Abstract
A series of N2,N8-bis(nitrogen-containing heterocycle)-yl-2,8-dicarboxamide-Tröger’s bases were synthesized. The most efficient one, N2,N8-di(4H-1,2,4-triazol-4-yl)-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine-2,8-dicarboxamide (4d), was used as bifunctional catalyst to promote the one-step one-pot preparation of the chromeno[3′,4′:4,5]furo[2,3-b]indoles or naphtho[2′,3′:4,5]furo[2,3-b]indoles with CuI via the cascade Aldol-[4 + 1]cycloaddition-intramolecular Ullmann reaction of 4-hydroxycoumarin (or 2-hydroxy-1,4-naphoquinone), substituted benzaldehydes and isocyanide. A reasonable catalysis mechanism was investigated by the 1H NMR titration and control experiments.
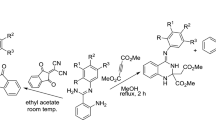
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Furoindole is an important framework of many natural products and synthetic intermediates [1,2,3] with many biological activities including anti-inflammatory [4], analgesic [5], anti-allergy [6], removing fever [7], and so on. Some furo[2,3-b]indole derivatives have also been used as the potent openers of Ca2+-activated K+ (BKCa) channels [8] to treat relative diseases [9]. It is important to note that the furo[2,3-b]indole derivatives have excellent luminescent properties due to the large conjugation system and plane rigidity. Therefore, their simple and efficient synthesis is of great significance.
In general, there are three methods to prepare furo[2,3-b]indoles: (1) to construct the furan ring based on a functional indole skeleton [10,11,12,13]; (2) to form the indole ring from the reactions between anilines and functional furans [14,15,16]; and (3) to close the ring of opening-chain structures [17]. It is necessary to synthesize at least one substrate in advance in these strategies. Ji and co-workers [18] reported a two-step one-pot reaction, including an isocyanide-based multicomponent reaction (I-MCR) followed by a copper-catalyzed intramolecular Ullmann reaction, to construct the furo[2,3-b]indole derivatives. They firstly carried out the reaction of 4-hydroxycoumarin, 4-hydroxy-1-methylquinolinone or 2-hydroxy-1,4-naphthoquinone with aldehyde and isocyanide to give relative furan derivatives at 110 °C in anhydrous toluene for 6–48 h. Without separating and purifying the produced furan derivatives, CF3COOH (TFA), CuI, L-proline and K2CO3 were added in sequentially under nitrogen atmosphere and the mixture was refluxed for another 24 h to afford the target furo[2,3-b]indole derivatives by recrystallization from acetone or by silica gel column chromatography. An organic acid L-proline was used as the ligand in the second step. Therefore, it is necessary to develop alkaline ligand for the acid-sensitive substrates. In Ji’s work, DABCO (1,4-diazabicyclo[2.2.2]octane) was also found to promote the Cu-catalyzed Ullmann reaction as the ligand, which reminds us of the Tröger’s base (TB, Fig. 1) derivatives that also contains two bridge nitrogen atoms.
Because of the unique V-type structure, molecular rigidity and C2-axial chirality, TB and its derivatives have been applied in fluorescent material [19,20,21,22], molecular recognition [23,24,25], DNA probe [26,27,28], bionic molecular receptor [29, 30], etc., since the synthesis of TB in 1887. Recently, they become the research focus of polymer chemistry and gas separation membrane materials [31,32,33,34,35,36]. However, it is worth noting that the advantages of TB and its derivatives served as catalysts are far from being developed. Enlightened by the unique structure of TB framework, which enables TB and its derivatives to capture appropriate molecules and then shows catalytic activities, we have synthesized several series of TB derivatives by increasing catalytic active sites and basicity and then applied them successfully in organic synthesis as organocatalyst or ligand [37,38,39,40,41,42].
Amide is the main structural unit of peptide chains. Acting both as a hydrogen bond donor and acceptor, it has been utilized as the main segment of many types of catalysts, especially peptide catalysts [43,44,45,46,47,48].
In this paper, a series of new TB derivatives (4) were synthesized by introducing N-heterocycles (including pyridine, pyrimidine, isoxazole, pyrazole, triazole, 1,3,5-triazin-2(1H)-one and tetrazole) into TB skeleton through amide bond (Scheme 1). They were used both as the basic catalyst for the Aldol-[4 + 1]cycloaddition and the ligand for the following copper-catalyzed intramolecular Ullmann reaction of coumarins or 2-hydroxy-1,4-naphthoquinone (5), isocyanide (6) and substituted benzaldehydes (7) to promote the real one-pot preparation of the chromeno[3′,4′:4,5]furo[2,3-b]indoles or naphtho[2′,3′:4,5]furo[2,3-b]indoles (8) in good yield (Scheme 2).
Results and discussion
The reaction conditions were optimized based on the yield of 8a (Table 1). The results showed that the reaction did not occur without catalyst (Table 1, Entry 1) in toluene. TB derivative 2 (Table 1, Entry 2) could only afford trace product, while 4a-4 g (Table 1, Entries 3–9) could promote the synthesis of 8a, in which 4d gave the best result (61%, Table 1, Entry 6). When the loading of CuI was 5 mol%, increasing the loading of 4d from 5 to 10 then 15 mol% only caused 1% increment of the yield of 8a (Table 1, Entry 6 vs. Entries 10, 11). But when the loading of CuI was 10 mol%, adding the loading of 4d from 5 to 10 mol% led to an increase in the yield of 8a from 61 to 85% (Table 1, Entry 6 vs. Entry 13). Based on the principle of economic and environmental benefits, the combination of 10 mol% of 4d and 10 mol% of CuI was the best choice. Subsequently, it was found that the highest yield of 8a was obtained in the presence of K2CO3 (2 eq., 85%, Table 1, Entry 13 vs. Entries 16–17). The results also indicated that toluene was the most suitable solvent for the reaction (Table 1, Entry 13 vs. Entries 18–20).
Above all, in the presence of K2CO3 (2 eq.), using 10 mol % of 4d and 10 mol% CuI as catalyst and refluxing in toluene was the optimum condition.
With the optimized conditions in hand, we proceeded to explore the scope of substrates (Table 2). According to the results, in general, coumarins 5a-5c gave higher yield than 2-hydroxy-1,4-naphthoquinone 5d. The possible reason is that the deprotonation of 5a-5c carries out more easily than the latter. As for isocyanides, benzyl isocyanide gave a higher yield of product than tert-butyl-substituted or cyclohexyl-substituted isocyanide, which maybe due to the larger steric hindrance of cyclohexyl and tert-butyl than that of benzyl.


To understand the catalysis mechanism, 1H NMR analysis (400 MHz) was applied to monitor the reaction process. The specific steps were as follows: catalyst 4d (0.0133 g, 0.3 mmol), 5a (0.0486 g, 0.3 mmol), 6a (0.0328 g, 0.3 mmol), 7a (0.0555 g, 0.3 mmol), CuI (0.0057 g, 10 mol%, 0.03 mmol), K2CO3 (0.0829 g, 0.6 mmol) and toluene (15 mL) were added in a 25 mL two-neck round-bottom flask and stirred at 110 °C. 0.50 mL of the mixture was taken out and the solvent was removed under vacuum, and then the residue was dissolved in DMSO-d6 for NMR analysis. The reaction process was monitored by repeating the operation at five-minute intervals (Fig. 2).
From Fig. 2, as the reaction proceeded, the signal of hydrogen atoms on the amide (δ = 11.94 ppm) and triazole (δ = 8.70 ppm) group of 4d became weaker gradually. And it is also clear that the signal of three methylene groups (δ = 4.77 and 4.36–4.30 ppm) on the eight-member ring shifted to the low field. Therefore, it can be speculated that the electron cloud density of eight-member ring, amide and triazole group in 4d have changed. The possible reason is that the 4d and Cu(I) formed a complex and the coordination cause the change of electron cloud density [38].
Several catalyst systems were also used to shed light on the reaction mechanism (Table 3).
From Table 3, CuI alone cannot promote the cascade reaction to give the intermediate product I or the target product 8a (Table 3, Entry 1), indicating that 4d is necessary whether as a catalyst in the first stage or as a ligand in the second stage. Without CuI, 4d itself can only promote the first stage to afford I (Table 3, Entry 2). Compound 4H-1,2,4-triazol-4-amine can also catalyze the first stage reaction to obtain I, but the yield is very low (28%, Table 3, Entry 3), indicating that the synergy of TB skeleton and aminotriazole fragment works and the TB framework can increase the yield apparently.
Based on the results mentioned above and the literature [45], a possible mechanism was then proposed in Scheme 3. Firstly, the catalyst 4d captured hydrogen atom of hydroxyl on 5a to produce intermediate A. Then, intermediate A acted as nucleophile to attack 7a to form intermediate B. Intermediate C was formed via dehydration of intermediate B. 6 attacked intermediate C as nucleophile to afford intermediate D after [4 + 1] cycloaddition. Then, the catalyst 4d-Cu formed complex I with D, and the N atom in I reacted with Ar-Br by intramolecular addition to form intermediate II. The bromine atom in II was replaced by carbonate to form intermediate III and left in the form of bromine ion. The N–Cu interaction in III cleaved to form the final product 8, and meanwhile, a carbonate was lost and the catalyst 4d-Cu was recovered to complete a cycle.
Conclusion
In summary, a series of N2,N8-bis(nitrogen-containing heterocycle)-yl-2,8-dicarboxamide-Tröger’s bases were synthesized. The most efficient 4d was used to promote reaction of 4-hydroxycoumarin (or 2-hydroxy-1,4-naphoquinone), substituted benzaldehydes and isocyanide in mild condition. The results of catalysis mechanism investigation by 1H NMR titration and control experiments showed the high catalytic efficacy of 4d comes from its bifunction (the basic catalyst for the Aldol-[4 + 1]cycloaddition reaction and the ligand for the following copper-catalyzed intramolecular Ullmann reaction). Based on the high catalytic ability, the one-step one-pot preparation of the chromeno[3’,4’:4,5]furo[2,3-b]indoles and naphtho[2′,3′:4,5]furo[2,3-b]indoles was realized.
References
W.H. Pearson, Y. Mi, I.Y. Lee, P. Stoy, J. Am. Chem. Soc. 123, 6724 (2001)
C.M. Li, C. Chan, A.C. Heimann, S.J. Danishefsky, Angew. Chem. Int. Ed. 46, 1448 (2007)
J.D. Trzupek, D. Lee, B.M. Crowley, V.M. Marathias, S.J. Danishefsky, J. Am. Chem. Soc. 132, 8506 (2010)
Y. Kawashima, M. Sato, Y. Hatada, S. Okuyama, F. Amanuma, Y. Nakashima, K. Sota, I. Moriguchi, Chem. Pharm. Bull. 34, 3267 (1986)
Y. Kawashima, F. Amanuma, M. Sato, S. Okuyama, Y. Nakashima, K. Sota, I. Moriguchi, J. Med. Chem. 29, 2284 (1986)
P.C. Unangst, M.E. Carethers, K. Webster, G.M. Janik, L.J. Robichaud, J. Med. Chem. 27, 1629 (1984)
T. Kameyama, F. Amanuma, S. Okuyama, S. Higuchi, H. Aihara, J. Pharmacobio-Dyn. 8, 477 (1985)
T.S. Ha, H.H. Lim, G.E. Lee, Y.C. Kim, C.S. Park, Mol. Pharmacol. 69, 1007 (2006)
K. Kanbe, M. Okamura, S. Hattori, H. Naganawa, M. Hamada, Y. Okami, T. Takeuchi, Biosci. Biotechnol. Biochem. 57, 632 (1993).
T. An I, C.H. Lin, C.P. Chuang, Heterocycles 65, 2381 (2005).
C. Chan, C. Li, F. Zhang, S.J. Danishefsky, Tetrahedron Lett. 47, 4839 (2006)
M. Sattar, V. Rathore, C.D. Prasad, S. Kumar, Chem. Asian J. 12, 734 (2017)
S.P. Nikumbh, A. Raghunadh, T.S. Rao, V.N. Murthy, S.C. Joseph, Y.L.N. Murthy, M. Pal, RSC Adv. 6, 23489 (2016)
M. Pudlo, D. Csányi, F. Moreau, G. Hajós, Z. Riedl, J. Sapi, Tetrahedron 63, 10320 (2007)
H. Gao, Q. Xu, M. Yousufuddin, D.H. Ess, L. Kürti, Angew. Chem. Int. Ed. 53, 2701 (2014)
M. Kienle, A.J. Wagner, C. Dunst, P. Knochel, Chem. Asian J. 6, 517 (2011)
K. Muñiz, J. Am. Chem. Soc. 129, 14542 (2007)
X. Zhu, X.P. Xu, C. Sun, T. Chen, Z.L. Shen, S.J. Ji, Tetrahedron 67, 6375 (2011)
Y.H. Chu, Y. Wan, Z.T. Liu, X.E. Han, H. Wu, Tetrahedron Lett. 56, 7046 (2015)
Q. Xin, X.T. Tao, F.Z. Wang, J.L. Sun, D.C. Zou, F.J. Wang, H.J. Liu, Z. Liu, Y. Ren, M.H. Jiang, Org. Electron. 9, 1076 (2008)
C.A.M. Abella, F.S. Rodembusch, V. Stefani, Tetrahedron Lett. 45, 5601 (2004)
D.M.P. Aroche, J.M. Toldo, R.R. Descalzo, P.F.B. Gonçalves, F.S. Rodembusch, New J. Chem. 39, 6987 (2015)
S. Goswami, K. Ghosh, Tetrahedron Lett. 38, 4503 (1997)
Ö.V. Rúnarsson, J. Artacho, K. Wärnmark, Eur. J. Org. Chem. 7015 (2012).
D.M.P. Aroche, J.P. Vargas, P.A. Nogara, F. da Silveira Santos, J.B.T. da Rocha, D.S. Lüdtke, F.S. Rodembusch, ACS Omega 4, 13509 (2019).
C. Bailly, W. Laine, M. Demeunynck, J. Lhomme, Biochem. Biophys. Res. Commun. 273, 681 (2000)
B. Baldeyrou, C. Tardy, C. Bailly, P. Colson, C. Houssier, F. Charmantray, M. Demeunynck, Eur. J. Med. Chem. 37, 315 (2002)
E.B. Veale, T. Gunnlaugsson, J. Org. Chem. 75, 5513 (2010)
R. Kaplánek, M. Havlík, B. Dolenský, J. Rak, P. Džubák, P. Konečný, M. Hajdúch, J. Králová, V. Král, Bioorg. Med. Chem. 23, 1651 (2015)
A. Paul, B. Maji, S.K. Misra, A.K. Jain, K. Muniyappa, S. Bhattacharya, J. Med. Chem. 55, 7460 (2012)
Z.Y. Zhu, J.J. Zhu, J.X. Li, X.H. Ma, Macromolecules 53, 1573 (2020)
Y.J. Zhao, K.P. Chen, E.A. Yildiz, S.J. Li, Y.Q. Hou, X. Zhang, Z.J. Wang, J.Z. Zhao, A. Barbon, H.G. Yaglioglu, H.J. Wu, Chem. Eur. J. 26, 3591 (2020)
X.C. Xu, J.J. Wang, J. Dong, H.B. Li, Q.H. Zhang, X. Zhao, J. Membr. Sci. 602, 117967 (2020).
X.X. Zhuge, R.C. Liu, J.Y. Li, J. Zhang, Y.X. Li, C.X. Yuan, Dyes Pigm. 171, 107678 (2019).
I. Kammakakam, K.E. O’Harra, J.E. Bara, E.M. Jackson, ACS Omega 4, 3439 (2019)
S. Shanmugaraju, D. Umadevi, L.M. González Barcia, J.M. Delente, K. Byrne, W. Schmitt, G.W. Watson, T. Gunnlaugsson, Chem. Commun. 55, 12140 (2019).
H. Wu, X.M. Chen, Y. Wan, L. Ye, H.Q. Xin, H.H. Xu, C.H. Yue, L.L. Pang, R. Ma, D.Q. Shi, Tetrahedron Lett. 50, 1062 (2009)
R. Yuan, M.Q. Li, X.X. Ren, W. Chen, H. Zhou, Y. Wan, P. Zhang, H. Wu, Res. Chem. Intermed. 46, 2275 (2020)
R. Yuan, H. Cui, W. Chen, X.X. Ren, H. Zhou, H. Xu, Y.W. Sun, Y.N. Liang, Y. Wan, J.J. Liu, H. Wu, Chin. J. Org. Chem. 40, 1017 (2020)
W. Chen, R. Yuan, Y. Fang, H.R. Dong, Y. Bumaryam, L.J. Su, X.X. Ren, H. Zhou, Y. Wan, P. Zhang, S.L. Zhou, W. Hui, Chin. J. Org. Chem. 40, 988 (2020)
X.X. Ren, R. Yuan, W. Chen, H. Zhou, F. Ye, X.Y. Shi, J. Hu, P. Zhang, S.L. Zhou, Y. Wan, H. Wu, Chin. J. Org. Chem. 40 (2020).
R. Yuan, M.Q. Li, H. Zhou, Y.W. Sun, Y.N. Liang, H. Xu, Y. Wan, H. Wu, Tetrahedron Lett. 61, 152388 (2020).
K. Akagawa, N. Nishi, J. Sen, K. Kudo, Org. Biomol. Chem. 12, 3581 (2014)
A.J. Metrano, S.J. Miller, J. Org. Chem. 79, 1542 (2014)
A. Albrecht, A. Skrzyńska, A. Przydacz, Ł Albrecht, Synlett 26, 2679 (2015)
R.J.H. Scanes, O. Grossmann, A. Grossmann, D.R. Spring, Org. Lett. 17, 2462 (2015)
K. Akagawa, J. Satou, K. Kudo, J. Org. Chem. 81, 939 (2016)
A. Ueda, T. Umeno, M. Doi, K. Akagawa, K. Kudo, M. Tanaka, J. Org. Chem. 81, 6343 (2016)
Acknowledgements
We are grateful to the foundation of the “Priority Academic Program Development of Jiangsu Higher Education Institutions” for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, H., Sun, Yw., Xu, Jb. et al. Tröger’s base derivative-catalyzed one-step one-pot synthesis of chromenofuroindoles and naphthofuroindoles. Res Chem Intermed 48, 1763–1772 (2022). https://doi.org/10.1007/s11164-022-04664-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04664-2