge (TD5A) and ultrasonically rinsed in deionized water for 3 times at the end of each experiment. A similar cyclic photocatalytic activity was obtained with the method described above.
Active species trapping experiment
Oxalic acid, 2-propanol (IPA), and 1,4-benzoquinone (BQ) were used as trapping agents for hole (h+), hydroxyl radical (·OH), and superoxide radical (·O2−), respectively. The active species trapping experiments were similar to that of the photocatalytic experiments except for the addition of 1 mmol of trapping agent. The inhibitory effects of the active species were characterized by the degradation efficiency of RhB in visible light irradiation system under a Xe lamp in open air and at room temperature.
Results and discussion
Microstructure of photocatalysts
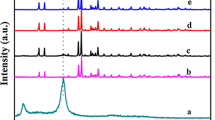
XRD patterns of the samples are shown in Fig. 2. The diffraction peaks for o-CNO obtained at 24.7°, 30.3°, and 35.0° could be assigned to the (310), (311), and (002) reflections of orthorhombic CuNb2O6 (JCPDS 83-1201, space group Pbcn), respectively, suggesting that the sample had orthorhombic structure and high purity. The peaks for Ag/g-C3N4/CNO-4 composites at 27.5° could be assigned to the (110) reflection of g-C3N4, indicating that the graphite-like lamellar structure of the g-C3N4 phase was intact. Moreover, none of the Ag/g-C3N4/CNO-4 samples showed diffraction peaks from the Ag phase because of the nanocrystalline structure and high dilution effect of the Ag phase [28, 29].
Figure 3 shows SEM morphologies of the samples. As shown in Fig. 3a, the o-CNO sample was granular, and the particle diameter ranged from 0.2 to 0.6 μm. Usually, the porous structure can increase the specific surface area of powders and facilitate the formation of heterojunction with other phases. In Fig. 3b, the C3N4 phase in the g-C3N4/CNO-4 composite consisted of clustered g-C3N4 rods, and the CNO particles were coated on the surface of the C3N4 phase to form a heterojunction. As shown in Fig. 3c–e, the rod-like C3N4 was well-coated on the surface of granular copper niobate to form a composite with nanoscale Ag particles distributed throughout the surface.
Figure 4 shows TEM images of 11% Ag/g-C3N4/CNO-4 composite. The obvious rod-like structures of the g-C3N4 phase as shown in Fig. 4a and the corresponding electron diffraction pattern (Fig. 4b) suggests an amorphous structure for the g-C3N4 phase. As shown in Fig. 4c, the Ag particles (see the lattice lines of metallic Ag in Fig. 4d) with a diameter smaller than 20 nm were uniformly dispersed on the amorphous g-C3N4 components. The EDS element mapping (Fig. 4e) further confirmed the distribution of Ag nanoparticles. The TEM image of the g-C3N4/CNO-4 heterojunction (Fig. 4f) and corresponding high-resolution image (Fig. 4g) demonstrated a crystalline CNO phase (the electron diffraction pattern as shown in Fig. 4h) above the dashed line and an amorphous g-C3N4 phase below the dashed line in the heterojunction.
In addition, according to the adsorption–desorption curves in BET tests (Fig. 5), the specific surface area of g-C3N4, o-CNO, g-C3N4/CNO-4, and 11% Ag/g-C3N4/CNO-4 sample was 22.7, 2.74, 39.2, and 38.4 m2/g, respectively. This further suggests that the heterojunction had a large specific surface area which increased after the two phases recombined. However, no significant change in the specific surface area was seen after the addition of silver. Therefore, the deposition of silver on the g-C3N4/CNO-4 heterojunction was not conducive to improve the specific surface area of the photocatalysts.
Figure 6 shows the X-ray photoelectron spectrum of pure g-C3N4, o-CNO and 11% Ag/g-C3N4/CNO-4 nanocomposite. The charging effect of the samples was corrected by referring to C1s 284.6 eV. The fitting results related to the position of peaks are shown in Table 1. In the Ag3d spectrum, the binding energy centered at 368.1 and 374.1 eV corresponds to metallic Ag (3d5/2) and Ag (3d3/2), respectively, which suggests the presence of metallic Ag in 11% Ag/g-C3N4/CNO-4 catalyst [30, 31]. In the C1s spectrum, the peak located at 284.6 eV was assigned to the sp2 C–C bond in g-C3N4, and the peaks at 287.9 and 288.0 eV were assigned to the N–C=N groups in g-C3N4 for 11% Ag/g-C3N4/CNO-4 nanocomposite and pure g-C3N4, respectively [30]. For the N1s spectrum, the two peaks centered at 398.5 and 400.2 eV for 11% Ag/g-C3N4/CNO-4 nanocomposite, and the peaks at 398.5 and 400.0 eV for pure g-C3N4, correspond to the C=N–C and N–(C)3 groups, respectively [32]. The results from N1s and C1s spectrum clearly proved the existence of g-C3N4 in the catalysts. In the Cu2p spectrum, the peaks centered at 934.0 and 953.9 eV for 11% Ag/g-C3N4/CNO-4 nanocomposite were attributed to Cu (2p3/2) and Cu (2p1/2) from Cu–O bonds with a 19.9 eV splitting spacing of 2p doublet [33, 34], whereas the additional peaks at 941.9 and 961.9 eV correspond to the shake-up satellites from Cu–O bonds in the catalysts [35]. For pure o-CNO, the peaks for Cu (2p3/2) and Cu (2p1/2) were located at 934.3 and 954.4 eV, respectively, with a splitting spacing of 20.1 eV, which caused a slight shift compared to those of 11% Ag/g-C3N4/CNO-4 nanocomposite. The Nb3d spectrum of 11% Ag/g-C3N4/CNO-4 nanocomposite showed the two main peaks, Nb (3d3/2) and Nb (3d5/2), located at 209.6 and 206.9 eV, respectively. The splitting energy between Nb (3d3/2) and Nb (3d5/2) was 2.7 eV. According to previous reports, this splitting energy was 2.72 eV in LiNbO3 [36] and 2.70 eV in CaNb2O6 [37]. For pure o-CNO, the two peaks were located at 209.7 and 207.0 eV, and the splitting spacing of the doublet was the same as that of the 11%Ag/g-C3N4/CNO-4 nanocomposite. For 11% Ag/g-C3N4/CNO-4 nanocomposite, the O1s spectrum was deconvoluted into three peaks located at 530.1, 531.7, and 532.9 eV, which could be successively attributed to the Nb–O and Cu–O bonds in o-CNO, and H–O bonds from water vapor adsorbed on the surface [38]. However, these peaks caused a slight shift compared to those of pure o-CNO. Based on the above results, a certain chemical shift was observed in the binding energy of bonds in 11% Ag/g-C3N4/CNO-4 nanocomposite, which further suggests that a chemical interaction was involved.
Figure 7a shows the UV/Vis spectra of o-CNO and Ag/g-C3N4/CNO-4 composites. It was seen that o-CNO mainly absorbed light of wavelength below 550 nm and the g-C3N4/CNO-4 composite exhibited a lower light absorption intensity than o-CNO. The light absorption intensities of Ag/g-C3N4/CNO-4 composites with Ag content of 1% and 11% were higher than that of g-C3N4/CNO-4 due to the loading of Ag, and their absorption edge wavelengths were ~ 470 nm. The 11% Ag/g-C3N4/CNO-4 composite exhibited the highest absorption intensity among these composites, which further suggests that the 11% Ag/g-C3N4/CNO-4 composite had a better utilization rate of visible light. The reasonable explanation was that, after loading Ag, the more refined grains of g-C3N4 and o-CNO and the close interaction between g-C3N4 and o-CNO increased the light scattering, thereby increasing the transmission length of incident light and enhancing the visible light capturing ability of g-C3N4/CNO composites. Due to the excessive metallic Ag on its surface, the composite 30% Ag/g-C3N4/CNO-4 demonstrated a relatively low light absorption intensity.
In addition, the Kubelka–Munk formula (2) was used to calculate the band gap energy of the photocatalysts:
In formula (2), α, h, ν, A, and Eg are the absorption coefficient, Planck constant, optical frequency, constant, and band gap, respectively. The value of n was determined by the band gap type of semiconductors and for an indirect transition semiconductor, n equals 4. Figure 7b shows the curves of (αhν)0.5 on hν for o-CNO and Ag/g-C3N4/CNO composites. Consequently, the band gap of o-CNO was 1.90 eV, similar to the previous reports [20], and the band gaps of g-C3N4/CNO-4 and Ag/g-C3N4/CNO-4 composites were ~ 2.31 eV.
Figure 8 shows the PL spectra of o-CNO and Ag/g-C3N4/CNO-4 composites. In general, the photoluminescence emission is generated by the recombination of photoelectron–hole pairs. The higher the recombination rate of electron–hole pairs, the higher is the fluorescence intensity, and the lower is the photocatalytic performance. Recent research studies showed that the PL spectrum of g-C3N4 demonstrated a relatively wide and strong fluorescence emission peak at 450 nm [39, 40]. The peak around 450 nm which occurs in the PL spectrum of Ag/g-C3N4/CNO-4 composites was majorly because of the presence of g-C3N4 component in the composites. However, it was observed that Ag-loading reduced the photoluminescence emission effect and thus the photoluminescence intensity was negatively correlated with Ag content. After the formation of the composite, the absorption peak showed a slight blue shift, which might be attributed to the enhanced absorption ability of the composite to UV–visible light. These results indicate that Ag-loading effectively inhibited the recombination of photoelectron–hole pairs in g-C3N4/CNO composite catalyst, which was conducive to the separation of photocarriers, thus improving the photocatalytic activity of the composite.
Analysis of photocatalytic activity of g-C3N4-based heterostructured composites
The photodegradation efficiency directly expressed the photocatalytic performance of materials as one of the important indexes of composite photocatalysts. Figure 9 shows the photocatalytic activity of the Ag/g-C3N4/CNO-4 composite with different Ag contents for degradation of RhB. The degradation efficiency of o-CNO and g-C3N4/CNO-4 was found to be 11.47% and 27.7%, respectively, and each of the Ag/g-C3N4/CNO-4 composites exhibited a higher degradation efficiency than the above two, which further suggests that the heterojunction was successfully fabricated. It was noteworthy that 11%Ag/g-C3N4/CNO-4 composite showed the highest degradation efficiency of 82.07%, which was about 7.1 times that of o-CNO and 3.8 times that of g-C3N4/CNO-4, and as a result showed the best photocatalytic performance. However, the decrease in degradation efficiency with an excessive Ag-loading content for the samples might be attributed to the reduction in light reflection and to the amount of active sites present on the composite surface owing to the shadowing effect of Ag particles. As a result, the 30% Ag/g-C3N4/CNO-4 sample showed reduced fluorescence intensity in PL test whereas o-CNO showed low photocatalytic efficiency but better light absorption in the UV/Vis experiment.
According to the Langmuir–Hinshelwood model, the rate constant (k) during the degradation process was used to characterize the change of dye concentration with irradiation time by measuring the change in absorbance of RhB. As shown in Fig. 10, the rate constant of 11% Ag/g-C3N4/CNO-4 composite showed the highest value of 0.859 h−1, and the degradation rate gradually decreased with increasing test time. The results imply that the degradation rate of RhB decreases with a decrease in RhB concentration.
The stability of photocatalyst is an important property. Figure 11a shows the XRD patterns of the 11% Ag/g-C3N4/CNO-4 photocatalyst before and after cyclic experiments. On comparing with the pattern before cyclic experiments it was found that the phase structure in the catalysts hardly changed after cyclic experiments. Meanwhile, the photocatalytic degradation efficiency showed a slight decrease with the number of cycles (Fig. 11b), which clearly suggests that the prepared catalysts have good stability.
According to the data from research studies, the comparison of photocatalytic performance of g-C3N4-based and CuNb2O6-based materials for degradation of various dyes is shown in Table 2. The Ag/g-C3N4/CuNb2O6 composite prepared in this work demonstrates an outstanding degradation efficiency and expectable low cost.
Electrochemical analysis
Electrochemical testing can effectively characterize the catalytic activity and band structure of photocatalytic materials [41]. Mott–Schottky (MS) tests were carried out to estimate the band structure of the catalysts. As shown in Fig. 12a, CuNb2O6 is an N-type semiconductor with a flat band potential (VFB) of − 1.53 V (vs. Ag/AgCl). Considering that the value of VFB of N-type semiconductors is usually about 0.1 V higher than the conduction band edge potential (ECB) and Ag/AgCl potential is 0.20 V [42], the CB edge potential of CuNb2O6 was estimated to be − 1.02 V (vs. NHE, pH = 7). Similarly, the CB edge potential of 11% Ag/g-C3N4/CuNb2O6-4 composite and g-C3N4 was about − 0.91 and − 0.83 V, respectively (vs. NHE, pH = 7).
Photocurrent measurement can characterize the photocatalytic activity of the catalyst. As shown in Fig. 12b, the photocurrent response of o-CNO was significantly lower than that of g-C3N4 and 11% Ag/g-C3N4/CuNb2O6-4 composite. The photocurrent response of 11% Ag/g-C3N4/CuNb2O6-4 composite was slightly higher than that of g-C3N4, which could be attributed to the formation of g-C3N4/CuNb2O6-4 heterojunction.
The electrochemical impedance spectra (EIS) of the samples are shown in Fig. 12c. Compared with the other samples, 11% Ag/g-C3N4/CuNb2O6-4 composite demonstrates the smallest radius in the Nyquist diagram, suggesting the smallest electric resistance and the best charge transfer efficiency [43]. This indicates that Ag shows a charge transfer effect in the photocatalyst after the formation of composite semiconductor.
Active species
To further study the photocatalytic mechanism of 11% Ag/g-C3N4/CNO-4 composite, free radical trapping experiments were carried out to determine the main active components involved in the photocatalytic process. The procedure was similar to the measurement of photocatalytic activity, except that the trapping agent was added in RhB dye solution. In the experiment, oxalic acid, benzoquinone and isopropanol (IPA) were used as the traps for h+, ·O2−, and ·OH radicals, respectively. The capture experiment results of 11% Ag/g-C3N4/CNO-4 composite for the degradation of RhB are shown in Fig. 13a. The degradation efficiency of 11% Ag/g-C3N4/CNO-4 on RhB inhibited significantly after the addition of oxalic acid, indicating that h+ was the main reactive species in the photocatalytic process. In addition, the photocatalytic decolorization of RhB also inhibited after the addition of benzoquinone, indicating that ·O2− radical also played a main role in the photocatalytic process. However, when IPA was added, the photocatalytic efficiency of the sample changed slightly. It indicates that ·OH plays a minor role in the photocatalytic process. In addition, according to the results of the ESR test shown in Fig. 13b–d, the presence of active free radicals, such as h+, ·O2−, and ·OH, was confirmed [44]. As a result, the h+ and ·O2− were the main reactive radicals whereas the ·OH radical played an auxiliary role in the photocatalytic process for degradation of RhB.
Degradation mechanism analysis
As the CB edge potentials of g-C3N4, 11% Ag/g-C3N4/CNO-4 composite, and o-CNO were − 0.83, − 0.91, and − 1.02 V, respectively (vs. NHE, pH = 7), the valence band (VB) edge potentials of g-C3N4, 11% Ag/g-C3N4/CNO-4 composite, and o-CNO can be easily calculated and were found to be + 1.87, + 1.40, and + 0.88 V, respectively (Fig. 14).
It is known that there are two common routes for charge transfer between two different kinds of semiconductors. The first is the traditional type-II heterojunction and the second is the Z-scheme heterojunction. In the former case, electrons are transferred from the CB of the semiconductor with high CB edge potential to the CB of another semiconductor with low CB edge potential, and the holes move from the low potential VB to the high potential VB. However, in Z-scheme mechanism, the electrons in the CB of a semiconductor directly recombine with the holes in the VB of the other semiconductor with a relatively lower oxidation potential. Therefore, the electrons and holes with high reduction and oxidation capacities are retained.
In the present work, according to the band structure of 11% Ag/g-C3N4/CNO-4 composite, ·OH radical was not produced during the photocatalytic process, because its VB edge potential was more negative than the potential of OH−/·OH electrode. However, the presence of ·OH radical was confirmed in the active species test and ESR test. Therefore, the hypothesis that the Ag/g-C3N4/CNO composite forms a traditional type-II heterojunction was inconsistent with the results of active species trapping and ESR experiments. We proposed a possible all-solid-state Z-scheme photocatalytic mechanism. Under visible light irradiation, g-C3N4 and o-CNO can be excited to form electron–hole pairs and the photogenerated electrons can be transferred from VB to CB of g-C3N4 phase. Subsequently, the Ag nanoparticles dispersed on the interface of g-C3N4 and o-CNO act as a bridge for the transfer of electrons from CB of the g-C3N4 phase to the VB of o-CNO phase, thereby inhibiting the recombination of photogenerated electrons and holes in individual semiconductors. Consequently, the electrons were excited to the CB of the CNO phase, and holes generated in the g-C3N4 phase were completely spatially segregated, and therefore, the 11% Ag/g-C3N4/CNO composite provides a much stronger redox capacity and demonstrates better photocatalytic performance. Meanwhile, due to the formation of heterojunction, a built-in electric field was formed inside the catalyst, and the band edge of g-C3N4 bends toward the side of higher oxidation potential, thus producing ·OH radical. Moreover, as a charge transfer channel, silver effectively retains the REDOX capability of all-solid-state Z-scheme heterojunction.
Conclusion
In summary, an orthorhombic CuNb2O6 phase was obtained in high purity via the calcination of hydrothermally synthesized CuNb2O6 and Ag/g-C3N4/CuNb2O6-4 (mass ratio of g-C3N4 to CuNb2O6 was 1:4) composites with different Ag contents which were successfully prepared using the hydrothermal and photo-deposition method. The Ag content in Ag/g-C3N4/CuNb2O6-4 composites showed a significant effect on its photocatalytic efficiency and the 11% Ag/g-C3N4/CuNb2O6-4 composite showed the highest photocatalytic activity. An all-solid-state Z-scheme heterojunction was successfully fabricated by loading Ag onto the g-C3N4/CuNb2O6 composite. The h+ and ·O2− were found to be the main active radicals in the photocatalytic degradation of RhB solution. Although the photocatalytic efficiency of Ag/g-C3N4/CuNb2O6-4 composites improved greatly as seen in the experiments, further study should be carried out to reduce the cost of photocatalysts in industrial applications.
References
S. Lin, W. Liu, J. Environ. Eng. 120, 2 (1994)
S.S. Lee, H. Bai, Z. Liu, D.D. Sun, Environ. Sci. Technol. 49, 4 (2015)
V. Vaiano, O. Sacco, D. Sannino, P. Ciambelli, Appl. Catal. B Environ. 170–171, 153 (2015)
A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard, J.-M. Herrmann, Appl. Catal. B 31, 2 (2001)
N. Al-Bastaki, Chem. Eng. Process. 43, 12 (2004)
A.N. Soon, B.H. Hameed, Desalination 269, 1 (2011)
G. Liu, M. Feng, M. Tayyab, J. Gong, M. Zhang, M. Yang, K. Lin, J. Hazard. Mater. 412, 125224 (2021)
C. Rafols, D. Barcelo, J. Chromatogr. A 777, 1 (1997)
I. Oller, S. Malato, J.A. Sánchez-Pérez, Sci. Total Environ. 409, 20 (2011)
N. Fajrina, M. Tahir, Chem. Eng. J. 374, 1076 (2019)
C. Namasivayam, D. Sangeetha, J. Hazard. Mater. 135, 1 (2006)
V.K. Gupta, Suhas, J. Environ. Manag. 90, 8 (2009)
E. Forgacs, T. Cserháti, G. Oros, Environ. Int. 30, 7 (2004)
L. Zhang, H.H. Mohamed, R. Dillert, D. Bahnemann, J. Photochem. Photobiol. C 13, 4 (2012)
Y. Liu, Q. Zhu, M. Tayyab, L. Zhou, J. Lei, J. Zhang, Solar RRL 5, 11 (2021)
Y.C. Zhang, B. Fu, Q. Liu, J. Alloys Compd. 477, 1 (2009)
A. Kormányos, A. Thomas, M.N. Huda, P. Sarker, J.P. Liu, N. Poudyal, C. Janáky, K. Rajeshwar, J. Phys. Chem. C 120, 29 (2016)
X. Ye, Z. Wen, R. Zhang, H. Ling, J. Xia, X. Lu, J. Power Sources 483, 22919 (2021)
M.G.B. Drew, R.J. Hobson, V.T. Padayatchy, J. Mater. Chem. 3, 8 (1993)
S. Kamimura, S. Abe, T. Tsubota, T. Ohno, J. Photochem. Photobiol. A Chem. 356, 263 (2018)
Y. Tang, D. Zhang, Y. Li, B. Huang, H. Li, X. Pu, Y. Geng, Sep. Purif. Technol. 220, 78 (2019)
Y. Kang, Y. Yang, L.-C. Yin, X. Kang, G. Liu, H.-M. Cheng, Adv. Mater. 27, 31 (2015)
W.-J. Ong, L.-L. Tan, Y.H. Ng, S.-T. Yong, S.-P. Chai, Chem. Rev. 116, 12 (2016)
J. Fu, J. Yu, C. Jiang, B. Cheng, Adv. Energy Mater. 8, 3 (2018)
L. Guan, N. Xu, X. Liu, Y. Zhao, H. Li, J. Sun, J. Wu, Z. Ying, Carbon 79, 578 (2014)
Y. Chen, X. Bai, Catalysts 10, 1 (2020)
K. Qi, Y. Li, Y. Xie, S.-Y. Liu, K. Zheng, Z. Chen, R. Wang, Front. Chem. 7, 91 (2019)
Y. Fu, T. Huang, L. Zhang, J. Zhu, X. Wang, Nanoscale 7, 32 (2015)
X. Zhou, Z. Luo, P. Tao, B. Jin, Z. Wu, Y. Huang, Mater. Chem. Phys. 143, 3 (2014)
M. Faisal, A.A. Ismail, F.A. Harraz, S.A. Al-Sayari, A.M. El-Toni, M.S. Al-Assiri, Mater. Des. 98, 223 (2016)
S. Ma, S. Zhan, Y. Jia, Q. Shi, Q. Zhou, Appl. Catal. B Environ. 186, 77 (2016)
L. Ge, C. Han, J. Liu, Y. Li, Appl. Catal. A Gen. 409–410, 215 (2011)
M. Arfan, I. Hussain, Z. Ahmad, A. Afzal, T. Shahid, A.G. Wattoo, M. Rafi, A. Zeb, M.I. Shahzad, S. Zhenlun, Cryst. Res. Technol. 57, 2100230 (2022)
X.-C. Zheng, S.-P. Wang, S.-R. Wang, S.-M. Zhang, W.-P. Huang, S.-H. Wu, Mater. Sci. Eng. C 25, 4 (2005)
M.T. Qamar, M. Aslam, I.M.I. Ismail, N. Salah, A. Hameed, ACS Appl. Mater. Interfaces 7, 16 (2015)
P. Steiner, H. Höchst, Zeitschrift für Phys. B Condens. Matter 35, 1 (1979)
Y. Wang, L. Zhang, R. Cao, Q. Miao, J. Qiu, Appl. Phys. A 115, 4 (2014)
M. Grobelny, M. Kalisz, M. Mazur, D. Wojcieszak, D. Kaczmarek, J. Domaradzki, M. Świniarski, P. Mazur, Thin Solid Films 616, 64 (2016)
Y. Shi, S. Jiang, K. Zhou, B. Wang, B. Wang, Z. Gui, Y. Hu, R.K.K. Yuen, RSC Adv. 4, 6 (2014)
N.K.R. Bogireddy, S. Godavarthi, M.K. Kesarla, V. Agarwal, L. Martínez-Gómez, J. Environ. Chem. Eng. 8, 6 (2020)
M. Tayyab, Y. Liu, S. Min, R. Muhammad-Irfan, Q. Zhu, L. Zhou, J. Lei, J. Zhang, Chin. J. Catal. 43, 4 (2022)
W. Zhang, P. Xing, J. Zhang, L. Chen, J. Yang, X. Hu, L. Zhao, Y. Wu, Y. He, J. Colloid Interface Sci. 590, 548 (2021)
H. Liang, T. Li, J. Zhang, D. Zhou, C. Hu, X. An, R. Liu, H. Liu, J. Colloid Interface Sci. 558, 85 (2020)
D. Dvoranová, V. Brezová, M. Mazúr, M.A. Malati, Appl. Catal. B 37, 2 (2002)
D. Peng, H. Wang, K. Yu, Y. Chang, X. Ma, S. Dong, RSC Adv. 6, 81 (2016)
M. Sierra, E. Borges, P. Esparza, J. Méndez-Ramos, J. Martín-Gil, P. Martín-Ramos, Sci. Technol. Adv. Mater. 17, 1 (2016)
Y. Chen, W. Huang, D. He, Y. Situ, H. Huang, ACS Appl. Mater. Interfaces 6, 16 (2014)
H. Huang, C. Liu, H. Ou, T. Ma, Y. Zhang, Appl. Surf. Sci. 470, 205 (2019)
Y. Zheng, C. Wei, Q. An, J. Yu, S. Xu, L. Li, New J. Chem. 45, 36 (2021)
Acknowledgements
This work was supported by Zhejiang provincial key R&D program under Grant No. 2019C01088.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, F., Zhang, H., Tang, Q. et al. Z-scheme Ag-loaded g-C3N4/CuNb2O6 composite photocatalyst for RhB dye degradation. Res Chem Intermed 48, 4163–4182 (2022). https://doi.org/10.1007/s11164-022-04812-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04812-8