Abstract
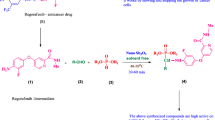
A new class of thiazolyl α-aminophosphonate derivatives was synthesized by one-pot Kabachnik–Fields reaction of ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate with various aryl amines and diethyl phosphite under solvent-free conditions using β-cyclodextrin supported sulfonic acid (β-CD-SO3H) as an efficient, reusable and heterogeneous solid acid catalyst. The products were obtained in good to excellent yields at shorter reaction time. All the title compounds were screened for cytotoxic activity against human breast cancer (MCF-7 and MDA-MB-231), prostate cancer (DU-145) liver cancer (HepG2) and HeLa cancer cell lines using sulfarodamine-B (SRB assay). Compounds (8b, –4OMe), (8h, –4NO2) and (8j, –2I, –4CF3) showed better anticancer activity when compared with standard drug Adriamycin. Further in-silico target hunting reveals the anticancer activity of the designed compounds by inhibiting DNA topoisomerase II.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The structural diversity and biocidal importance of phosphorylated heterocycles have made them attractive scaffolds for the synthesis of pharmacological agents in medicinal chemistry [1,2,3]. In recent years, some of the α-aminophosphonate derivatives containing heterocycle moieties have been synthesized which have shown interesting biological activities. It has been shown that the existence of heterocyclic motifs in the structure of the α-aminophosphonate molecules significantly enhanced their bioactivities [4,5,6,7].
α-Aminophosphonates (APs) are an important class of organophosphorus compounds because of their applications in various fields like catalysis [8] and materials chemistry [9, 10]. Due to the structural similarity of α-aminophosphonates to that of natural α-amino carboxylic acids and tetrahedral configuration at phosphorus [11], they can act as enzyme inhibitors [12], antibiotics [13] herbicides [14], insecticides [15], plant growth regulators [16], antitumor [17], anti-inflammatory [18], antimicrobials [19], antioxidants [20], antivirals [21] and antitubercular agents [22]. Furthermore, the introduction of aminophosphonate group to a pharmacophore is capable of enhancement of the anticancer activity and many of them have demonstrated potent inhibitory activities against various human cancers including human cervical cancer (HeLa), human breast adenocarcinoma (SK-BR-3) [23] human chronic myeloid leukemia (K 562) and human colon carcinoma cells (Colo 205) [24].
In the field of medicinal chemistry, thiazole is an important class of heterocyclic compound which is known to be present in many commercially available products with a wide range of pharmacological activities [25]. Because of its low toxicity, thiazole moiety is a key pharmacophore for the synthesis of several biological molecules like vitamin B1 (thiamine) which helps in the regular functioning of the nervous system by its role in the synthesis of acetylcholine [26]. Furthermore, thiazole derivatives play a key role in the field of medicinal chemistry and normally present in the structure of various natural products and bioactive compounds including antimicrobial [27], anticonvulsant [28], anti-inflammatory [29], antiviral [30], insecticidal [31], antioxidant [32], antihypertensive [33], anticancer [34], analgesic [35], anti-HIV [36], anti-filarial [37], antimalarial [38], anti-leishmanial [39] and enzyme inhibition activities [40]. Thiazole is present in natural products as a subunit in numerous terrestrial and marine compounds with diverse bioactivities that signifies its key role in drug discovery [41, 42]. Apart from biological activities, thiazoles also have applications in liquid crystals, polymers, fluorescent dyes and photo-nucleases and so on [43,44,45].
Molecular docking studies were carried out to understand their possible target-based interactions that led to the anticancer activity of α-aminophosphonates against human Topo IIa. DNA helix and tubulin polymerization inhibitory activity of α-aminophosphonates showed promising antitumor activity. These results encouraged us to design and synthesis novel derivatives of thiazolyl α-aminophosphonates and evaluated their anticancer activity. The design strategy is to club the aminophosphonate and thiazole moieties and generate novel thiazolyl α-aminophosphonates (Fig. 1) [17, 46,47,48,49].
Encouraged by the aforesaid findings and in continuation of our research in the development of new methodologies for the construction of bio-potent heterocyclic α-aminophosphonates, it was thought worthwhile to synthesize some novel thiazolyl α-aminophosphonate derivatives and test their antitumor activities in vitro against several selected tumor cell lines in addition to a study of their structure–activity relationship (SAR) in order to develop new potentially bioactive synthetic drugs. Besides this, molecular docking studies were also carried out against X-ray crystal structure of human type IIA DNA topoisomerase and colchicine binding site at α/β-tubuline interface by using AutoDock 4.2.
Results and discussion
Chemistry
The synthesis of thiazolyl α-aminophosphonate derivatives (8a–8j) has been accomplished by a two-step process. Initially, the cyclization of 4-hydroxythiobenzamide (1) with 2-bromoacetoacetic acid ethyl ester (2) in refluxing ethanol provides 2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester (3), which is formylated by reaction with hexamethylenetetramine (HMTA) and trifluoroacetic acid (TFA) in hot HOAc/water to afford 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester (4) [50]. Alkylation of (4) with isobutyl bromide, K2CO3 and KI in DMF gives ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (5) as starting compound (Scheme 1) [51]. The structure elucidation of compound (5) was based on the full sets of IR, 1H NMR, 13C NMR and mass spectral data.
At the outset, an improved one-pot, three-component Kabachnik–Fields reaction of the starting compound ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (5) with diversely substituted aromatic amines (6a–6j) and diethyl phosphite (7) was taken as a typical reaction and run it in the presence of β‐CD‐SO3H under solvent-free conditions for about 30 min at 50 °C. The desired products (8a–8j) were obtained in good to excellent (90–96%) yields (Scheme 2).
To accomplish optimum reaction conditions for the synthesis of thiazolyl α-aminophosphonates (8a–8j), the model reaction was carried out by taking ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (5), aniline (6a) and diethyl phosphite (7) as reactants under catalyst and solvent-free conditions at r.t. Even after heating the reaction mixture for about 24 h, the desired product was not obtained (Table 1, entry 1). Subsequently, the reaction was conducted in the presence of various catalysts like FeCl3, AlCl3, LaCl3, ZnCl2, NiCl2, CuCl2, CuBr2, BF3.SiO2, Fe3O4, TiO2 and β‐CD‐SO3H in the absence of solvent (Table 1, Entries 2–12). Remarkably, the model reaction showed excellent product yields (95%) within 30 min reaction time in the presence of β‐CD‐SO3H as catalyst under solvent-free conditions at 50 °C (Table 1, entry 12). Later, the solvent effect on the reaction was also studied by running the reaction with various protic and non-protic solvents like methanol, ethanol, THF, DCM, DMF and toluene (Table 1, Entry 13–18) and also in solvent-free conditions. To our delight, high product (8a) yield was obtained in solvent-free conditions with β‐CD‐SO3H as catalyst.

After establishment of the optimal reaction conditions, we investigated the scope of the reaction by condensing ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (5) with various commercially available substituted aryl amines having different electronically activating or deactivating substituents (6a–6j) and diethyl phosphite (7) to obtain corresponding thiazolyl α-aminophosphonates (8a–8j). The details of physical data like yield and melting points are illustrated in (Table 2).
Formation of α-aminophosphonates (8a–8j) in the presence of β‐CD‐SO3H as catalyst involves nucleophilic amine addition to the electrophilic carbonyl carbon of the aldehyde with simultaneous elimination of water from it. The β‐CD‐SO3H (a) as catalyst initially protonates the carbonyl oxygen of the aldehyde (b) and renders the carbon more electrophilic and this factor facilitates the amine NH2 (c) nucleophilic attack on the carbonyl carbon to form the imine intermediate (e). Nucleophile [P] of phosphite (f) attacks the electrophilic C of the imine intermediate (e) leading to the formation of C–P bond. Abstraction of the proton from phosphonate (f) by sulfonate anion of the catalyst (g) makes the P of the phosphonate (f) more nucleophile and accelerates P–C bond formation leading to α-aminophosphonates (h) (Scheme 3).
The title compounds (8a–8j) were characterized by physical and spectral (IR, NMR and Mass) data. They showed strong IR absorption bands in the region of 3296–3260 cm−1, 1710–1690, 1258–1228 cm−1, 1026–1012 cm−1 and 760–730 cm−1 for –NH, –C=O, –P=O, –P–O–C and P–C aliphatic stretching frequencies, respectively. In 1H NMR spectra, the chemical shifts in the region of 8.50–6.10 ppm are due to aromatic protons and the doublet at 5.50–5.10 ppm corresponds to HC–P proton. The singlet at δ 5.00–4.82 ppm confirmed the presence of –NH proton. The multiplet in the region of 4.40–3.60 ppm is due to the –O–CH2–CH3 protons. The singlet in the region of 2.80–2.60 ppm and a multiplet in the region 2.30–2.10 ppm are due to the –CH3 and –CH protons, respectively. Two triplets in the region of 1.40–1.20 ppm confirmed the presence of –O–CH2–CH3 protons. The doublet and triplet in the range of 1.14–1.12 and 1.10–1.00 ppm confirmed the presence of –CH3 protons. In 13C NMR spectra, the chemical shifts in the region of 184.50–110.00 ppm are assigned to carbons of aromatic ring; the signals in the region of 64.30–58.00, 52.00–46.00, 29.00–26.00, 19.60–19.20, 16.70–16.10 and 14.60–14.10 ppm confirmed the presence of –OCH2–CH3, HC–P, –CH–, (CH3)2, –OCH2–CH3 and –CH3 carbons. The 31P NMR chemical shifts of the title compounds appeared in the range of 22.00 to 24.80 ppm.
Pharmacology
In vitro anticancer activity
The synthesized thiazolyl α-aminophosphonates (8a–8j) were tested against human breast (MCF-7 and MDA-MB-231), prostate cancer (DU-145) liver cancer (HepG2) and HeLa cancer cell lines sulforhodamine-B (SRB) cytotoxic assay [52] to investigate the effectiveness of the in vitro cell cytotoxic properties (Table 3). All the data were expressed as IC50 values. The obtained data revealed that the compound 8h with nitro substitution exhibited highest cell growth inhibitory effects on MCF-7, MDA-MB-231, DU-145 HepG2 and HeLa cell lines with IC50 values when compared to that of IC50 of the Adriamycin standard used. Compound 8b with 4-OMe and compound 8j having 2I, 4-CF3has exhibited excellent growth inhibitory effects on all the cell lines. While compounds 8d, 8c, 8a and 8g showed moderate cytotoxic activity remaining compounds 8e, 8f and 8i could not show effective cytotoxic activity on all the five cell lines (Fig. 2).
Anticancer activities of tested compounds (quantified version of Table 3)
Molecular docking studies against DNA topoisomerase II
All the synthesized compounds were docked with X-ray crystal structure of tubulin inhibitor (PDB ID: 3E22), and human type IIA DNA topoisomerase (PDB: 1ZXM), using AutoDock, protocol reported in earlier communications [53,54,55,56,57,58,59]. Human type IIA DNA topoisomerase tremendously expressed cancer proliferating cells and plays an important function in cellular processes such as replication, transcription, chromatin assembly. In order to understand the possible mechanism of action, molecular docking studies were carried out against X-ray crystal structure of Topo IIa (DNA gyrase, PDB: 1ZXM) [60]. All the synthesized compounds completely occupied the active site residue (Leu89, ALA93, Arg98, Ala92, Cys104, Lys96, Trp119, Tyr214 and Val90) of human Topo IIa and shown excellent fee energy of binding from − 7.08 to − 8.69 kcal/mole with good inhibitory constant up to ki = 0.42 µM (Table 4). The active compound 8b the methoxy oxygen interacts with terminal amino hydrogen of Lys157 (bond distance 2.10 Å), with free binding energy − 7.55 kcal/mole, while in the compound 8h, both the oxygen of nitro group establish two hydrogen bonds one with terminal amine hydrogen of Lys168 (bond distance 2.08 Å), another with amine hydrogen of Ala167 (bond distance 1.97 Å), with free binding energy of binding − 7.28kacl/mole (Fig. 3).
Molecular docking with colchicine binding site
Tubulin is recognized as an important target for anticancer drug development. All the synthesized compounds interacted with both α, β interface of tubulin [61,62,63] in the colchicine binding pocket, i.e., αAsn101, αSer178, αVal181, αThr179, βLys352, βThr353, βLeu248, βGln247 and exhibit significant fee energy of binding from − 7.24 to − 7.88 kcal/mole with good inhibitory constant up to Ki = 1.67 µM (Table 4). Compound 8h oxygen of nitro group established a hydrogen bond with amine hydrogen of Cys352, i.e., bond distance 1.78 Å with free binding energy of binding-7.28kacl/mole, while compound 8j, the sulfur of thiazole ring established one hydrogen bond with carbonyl oxygen of Thr353, i.e., bond distance of 2.95 Å, with free binding energy of binding-7.34kacl/mole (Fig. 4).
The reason behind the cytotoxic activity of compounds (8b, 8h and 8j) may be due to the additional hydrogen bonding with the active site residue in the target, and we are not trying to correlate our observation with the experimental results as other modes of action are possible for this type of chemical scaffold. This study has clearly shown that the compounds are well accommodated in the active site of DNA topoisomerase II and colchicine binding site in tubulin protein, though activity can be ascertained only through in vitro enzyme-based assay methodology.
Structure activity relation (SAR) studies
The SAR studies related to the in vitro cytotoxicity of the title compounds (8a–8j) against MCF-7, MDA-MB-231, DU-145, HepG2 and HeLa cancer cell lines revealed that though the basic skeletal structure of title compounds remains same among all the compounds, diverse groups on the phenyl ring substituted at the α-carbon of the phosphonates shows varying percent of inhibition among all the cancer cell lines. Furthermore, the same phosphonate is not continuously active on the cancer cell lines. This shows that not only the basic skeleton structure of compounds, but also its substituent groups are the deciding factor for the activity of a certain compound. Amongst all, the compound 8h (R = 4–NO2) exerts extremely higher percentage of inhibition among all the five cancer cell lines than the standard Adriamycin. After that, compounds 8b (R = 4–OMe) and 8j (R = 2I, 4-CF3) exhibit higher percentage of inhibition on five cell lines, respectively. The results of the docking studies are also in support with the cytotoxic studies having good binding energies. Thus, these compounds form a colony of novel cytotoxic lead molecules and by further fine tuning their structure with respect to bioactivity, they may offer a new generation of potential and safe cytotoxic compounds.
Conclusion
In summary, we have developed an efficient and eco-friendly protocol for the synthesis of thiazolyl α-aminophosphonate derivatives through the β‐CD‐SO3H catalyzed reaction of an aldehyde with various aryl amines and diethyl phosphite. This new method is endowed with green reaction conditions such as low cost, use of non-toxic catalyst, solvent-free medium, easy work-up process and good yields. The title compounds were screened for anticancer activity against human breast (MCF-7 and MDA-MB-231), prostate cancer (DU-145) liver (HepG2) and HeLa cancer cell lines with sulfarodamine-B (SRB) assay. The compounds 8b, 8g and 8j showed better anticancer activity when compared with the standard drug Adriamycin. In-silico target hunting reveals the anticancer activity of the designed compounds by inhibiting DNA topoisomerase II and tubulin polymerase inhibition. The design strategy provides a base for evaluating this class of molecules for their anticancer activity and also be utilized to rationale design and synthesis of a new library of compounds for anticancer activity.
Experimental
Analysis and instruments
All the required chemicals were purchased from Sigma-Aldrich and the solvents from Merck and were used without further purification. The completion and purity of the reactions were monitored by TLC, performed on silica gel aluminum 60 F-254 thin layer plates procured from Merck, and visualization on TLC was achieved by UV light and iodine indicator. Melting points of the compounds were determined on Guna digital melting point apparatus using open capillary tubes and are uncorrected. Infrared spectra were recorded on FT-IR Bruker ALPHA Interferometer and wave numbers are given in cm−1. NMR spectra were recorded on a Bruker instrument operating at 400 MHz for 1H, 100 MHz for 13C and 161.9 MHz for 31P in CDCl3. TMS was used as an internal standard. Assignments of the signals are based on the chemical shifts and intensity patterns. Chemical shift (δ) and coupling constant (J) were expressed in ppm and Hertz, respectively. Mass spectra were recorded on a LC–MS/MS-TOF API QSTAR PULSAR spectrometer; samples were introduced by the infusion method using the electrospray ionization technique (ESI).
General procedure for synthesis of the starting compound ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (5)
Initially, 4-hydroxythiobenzamide (1) 2-bromoacetoacetic acid ethyl ester (2) and 10 ml of ethanol were taken in a round-bottomed flask and run the reaction under refluction conditions for about 2 h to get 2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester (3), which is formylated by reaction with hexamethylenetetramine (HMTA) and trifluoroacetic acid (TFA) in hot HOAc/water for about 2.5 h to afford 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester (4). Finally, alkylation of (4) with isobutyl bromide, in the presence of K2CO3 and KI in DMF, gives ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (5) as starting compound.
Ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (5)
White solid; Yield: 90%, M.p. 253–255 °C; FT-IR (neat, cm−1): ν 2979 (–C–H), 1704 (ester -C=O), 1696 (aldehydic –C=O). 1H-NMR (400 MHz, CDCl3, ppm): δ 10.52 (s, 1H, H–C=O), 8.35 (s, 1H, Ar–H), 8.19 (d, J = 8.0 Hz, 1H, Ar–H), 7.04 (d, J = 8.0 Hz, 1H, Ar–H) 4.36–4.31 (m, 2H –OCH2), 3.91 (d, J = 8.0 Hz, 2H, –OCH2), 2.75 (s, 3H, –CH3), 2.22–2.16 (m 1H, –CH), 1.37 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 3H), 1.06 (d, J = 8.0 Hz, 6H, –CH3). 13C-NMR (100 MHz, CDCl3, ppm): δ 188.88, 168.56, 163.29, 162.32, 161.12, 133.87, 127.13, 125.94, 125.08, 121.65, 113.18, 75.27, 61.32, 28.36, 19.25, 17.57, 14.41. HRMS (ESI)+ calcd. for C18H21NO4S [M + H]+: 348.1225 and found 348.1221.
General procedure for synthesis of thiazolyl α-aminophosphonates (8a–8j)
Ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (5) (1 mol), various heteroaryl amines (6a–6j) (1 mol), diethyl phosphite (7) (1.5 mmol) and 7.5 mol% of β‐CD‐SO3H were taken in a round-bottomed flask and the reaction was run under neat conditions at for about 30 min. The progress of the reaction was monitored by TLC (3:2; n-hexane/ethyl acetate) for every 10 min. After completion of reaction, the mixture was dissolved in 10 mL of DCM and filtered to remove the catalyst as residue. The organic layer was washed with water (2 × 5 mL) and the water layer was discarded. The combined organic mixture was washed with brine solution (5 mL), dried over anhydrous Na2SO4 and concentrated under reduced pressure. The solids obtained were washed with cold water, air-dried and recrystallized from ethanol to get the pure compounds.
Ethyl 2-(3-((diethoxyphosphoryl)(phenylamino)methyl)-4-isobutoxyphenyl)-4-methyl thiazole-5-carboxylate (8a)
White solid; Yield: 96%, M.p. 235–237 °C. FT-IR (neat, cm−1) ν 3290 (–NH) 2967 (–C–H), 1702 (–C=O), 1246 (–P=O), 1019 (–P–O–C), 749 (–P–C–). 1H-NMR (400 MHz, CDCl3, ppm): δ 8.04 (s, 1H, Ar–H), 7.85 (d, J = 8.0 Hz, 1H, Ar–H), 7.09 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 2H, Ar–H), 6.91(d, J = 8.0 Hz, 1H, Ar–H), 6.67 (d, J = 4.0 Hz, 2H, Ar–H), 6.63 (d, J = 8.0 Hz, 2H, Ar–H), 5.41 (d, J = 24.0 Hz, 1H, P–CH), 4.93 (s, 1H, –NH), 4.34–4.28 (dd, J = 8.0 Hz, 2H, –OCH2), 4.18–3.67 (m, 6H –OCH2), 2.72 (s, 3H, –CH3), 2.25–2.17 (m 1H, –CH), 1.35 (t, J1 = 8.0 Hz, J2 = 4.0 Hz 3H), 1.29 (t, J1 = 8.0 Hz, J2 = 4.0 Hz 3H, –OCH3), 1.12 (d, J = 8.0 Hz, 6H, –CH3) 1.05 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 3H, –CH3). 13C-NMR (100 MHz, CDCl3, ppm): δ 169.68, 162.45, 161.02, 159.00 (d, JP–C = 6.0 Hz), 153.75, 146.27 (d, JP–C = 14.0 Hz), 129.25, 127.83 (d, JP–C = 2.0 Hz), 127.21 (d, JP–C = 5.0 Hz), 125.95, 120.98, 113.57 (d, JP–C = 5.0 Hz), 111.39, 75.06, 63.26 (dd, J1P–C = 2.0 Hz, J2P–C = 2.0 Hz), 61.17, 55.75, 48.08 (d, JP–C = 153.0 Hz), 28.52, 19.44 (d, JP–C = 3.0 Hz), 17.54, 16.53 (d, JP–C = 5.0 Hz), 16.24 (d, JP–C = 5.0 Hz), 14.41. 31P-NMR (162 MHz, CDCl3, ppm): δ 23.5. HRMS (ESI)+ calcd. for C28H37N2O6PS [M + H]+: 561.2143 and found 561.2140.
Ethyl 2-(3-((diethoxyphosphoryl)((4-methoxyphenyl)amino)methyl)-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (8b)
Brown solid; Yield: 96%, M.p. 255–257 °C. FT-IR (neat, cm−1) ν 3288 (–NH) 2928 (–C–H), 1703 (–C=O), 1249 (–P=O), 1025 (-P-O-C), 751 (–P–C–). 1H-NMR (400 MHz, CDCl3, ppm): δ 8.03 (s, 1H, Ar–H), 7.85 (d, J = 8.0 Hz, 1H, Ar–H), 6.90 (d, J = 8.0 Hz, 1H, Ar–H), 6.67 (d, J = 8.0 Hz, 2H, Ar–H), 6.58 (d, J = 8.0 Hz, 2H, Ar–H), 5.33 (d, J = 28.0 Hz, 1H, P–CH), 4.93 (s, 1H, –NH), 4.33–4.28 (dd, J = 8.0 Hz, 2H, –OCH2), 4.18–3.69 (m, 6H –OCH2), 3.66 (s, 3H, –OCH3), 2.72 (s, 3H, –CH3), 2.24–2.18 (m 1H, –CH), 1.35 (t, J1 = 8.0 Hz, J2 = 4.0 Hz 3H), 1.29 (t, J1 = 8.0 Hz, J2 = 4.0 Hz 3H, –OCH3), 1.12 (d, J = 8.0 Hz, 6H, –CH3) 1.05 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 3H, –CH3). 13C-NMR (100 MHz, CDCl3, ppm): δ 169.71, 162.44, 160.99, 159.07 (d, JP–C = 6.0 Hz), 152.74, 140.33 (d, JP–C = 15.0 Hz), 127.75 (d, JP–C = 2.0 Hz), 127.23 (d, JP–C = 5.0 Hz), 120.95, 114.87 (d, JP–C = 5.0 Hz), 111.38, 75.04, 63.20 (dd, J1P–C = 15.0 Hz, J2P–C = 14.0 Hz), 61.17, 55.75, 48.95 (d, JP–C = 152.0 Hz), 28.51, 19.44 (d, JP–C = 3.0 Hz), 17.53, 16.54 (d, JP–C = 6.0 Hz), 16.26 (d, JP–C = 6.0 Hz), 14.41. 31P-NMR (162 MHz, CDCl3, ppm): δ 23.68. HRMS (ESI)+ calcd. for C29H39N2O7PS [M + H]+: 591.2249 and found 591.2246.
Ethyl 2-(3-((diethoxyphosphoryl)((4-(phenylthio)phenyl)amino) methyl)-4-isobutoxy phenyl) -4-methylthiazole-5-carboxylate (8c)
White solid; Yield: 96%, M.p. 286–288 °C. FT-IR (neat, cm−1) ν 3275 (–NH) 2967 (–C–H), 1699 (–C=O), 1245 (–P=O), 1023 (–P–O–C), 764 (–P–C–). 1H-NMR (400 MHz, CDCl3, ppm): δ 7.88 (d, J = 8.0 Hz, 1H, Ar–H), 7.75 (s, 1H, Ar–H), 7.49 (d, J = 8.0 Hz, 1H, Ar–H), 7.18–7.06 (m, 6H Ar–H) 6.91 (d, J = 8.0 Hz, 1H, Ar–H) 6.69 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 1H, Ar–H) 6.54 (d, J = 8.0 Hz, 1H, Ar–H), 6.14 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 1H, Ar–H), 5.43–5.35 (d, J = 32.0 Hz, 1H, –P–C–H), 4.96 (s, 1H, –NH), 4.38–4.33 (dd, J = 8.0 Hz, 2H, –OCH2), 4.02–3.68 (m, 6H –OCH2), 2.73 (s, 3H, –CH3), 2.26–2.19 (m 1H, –CH), 1.40 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 3H), 1.18–1.12 (m, 9H, –OCH3, –CH3), 1.05 (t, J1 = 8.0 Hz, J2 = 8.0 Hz, 3H, –OCH3) ppm; 13C-NMR (100 MHz, CDCl3): δ 169.65, 162.52, 161.05, 158.89 (d, JP–C = 6.0 Hz), 157.96, 147.83 (d, JP–C = 14.0 Hz), 137.76, 136.78, 131.30, 129.06, 127.78 (d, JP–C = 4.0 Hz), 127.07 (d, JP–C = 8.0 Hz), 125.60, 120.97, 118.21, 115.64, 111.36 (d, JP–C = 10.0 Hz), 75.07, 63.23 (dd, J1P–C = 15.0 Hz, J2P–C = 14.0 Hz), 61.18, 48.11 (d, JP–C = 152.0 Hz), 28.50, 19.46 (d, JP–C = 2.0 Hz), 17.59, 16.47 (d, JP–C = 6.0 Hz), 16.26 (d, JP–C = 6.0 Hz), 14.48. 31P-NMR (162 MHz, CDCl3): δ 22.28. HRMS (ESI)+ calcd. for C34H41N2O6PS2 [M + H]+: 669.2177 and found 669.2174.
Ethyl 2-(3-((diethoxyphosphoryl)((4-fluorophenyl)amino)methyl)-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (8d)
White solid; Yield: 94%, M.p. 242–244 °C. FT-IR (neat, cm−1) ν 3279 (–NH), 2965 (–C–H), 1700 (–C = O), 1248 (–P=O), 1026 (–P–O–C), 764 (–P–C–). 1H-NMR (400 MHz, CDCl3, ppm): δ 8.02 (s, 1H, Ar–H), 7.86 (d, J = 8.0 Hz, 1H, Ar–H), 6.91 (d, J = 8.0 Hz, 1H, Ar–H), 6.79 (t, J1 = 8.0 Hz, J2 = 8.0 Hz, 2H, Ar–H), 6.56–6.53 (m, 2H, Ar–H), 5.32 (d, J = 28.0 Hz, 1H, P–C-H), 4.80 (s, 1H, –NH), 4.36–3.64 (m, 8H –OCH2), 2.72 (s, 3H, –CH3), 2.24–2.15 (m 1H, –CH), 1.36 (t, J1 = 8.0 Hz, J2 = 8.0 Hz, 3H), 1.30 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 3H, –OCH3), 1.11 (d, J = 4.0 Hz, 6H, –CH3) 1.06 (t, J1 = 8.0 Hz, J2 = 4.0 Hz 3H, –CH3). 13C-NMR (100 MHz, CDCl3, ppm): δ 169.58, 162.44, 161.05, 159.03, 155.19, 133.89, 127.87, 127.13, 126.03, 125.62, 125.07, 122.57, 121.03, 116.08, 115.61, 114.50 (d, JP–C = 7.0 Hz), 113.19, 111.45, 75.07, 63.26 (dd, J1P–C = 3.0 Hz, J2P–C = 3.0 Hz), 48.45 (d, JP–C = 204.0 Hz), 28.50, 19.44 (d, JP–C = 3.0 Hz), 19.25, 17.56, 16.54 (d, JP–C = 6.0 Hz), 16.26 (d, JP–C = 7.0 Hz), 14.41. 31P-NMR (162 MHz, CDCl3, ppm): δ 23.42. HRMS (ESI)+ calcd. for C28H36FN2O6PS [M + H]+: 579.2049 and found 579.2046.
Ethyl 2-(3-(((3-chlorophenyl)amino)(diethoxyphosphoryl)methyl)-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (8e)
White solid; Yield: 92%, M.p. 247–249 °C. FT-IR (neat, cm−1) ν 3284 (–NH) 2966 (–C–H), 1703 (–C=O), 1245 (–P=O), 1020 (–P–O–C), 755 (–P–C–). 1H-NMR (400 MHz, CDCl3, ppm): δ 8.12 (s, 1H, Ar–H), 7.99 (d, J = 8.0 Hz, 1H, Ar–H), 7.77 (d, J = 8.0 Hz, 2H, Ar–H), 7.34 (s, 1H, Ar–H), 7.25 (t, J1 = 12.0 Hz, J2 = 16.0 Hz 1H, Ar–H) 7.20 (s, 1H, Ar–H), 6.90 (d, J = 16.0 Hz, 1H, Ar–H), 5.31 (d, J = 32.0 Hz, 1H, P–C-H), 4.83 (s, 1H, –NH), 4.23–3.50 (m, 8H –OCH2), 2.61 (s, 3H, –CH3), 2.24–2.09 (m, 1H, –CH), 1.27–1.04 (m, 15H, –OCH3, –CH3). 13C-NMR (100 MHz, CDCl3, ppm): δ 169.32, 162.13, 160.69, 158.64, 154.05, 145.81 (d, JP–C = 19.0 Hz),, 129.81, 128.89 (d, JP–C = 26.0 Hz), 127.37, 126.52, 126.01, 124.74, 123.42, 121.34, 111.68 75.23, 64.07 (d, JP–C = 10.0 Hz), 63.87 (d, JP–C = 10.0 Hz), 58.26, 47.10 (d, JP–C = 210.0 Hz), 28.35, 19.34 (d, JP–C = 10.0 Hz), 16.41 (d, JP–C = 7.0 Hz), 16.16 (d, JP–C = 8.0 Hz), 14.30. 31P-NMR (162 MHz, CDCl3, ppm): δ 21.59. HRMS (ESI)+ calcd. for C28H36ClN2O6PS [M + 2H]+: 596.1691 and found 596.1688.
Ethyl 2-(3-(((4-bromophenyl)amino)(diethoxyphosphoryl)methyl)-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (8f)
White solid; Yield: 90%, M.p. 275–277 °C. FT-IR (neat, cm−1) ν 3286 (–NH) 2964 (–C–H), 1701 (–C=O), 1248 (–P=O), 1025 (–P–O–C), 756 (–P–C–). 1H-NMR (400 MHz, CDCl3, ppm): δ 8.00 (s, 1H, Ar–H), 7.84 (d, J = 8.0 Hz, 1H, Ar–H), 6.90 (d, J = 8.0 Hz, 1H, Ar–H), 6.76 (t, J1 = 8.0 Hz, J2 = 8.0 Hz, 2H, Ar–H), 6.58–6.50 (m, 2H, Ar–H), 5.34 (d, J = 32.0 Hz, 1H, P–C-H), 4.86 (s, 1H, –NH), 4.34–3.62 (m, 8H –OCH2), 2.74 (s, 3H, –CH3), 2.26–2.14 (m 1H, –CH), 1.34 (t, J1 = 8.0 Hz, J2 = 8.0 Hz, 3H), 1.33 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 3H, –OCH3), 1.10 (d, J = 4.0 Hz, 6H, –CH3) 1.02 (t, J1 = 8.0 Hz, J2 = 4.0 Hz 3H, –CH3). 13C-NMR (100 MHz, CDCl3, ppm): δ 169.52, 162.45, 161.02, 159.01, 155.13, 133.80, 127.78, 127.31, 126.04, 125.68, 125.00, 122.55, 121.09, 116.02, 115.58, 114.52 (d, JP–C = 6.0 Hz), 113.10, 111.49, 75.02, 63.28 (d d, J1P–C = 7.0 Hz, J2P–C = 7.0 Hz), 48.48 (d, JP–C = 158.0 Hz), 28.58, 19.46 (d, JP–C = 6.0 Hz), 19.26, 17.62, 16.56 (d, JP–C = 6.0 Hz), 16.23 (d, JP–C = 7.0 Hz), 14.56. 31P-NMR (162 MHz, CDCl3, ppm): δ 22.1. HRMS (ESI)+ calcd. for C28H36BrN2O6PS [M + 2H]+: 640.1195 and found 640.1192.
Ethyl 2-(3-((diethoxyphosphoryl)((2-iodophenyl)amino)methyl)-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (8g)
Brown solid; Yield: 91%, M.p. 290–292 °C. FT-IR (neat, cm−1) ν 3286 (–NH) 2927 (–C–H), 1702 (–C=O), 1246 (–P=O), 1024 (–P–O–C), 751 (–P–C). 1H-NMR (400 MHz, CDCl3, ppm): δ 7.98 (s, 1H, Ar–H), 7.91 (d, J = 8.0 Hz, 1H, Ar–H 1H, Ar–H), 7.65 (dd, J1 = 4.0 Hz, J2 = 4.0 Hz 1H, Ar–H), 7.05 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 1H, Ar–H), 6.94 (d, J = 8.0 Hz, 1H, Ar–H) 6.43 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 1H, Ar–H), 5.44 (d, J = 32.0 Hz, 1H, P–C-H), 4.98 (s, 1H, –NH), 4.33 (dd, J1 = 8.0 Hz, J2 = 8.0 Hz, 1H, –OCH2), 4.24–3.78 (m, 6H –OCH2), 2.73 (s, 3H, –CH3), 2.28–2.19 (m 1H, –CH), 1.38 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 3H, –OCH3), 1.33 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 3H, –OCH3), 1.15 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 3H, –OCH3), 1.12 (d, J = 4.0 Hz, 6H, –CH3). 13C-NMR (100 MHz, CDCl3, ppm): δ 169.53, 162.43, 160.96, 158.89 (d, JP–C = 6.0 Hz),, 145.78 (d, JP–C = 14.0 Hz), 139.11, 129.26, 127.80 (d, JP–C = 3.0 Hz), 127.06 (d, JP–C = 4.0 Hz), 126.02 (d, JP–C = 3.0 Hz), 125.25, 121.04, 119.83, 111.67,86.17, 75.03, 63.34 (dd, J1P–C = 7.0 Hz, J2P–C = 7.0 Hz), 61.11, 48.95 (d, JP–C = 154.0 Hz), 28.43, 21.32, 19.38 (d, JP–C = 3.0 Hz), 17.52, 16.54 (d, JP–C = 6.0 Hz), 16.29 (d, JP–C = 6.0 Hz), 14.36. 31P-NMR (162 MHz, CDCl3, ppm): δ 22.35. HRMS (ESI)+ calcd. for C28H36IN2O6PS [M + H]+: 687.1110 and found 687.1107.
Ethyl 2-(3-((diethoxyphosphoryl)((4-nitrophenyl)amino)methyl)-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (8h)
White solid; Yield: 92%, M.p. 288–290 °C. FT-IR (neat, cm−1) ν 3288 (–NH) 2920 (–C–H), 1708 (–C=O), 1245 (–P=O), 1024 (–P–O–C), 753 (–P–C–). 1H-NMR (400 MHz, CDCl3, ppm): δ 8.04 (s, 1H, Ar–H), 7.87 (d, J = 4.0 Hz, 1H, Ar–H), 6.93 (d, J = 8.0 Hz, 2H, Ar–H), 6.80 (t, J1 = 16.0 Hz, J2 = 16.0 Hz, 2H, Ar–H), 6.57 (t, J1 = 16.0 Hz, J2 = 16.0 Hz, 2H, Ar–H), 5.35 (d, J = 24.0 Hz, 1H, P–C-H), 4.82 (s, 1H, –NH), 4.40–3.70 (m, 8H –OCH2), 2.73 (s, 3H, –CH3), 2.24–2.18 (m 1H, –CH), 1.43–1.00 (m, 15H OCH3 & –CH3). 13C-NMR (100 MHz, CDCl3, ppm): δ 169.58, 168.58, 165.78, 163.30, 162.44, 159.03, 157.54, 155.19, 149.94, 138.07, 133.89, 130.46, 127.87, 127.13, 126.03, 125.62, 125.07, 122.52 (d, JP–C = 9.0 Hz), 121.03, 116.08, 115.83, 114.54, 113.19, 75.07, 63.30 (d, JP–C = 3.0 Hz), 63.23 (d, JP–C = 3.0 Hz), 48.70 (d, JP–C = 153.0 Hz), 28.50, 19.43 (d, JP–C = 3.0 Hz), 17.56, 16.54 (d, JP–C = 6.0 Hz), 16.25 (d, JP–C = 4.0 Hz), 14.41. 31P-NMR (162 MHz, CDCl3): δ 23.53. HRMS (ESI)+ calcd. for C28H36N3O8PS [M + H]+: 606.1994 and found 606.1991.
Ethyl2-(3-((diethoxyphosphoryl)((4-(trifluoromethyl)phenyl)amino) methyl)-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (8i)
Brown solid; Yield: 95%, M.p. 276–278 °C. FT-IR (neat, cm−1) ν 3284 (–NH) 2967(–C–H), 1699 (–C=O), 1248 (–P=O), 1023 (–P–O–C), 746 (–P–C–). 1H-NMR (400 MHz, CDCl3, ppm): δ 7.96 (s, 1H, Ar–H), 7.82 (d, J = 8.0 Hz, 1H, Ar–H), 7.34 (d, J = 8.0 Hz, 2H, Ar–H), 6.90 (d, J = 8.0 Hz, 1H, Ar–H), 6.56 (d, J = 8.0 Hz, 2H, Ar–H), 5.26 (d, J = 36.0 Hz, 1H, P–CH), 4.89 (s, 1H, –NH), 4.32–4.26 (dd, J = 8.0 Hz, 2H, –OCH2), 4.16–3.68 (m, 6H –OCH2), 3.62 (s, 3H, –OCH3), 2.78 (s, 3H, –CH3), 2.26–2.16 (m 1H, –CH), 1.33 (t, J1 = 8.0 Hz, J2 = 4.0 Hz 3H), 1.27 (t, J1 = 8.0 Hz, J2 = 4.0 Hz 3H, –OCH3), 1.10 (d, J = 8.0 Hz, 6H, –CH3) 1.02 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 3H, –CH3). 13C-NMR (100 MHz, CDCl3, ppm): δ 169.78, 162.38, 160.96, 159.06 (d, JP–C = 7.0 Hz), 152.72, 140.34 (d, JP–C = 17.0 Hz), 127.76 (d, JP–C = 2.0 Hz), 127.24, 124.46 (d, JP–C = 17.0 Hz), 123.19 (d, JP–C = 15.0 Hz), 120.95, 116.33 (d, JP–C = 7.0 Hz), 111.36, 75.10, 63.24 (dd, J1P–C = 14.0 Hz, J2P–C = 14.0 Hz), 61.16, 55.74, 48.98 (d, JP–C = 136.0 Hz), 28.50, 19.46 (d, JP–C = 7.0 Hz), 17.52, 16.58 (d, JP–C = 6.0 Hz), 16.28 (d, JP–C = 6.0 Hz), 14.61. 31P-NMR (162 MHz, CDCl3, ppm): δ 22.87. HRMS (ESI)+ calcd. for C29H36F3N2O6PS [M + H]+: 629.2017 and found 629.2014.
Ethyl 2-(3-((diethoxyphosphoryl)((2-iodo-4-(trifluoromethyl)phenyl)amino)methyl)-4-iso butoxyphenyl)-4-methylthiazole-5-carboxylate (8j)
White solid; Yield: 94%, M.p. 288–290 °C. FT-IR (neat, cm−1) ν 3290 (–NH) 2980 (–C–H), 1702 (–C=O), 1256 (–P=O), 1028 (-P-O-C), 756 (–P–C–). 1H-NMR (400 MHz, CDCl3, ppm): δ 7.84 (s, 1H, Ar–H), 7.76 (s, 1H, Ar–H), 7.63 (d, J = 8.0 Hz, 1H, Ar–H), 7.38 (d, J = 8.0 Hz, 1H, Ar–H), 7.12 (d, J = 8.0 Hz, 1H, Ar–H), 6.74 (d, J = 8.0 Hz, 1H, Ar–H), 5.24 (d, J = 28.0 Hz, 1H, P–C–H), 4.98 (s, 1H, –NH), 4.38 (d, J = 8.0 Hz, 1H, –OCH2), 4.18–3.86 (m, 6H –OCH2), 2.50 (s, 3H, –CH3), 2.48–2.30 (m 1H, –CH), 1.36 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 3H), 1.24 (t, J1 = 8.0 Hz, J2 = 8.0 Hz 3H, –OCH3), 1.02 (d, J1 = 12.0 Hz, 6H, –CH3). 13C-NMR (100 MHz, CDCl3, ppm): δ 171.36, 165.66, 159.24, 150.51, 149.92, 138.40, 135.96 (d, JP–C = 12.0 Hz), 130.44, 129.66 (d, JP–C = 12.0 Hz), 126.48, 124.14, 122.18, 117.32, 113.39 (d, JP–C = 10.0 Hz), 106.10, 75.94, 74.48, 64.12 (t, J1P–C = 11.0 Hz, J2P–C = 10.0 Hz), 61.56, 26.89, 19.76, 16.33 (d, JP–C = 13.0 Hz), 14.68. 31P-NMR (162 MHz, CDCl3, ppm): δ 22.86. HRMS (ESI)+ calcd. for C29H35F3IN2O6PS [M + H]+: 755.0984 and found 755.0981.
In vitro anticancer assay
The in vitro cytotoxicity of the title compounds (8a–8j) was tested against proliferation of human breast (MCF-7 and MDA-MB-231), prostate (DU-145) liver (HepG2) and HeLa cancer cell lines by performing sulforhodamine-B (SRB) assay [43]. The cell line of interest was seeded in disinfected flat-bottom 96-well plate (5000 cells/100 µL) in a medium containing 10% fetal bovine serum and antibiotics (penicillin and streptomycin). After incubation for about 18–20 h in an incubator continuously supplied with 5% CO2 to ensure appropriate adherence of the cells to the surface bottom of the wells, cells were treated with the compounds or reference standard Adriamycin. To treat cells, working dilutions of concentration of the compounds were prepared, of which 2 µL aliquot was added to every well, thereby creating the final concentration of compound 0 to 100 µM. Each compound was tested in triplicate and the cytotoxicity was determined as the average of that triplicate. DMSO and Adriamycin (as standard control anticancer drug) were taken as vehicle and positive controls, respectively. Further, the cells were allowed to grow for another 48 h in an incubator maintained at 37 °C with a constant supply of 5% CO2. The plates were then air-dried and 100 µL of 10 mM Tris base was added to each well to solubilize the SRB before reading the absorbance using Perkin-Elmer Multimode Reader at 510 nm. The amount of absorbance is directly relative to cell growth and is thus used to calculate the IC50 values. In this study for initial screening, five types of cancer cell lines, i.e., human breast cancer (MCF-7 and MDA-MB-231), prostate cancer (DU-145) liver cancer (HepG2) and HeLa cancer cell lines were tested for the cytotoxic effect of the title compounds.
Molecular docking studies
All the synthesized compounds were docked with X-ray crystal structure of tubulin inhibitor (PDB ID: 3E22) and human type IIA DNA topoisomerase (PDB: 1ZXM), using AutoDock. Ligand structures were drawn using build panel and prepared using Ligprep module implemented Maestro-8.4 (Schrodinger LLC). Energy minimization is carried out using OPLS-2005 force field. Structures were saved in. pdb format and rewritten using open babel for AutoDock compatible atom type. For docking, grid parameter file (.gpf) and docking parameter files (.dpf) were written using MGL Tools-1.5.6. Receptor grids were generated using 100 × 100 × 100 grid points in xyz with grid spacing of 0.20 Å. Grid box was generated by considering active residues. Map types were generated using autogrid 4.2. Docking was carried out with the following parameters with number of runs: 50, population size: 150, number of evaluations: 2,500,000 and number of generations: 27,000, using AutoDock 4.2. Analysis of docking results was done using MGL Tools-1.5.6. Top scoring molecule in the largest cluster was analyzed for its interaction with the protein.
References
G. Mohan, S. Santhisudha, S. Murali, N.B. Reddy, G. Sravya, Z.V. Grigory, C.S. Reddy, Res. Chem. Intermed. 44, 3475 (2018)
G. Mohan, S. Santhisudha, N.M. Reddy, S. Murali, T. Sreekanth, C.S. Reddy, Monatsh. Chem. 148, 1843 (2017)
G. Mohan, S. Santhisudha, S. Murali, T. Sreekanth, Y.S. Rao, N.B. Reddy, C.S. Reddy, J. Heterocyclic Chem. 57, 1414 (2020)
S. Murali, C. Venkataramaiah, N. Saichaithanya, G. Mohan, S.H. Yasmin, W. Rajendra, S.R. Cirandur, Med. Chem. Res. 28, 1740 (2019)
P. Sreelakshmi, M.R. Nadiveedhi, S. Santhisudha, G. Mohan, N. Saichaithanya, M. Sadik, K. Peddanna, S.R. Cirandur, Med. Chem. Res. 28, 528 (2019)
G. Mohan, S. Kumar, M. Sudileti, C. Sridevi, P. Venkatesu, C.S. Reddy, Int. J. Biol. Macromol. 165, 2010 (2020)
K.M.K. Reddy, S. Santhisudha, G. Mohan, K. Peddanna, C.A. Rao, C.S. Reddy, Phosphorus Sulfur Silicon 191, 933 (2016)
P. Dinéra, M. Amedjkouh, Org. Biomol. Chem. 4, 2091 (2006)
W.R. Gemmill, M.D. Smith, B.A. Reisner, J. Solid State Chem. 178, 2658 (2005)
L. Tusek-Bozic, Current. Med. Chem. 20, 2096 (2013)
A. Mucha, P. Kafarski, L. Berlicki, J. Med. Chem. 54, 5955 (2011)
M. Sienczyk, J. Oleksyszyn, Curr. Med. Chem. 16, 1673 (2009)
R. Damiche, S. Chafaa, J. Mol. Struct. 1130, 1009 (2017)
C. Jin-Long, W. Tang, C. Jian-Yi, C. Kai, Y. Gang, G. Yu-Cheng, S. De-Qing, J. Agri. Food Chem. 63, 7219 (2015)
Z.L. Ren, J. Zhang, H.D. Li, M.J. Chu, L.S. Zhang, X.K. Yao, Y. Xia, X.H. Lv, H.Q. Cao, Chem. Pharm. Bull. 64, 1755 (2016)
P. Kafarski, B. Lejczak, E. Slesak, J. Przetocki, Pest. Manag. Sci. 25, 137143 (1989)
L. Gu, C. Cheng, Org. Biomol. Chem. 10, 7098 (2012)
B. Sujatha, S. Mohan, Ch. Subramanyam, K.P. Rao, Phosphorus Sulfur Silicon 192, 1110 (2017)
S. Murali, G. Mohan, S. Santhisudha, T. Sreekanth, H. Balaji, M. Balaji C. S. Reddy, Monatsh. Chem. 150, 1101 (2019)
T. Sreekanth, G. Mohan, S. Santhisudha, N.M. Reddy, S. Murali, A. Rajasekhar, C.A. Rao, C.S. Reddy, Synth. Commun. 49, 563 (2019)
Y. Xu, K. Yan, B. Song, G. Xu, S. Yang, W. Xue, D. Hu, P. Lu, G. Ouyang, L. Jin, Z. Chen, Molecules 11, 666 (2006)
S.A.R. Mulla, M.Y. Pathan, S.S. Chavan, S.P. Gample, D. Sarkar, RSC Adv. 4, 7666 (2014)
R.R. Chinthaparthi, I. Bhatnagar, C.S. Gangireddy, S.C. Syama, S.R. Cirandur, Archive. Pharm Chem. Life Sci. 346, 667 (2013)
C.B. Reddy, K.S. Kumar, M.A. Kumar, M.V.N. Reddy, B.S. Krishna, M. Naveen, M.K. Arunasree, C.S. Reddy, C.N. Raju, C.D. Reddy, Eur. J. Med. Chem. 47, 553 (2012)
S.J. Kashyap, V.J. Garg, P.K. Sharma, N. Kumar, R. Dudhe, J.K. Gupta, Med. Chem. Res. 21, 2123 (2012)
C.A. Calderón-Ospina, M.O. Nava-Mesa, CNS Neurosci. Ther. 26, 5 (2020)
G.M. Reddy, J.R. Garcia, V.H. Reddy, A.M. Andrade, A. Camilo Jr., R.A.P. Ribeiro, S.R. Lazaro, Eur. J. Med. Chem. 123, 508 (2016)
N. Siddiqui, W. Ahsan, Eur. J. Med. Chem. 45, 1536 (2010)
M.H.M. Helal, M.A. Salem, M.S.A. El-Gaby, M. Aljahdali, Eur. J. Med. Chem. 65, 517 (2013)
P. Singh, S. Gupta, S. Kumar, Med. Chem. 16, 4 (2020)
H. Yu, Z. Qin, H. Dai, X. Zhang, X. Qin, T. Wang, J. Fang, J. Agric. Food Chem. 56, 11356 (2008)
V. Jaishree, N. Ramdas, J. Sachin, B. Ramesh, J. Saudi. Chem. Soc. 16, 371 (2012)
M. Bagheri, M. Shekarchi, M. Jorjani, M.H. Ghahremani, M. Vosooghi, A. Shafiee, Archive. Pharm. Chem. Life Sci. 337, 25 (2004)
S. Anuradha, R. Patel, P. Patle, A. Parameswaran, J.A. Shard, Eur. J. Pharma. Sci. 134, 20 (2019)
R.G. Kalkhambkar, G.M. Kulkarni, H. Shivkumar, R.N. Rao, European. J. Med. Chem. 42, 1272 (2007)
T.K. Venkatachalam, E.A. Sudbeck, C. Mao, F.M. Uckun, Bioorg. Med. Chem. Lett. 11, 523 (2001)
K.V. Sashidhara, K.B. Rao, V. Kushwaha, R.K. Modukuri, R. Verma, P.K. Murthy, Eur. J. Med. Chem. 81, 473 (2014)
F. Delmas, A. Avellaneda, C.D. Giorgio, M. Robin, E.D. Clercq, P. Timon-David, J.P. Galy, Eur. J. Med. Chem. 39, 685 (2004)
P. Makam, P.K. Thakur, T. Kannan, Eur. J. Pharma Sci. 52, 138 (2014)
H. Zhao, G. Cui, J. Jin, X. Chen, B. Xu, Bioorg. Med. Chem. 24, 5911 (2016)
S. Kumar, R. Aggarwal, Mini Rev. Org. Chem. 16, 26 (2019)
T.K. Khatab, A.M. Abdelghany, E.M. Kandil, D.E. Elsefy, A. El-Mekabaty, Biointer. Res. Appl. Chem. 10, 5182 (2020)
T.K. Khatab, A.M. Abdelghany, H.A. Soliman, Silicon 10, 703 (2018)
H.A. Soliman, T.K. Khatab, Silicon 10, 229 (2018)
R.M.F. Batista, S.P.G. Costa, E.L. Malheiro, M. Belsley, M.M.M. Raposo, Tetrahedron 63, 4258 (2007)
P.V.G. Reddy, Y.W. Lin, H.T. Chang, Arkivoc 16, 113 (2007)
D. Xie, A. Zhang, D. Liu, L. Yin, J. Wan, S. Zeng, D. Hu, Phosphorus Sulfur Silicon 192, 1061 (2017)
D. Ravikumar, S. Mohan, Ch. Subramanyam, K.P. Rao, Phosphorus Sulfur Silicon 193, 400 (2018)
F. Tamaddon, S.E. Tadayonfar, J. Mol. Liq. 283, 51 (2019)
N. Grimblat, A.M. Sarotti, T.S. Kaufmanaand, S.O. Simonetti, Org. Biomol. Chem. 14, 10496 (2016)
S. Lianyong, Z. Haibo, H. Tao, C. Lingwu, Chin. J. Pharma. 47, 22 (2016)
V. Vichai, K. Kirtikara, Nature Proto. 1, 1112 (2006)
S. Munusamy, V.N. Badavath, S. Maji, M. Sekar, M.N. Shabbir, New J. Chem. 44, 17231 (2019)
C. Nath, V.N. Badavath, A. Thakur, G. Ucar, O. Acevedo, M.U.M. Siddique, V. Jayaprakash, Med. Chem. Commun. 9, 1164 (2018)
B.V. Nayak, S. Ciftci-Yabanoglu, S. Bhakat, A.K. Timiri, B.N. Sinha, G. Ucar, M.E.S. Soliman, V. Jayaprakash, Bioorg. Chem. 58, 72 (2015)
V.N. Badavath, C. Nath, N.M. Ganta, G. Ucar, B.N. Sinha, V. Jayaprakash, Chin. Chem. Lett. 28, 1528 (2017)
V.N. Badavath, I. Baysal, G. Ucar, B.N. Sinha, V. Jayaprakash, A.C.S. Med, Chem. Lett. 7, 56 (2016)
V.N. Badavath, G. Ucar, B.N. Sinha, S.K. Mondal, V. Jayaprakash, Chem. Select. 1, 5879 (2016)
V.B. Nayak, S. Ciftci-Yabanoglu, S.S. Jadav, M. Jagrat, B.N. Sinha, G. Ucar, V. Jayaprakash, Eur. J. Med. Chem. 69, 762 (2013)
H. Wei, A.J. Ruthenburg, S.K. Bechis, G.L. Verdine, J. Biol. Chem. 280, 37041 (2005)
M. Mantipally, M.R. Gangireddy, R. Gundla, V.N. Badavath, S.R. Mandha, V.C. Maddipati, Bioorg. Med. Chem. Lett. 29, 2248 (2019)
G.M. Reddy, M. Mantipally, R. Gundla, B.V. Nayak, K. Paidikondala, A. Yamala, Chem.Select. 4, 13622 (2019)
B.V. Kumbhar, D. Panda, A. Kunwar, PLoS ONE 13, e0194934 (2018)
Acknowledgments
The author G. Mohan thanks to Prof. C. Devendranath Reddy, Department of Chemistry, S.V. University, Tirupati for his helpful discussions and acknowledges DST-PURSE 2nd Phase Programme facilitated in S.V. University, Tirupati funded by DST, New Delhi, India for providing instrumentation facility and funding (File No: 17118-UGC-III(3)/DST-PURSE 2nd Phase/2017, Dt: 23-08-2018). One of the authors S.H. Yasmin acknowledges DST, New Delhi, India for the financial support under DST-INSPIRE Fellowship Programme (File No. DST/INSPIRE Fellowship/2017/IF170317, Dated: 24/05/2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gundluru, M., Badavath, V.N., Shaik, H.Y. et al. Design, synthesis, cytotoxic evaluation and molecular docking studies of novel thiazolyl α-aminophosphonates. Res Chem Intermed 47, 1139–1160 (2021). https://doi.org/10.1007/s11164-020-04321-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04321-6