Abstract
A one-pot three-component condensation reaction of 3-amino-5-methylisoxazole, aryl aldehyde and 2-naphthol to afford the corresponding 3-amino isoxazolmethylnaphthols in good to excellent yields. The remarkable features of this new procedure are high conversions, clean reaction condition, short reaction time, nonhazardous and environmentally friendly reaction condition, inexpensive and easily commercially availability of the catalyst and simple work-up procedures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multi-component reactions (MCRs) are an effective strategy for the synthesis of complex structures and research into new processes can lead to the discovery of unreported reactions. [1,2,3,4]. Since they are one-pot processes in which three or more accessible components react to form a single product, the results of them can be achieved in an expedient manner without isolation of any intermediate [5, 6]. Biginelli [7, 8], Betti [9], Passerini [10, 11], and Mannich [12,13,14] reactions are some examples of MCRs. Nevertheless, development and discovery of new MCRs is still in demand. Recently, this strategy became important in drug discovery in the context of synthesis of biologically active compounds. This method increases the efficiency of the reactions by reducing the reaction time and increases the yield of products in comparison with normal multistep methods [15, 16]. Furthermore, isoxazole derivatives, especially 5-methylisoxazole, represent an interesting class of heterocycles possessing a wide range of biological activity [17,18,19,20,21,22].
A large number of isoxazole derivatives exhibited antibacterial [23], antifungal [24], anticonvulsant [25], analgesic and anticancer [26] activity (Fig. 1).
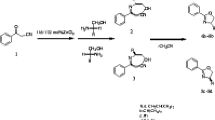
In continuation of our work on one-pot multi-component reactions (MCRs) [27,28,29,30,31], we embarked on the synthesis of a novel compound possessing 2-amino-5-methylisoxazole, aromatic aldehydes and 2-naphthol moieties embedded in a fused molecular framework via a three-component reaction under solvent-free conditions (Scheme 1).
Experimental section
General
Infrared (IR) spectra and melting points of all compounds were determined using an Electrothermal 9100 apparatus and an FT-IR-JASCO-460 plus spectrometer. The 1H and 13C NMR spectra of compounds were recorded on a Bruker DRX-300 Avance instrument in DMSO at 300 MHz. Mass spectra were obtained on Agilent-HP and Sciex-3200 Technology spectrometers operating at an ionization potential of 70 eV. We purchased all the chemicals from chemical producers Merck and Fluka and they were used without further purification.
General procedure for the preparation of 3-aminoisoxazolmethylnaphthols
To a mixture of aldehyde (1.0 mmol), 2-naphthol (1.0 mmol) and 3-amino-5-methylisoxazole (1.0 mmol) oxalic acid (10 mol%) were added. The mixture was stirred at 90 °C in an oil bath and the reaction was followed by thin layer chromatography (TLC). After completion of the reaction, the mixture was cooled to room temperature and washed with water to remove oxalic acid from the reaction mixture. The solid obtained was recrystallized from absolute EtOH to furnish the desired pure product.
Spectral data for the selected compounds
1-[(5-Methyl-isoxazol-3-ylamino)-phenyl-methyl]-naphthalen-2-ol ( 4a )
White powder, IR (KBr νmax, cm−1): 3403, 3058, 2925, 1628, 1600, 1581, 1541, 1516, 1493; 1H NMR (300 MHz, DMSO-d6): 2.23 (3H, s, CH3), 5.90 (1H, s, CHNH), 6.74–6.85 (2H, dd, Haromat), 7.14–7.41 (8H, m, Haromat), 7.76–7.83 (2H, m, Haromat), 8.01–8.04 (1H, d, NH), 10.10 (1H, s, OH); 13C NMR (75 MHz, DMSO-d6): 12.5, 53.0, 94.5, 118.9, 120.4, 122.8, 124.1, 126.4, 126.6, 128.3, 128.9, 129.0, 129.5, 132.6, 143.6, 153.2, 165.0, 167.7; MS m/z (%):77.1 (5), 98.1 (7), 115.2 (8), 202.2 (23), 215.2 (8), 231.2 (100), 246.2 (4), 330.2 (M+, 3).
1-[(5-Methyl-isoxazol-3-ylamino)-p-tolyl-methyl]-naphthalen-2-ol ( 4b )
White powder, IR (KBr νmax, cm−1): 3393, 3060, 1625, 1581, 1538, 1535, 1511, 1478, 1437; 1H NMR (300 MHz, DMSO-d6): 2.22, 2.24 (6H, s, 2CH3), 5.88 (1H, s, CHNH), 6.67–6.80 (2H, dd, Haromat), 7.05–7.08 (2H, d, Haromat), 7.19–7.29 (4H, m, Haromat), 7.34–7.39 (1H, t, Haromat), 7.74–7.82 (2H, m, Haromat), 7.99–8.02 (1H, d, NH), 10.06 (1H, s, OH); 13C NMR (75 MHz, DMSO-d6): 12.5, 21.0, 52.9, 94.4, 118.9, 120.5, 122.7, 124.2, 126.6, 128.9, 129.0, 129.4, 132.6, 135.3, 140.6, 153.1, 164.9, 167.7. MS m/z (%): 98.2 (10), 115.5 (8), 202.2 (20), 216.2 (9), 231.2 (100), 245.2 (46), 344.3 (M+, 2).
1-[(4-Methoxy-phenyl)-(5-methyl-isoxazol-3-ylamino)-methyl]-naphthalen-2-ol ( 4c )
White powder, IR (KBr νmax, cm−1): 3380, 2962, 1626, 1579, 1556, 1509, 1475, 1436; 1H NMR (300 MHz, DMSO-d6): 2.21 (3H, s, CH3), 3.69 (3H, s, OCH3), 5.86 (1H, s, CHNH), 6.62–6.64 (1H, d, Haromat), 6.76–6.85 (3H, m, Haromat), 7.20–7.39 (4H, m, Haromat), 7.73–7.81 (2H, m, Haromat), 7.99–8.02 (1H, d, NH), 10.06 (1H, s, OH); 13C NMR (75 MHz, DMSO-d6): 12.5, 52.7, 55.4, 94.4, 113.8, 118.9, 120.4, 122.7, 124.1, 126.5, 127.8, 128.9, 129.0, 129.4, 132.6, 135.4, 153.1, 158.0, 164.9, 167.7; MS m/z (%): 98.2 (20), 115.2(5), 165.2(10), 189.2 (45), 202.2 (10), 218.2 (30), 231.2 (95), 261.1 (100), 360.3 (M+, 2).
1-[(4-Chloro-phenyl)-(5-methyl-isoxazol-3-ylamino)-methyl]-naphthalen-2-ol ( 4d )
White powder, IR (KBr νmax, cm−1): 3389, 3064, 1624, 1580, 1572, 1532, 1516, 1488; 1H NMR (300 MHz, DMSO-d6): 2.23 (3H, s, CH3), 5.88 (1H, s, CHNH), 6.69–6.87 (2H, dd, Haromat), 7.25–7.42 (7H, m, Haromat), 7.77–7.83 (2H, t, Haromat), 7.95–7.98 (1H, d, NH), 10.16 (1H, s, OH); 13C NMR (75 MHz, DMSO-d6): 12.5, 52.5, 94.4, 118.8, 119.9, 122.9, 124.0, 126.8, 128.3, 128.5, 129.0, 129.8, 130.9, 132.5, 142.8, 153.2, 164.9, 167.8; MS m/z (%): 98.2 (8), 115.2 (9), 202.2 (32), 231.2 (100), 265.1 (58), 364.2 (M+, 1).
1-[(3-Chloro-phenyl)-(5-methyl-isoxazol-3-ylamino)-methyl]-naphthalen-2-ol ( 4e )
White powder, IR (KBr νmax, cm−1): 3414, 3066, 1629, 1592, 1551, 1537, 1516, 1473, 1437; 1H NMR (300 MHz, DMSO-d6): 2.23 (3H, s, CH3), 5.88 (1H, s, CHNH), 6.70–6.91 (2H, dd, Haromat), 7.19–7.34 (6H, m, Haromat), 7.39–7.44 (1H, t, Haromat), 7.78–7.84 (1H, t, Haromat), 7.97–8.00 (1H, d, NH), 10.16 (1H, s, OH); 13C NMR (75 MHz, DMSO-d6): 12.5, 52.6, 94.4, 118.8, 119.8, 122.9, 123.8, 125.4, 126.3, 126.4, 126.9, 129.0, 129.0, 129.9, 130.3, 132.4, 133.2, 146.5, 153.2, 164.8, 167.9; MS m/z (%): 98.2 (10), 115.2 (13), 202.2 (32), 231.2 (100), 265.1 (48), 364.2 (M+, 3).
1-[(2-2.3.6.1-[(3-Chloro-phenyl)-(5-methyl-isoxazol-3-ylamino)-methyl]-naphthalen-2-ol ( 4f )
White powder, IR (KBr νmax, cm−1): 3411, 3057, 2924, 1629, 1583, 1544, 1515, 1468, 1438; 1H NMR (300 MHz, DMSO-d6): 2.22 (3H, s, CH3), 5.78 (1H, s, CHNH), 6.72–6.89 (2H, dd, Haromat), 7.16–7.42 (6H, m, Haromat), 7.69–7.82 (2H, m, Haromat), 8.04–8.07 (1H, d, NH), 9.94 (1H, s, OH); 13C NMR (75 MHz, DMSO-d6): 12.5, 52.5, 94.4, 118.6, 120.0, 122.9, 123.6, 125.4, 126.5, 126.4, 126.9, 129.2, 129.5, 129.9, 130.3, 132.4, 134.2, 146.5, 154.2, 163.6, 168.2; MS m/z (%): 98.2 (5), 115.2 (7), 202.2 (20), 231.2 (100), 265.1 (8), 364.2 (M+, 1).
1-[(4-Bromo-phenyl)-(5-methyl-isoxazol-3-ylamino)-methyl]-naphthalen-2-ol ( 4 g )
White powder, IR (KBr νmax, cm−1): 3391, 3064, 1624, 1580, 1533, 1516, 1486, 1436. 1H NMR (300 MHz, DMSO-d6): 2.23 (3H, s, CH3), 5.88 (1H, s, CHNH), 6.67–6.87 (2H,dd, Haromat), 7.23–7.48 (7H, m, Haromat), 7.77–7.83 (2H,t, Haromat), 7.95–7.98 (1H, d, NH), 10.16 (1H, s, OH); 13C NMR (75 MHz, DMSO-d6): 12.5, 52.6, 94.4, 118.8, 119.4, 119.9, 122.9, 124.0, 126.8, 128.9, 129.0, 129.8, 131.2, 132.9, 143.3, 153.2, 164.9, 167.8; MS m/z (%): 98.2 (8), 101.2 (9), 115.3 (9), 202.2 (35), 215.2 (4), 231.2 (100), 311.2 (23), 408.2 (M+, 1).
1-[(3-Bromo-phenyl)-(5-methyl-isoxazol-3-ylamino)-methyl]-naphthalen-2-ol ( 4 h )
White powder, IR (KBr νmax, cm−1): 3417, 3065, 1629, 1586, 1555, 1516, 1504, 1474, 1437; 1H NMR (300 MHz, DMSO-d6): 2.23 (3H, s, CH3), 5.88 (1H, s, CHNH), 6.70–6.91 (2H, dd, Haromat), 7.19–7.49 (7H, m, Haromat), 7.78–7.84 (2H, t, Haromat), 7.98–8.00 (1H, d, NH), 10.14 (1H, s, OH); 13C NMR (75 MHz, DMSO-d6): 12.5, 52.6, 94.4, 118.8, 119.8, 121.9, 122.9, 123.8, 125.8, 126.9, 129.0, 129.0, 129.1, 129.3, 129.9, 130.6, 132.4, 146.7, 153.2, 164.8, 167.9; MS m/z (%): 98.2 (10), 101.2 (9), 115.3 (12), 202.2 (32), 231.2 (100), 311.1 (25), 408.2 (M+, 1).
1-[(4-Fluoro-phenyl)-(5-methyl-isoxazol-3-ylamino)-methyl]-naphthalen-2-ol ( 4i )
White powder, IR (KBr νmax, cm−1): 3400, 3061, 1626, 1604, 1582, 1539,1507, 1470, 1437; 1H NMR (300 MHz, DMSO-d6): 2.23 (3H, s, CH3), 5.87 (1H, s, CHNH), 6.68–6.86 (2H, dd, Haromat), 7.06–7012 (2H, t, Haromat), 7.25–7.33 (4H, m, Haromat), 7.36–7.41 (1H, t, Haromat), 7.76–7.83 (2H, m, Haromat), 7.97–7.99 (1H, d, NH), 10.11 (1H, s, OH); 13C NMR (75 MHz, DMSO-d6): 12.5, 52.5, 94.4, 114.9, 115.1, 118.8, 120.1, 122.8, 124.0, 126.7, 128.4, 128.5, 129.0, 129.7, 132.5, 139.7, 139.7, 153.1, 159.5, 162.7, 164.9, 167.8; MS m/z (%): 98.2 (10), 115.2 (8), 202.2 (8), 220.2 (21), 249.2 (100), 348.3 (M+, 1).
1-[(5-Methyl-isoxazol-3-ylamino)-(4-nitro-phenyl)-methyl]-naphthalen-2-ol ( 4j )
Yellow powder, IR (KBr νmax, cm−1): 3416, 3082, 1628, 1604, 1595, 1582, 1541, 1514, 1488; 1H NMR (300 MHz, DMSO-d6): 2.25 (3H, s, CH3), 5.89 (1H, s, CHNH), 6.79–7.00 (2H, dd, Haromat), 7.26–7.30 (2H, m, Haromat), 7.38–7.43 (1H, t, Haromat), 7.52–7.55 (2H, d, Haromat), 7.80–7.85 (2H, m, Haromat), 7.93–7.96 (1H, d, NH), 8.15–8.18 (2H, d, Haromat), 10.25 (1H, s, OH); 13C NMR (75 MHz, DMSO-d6): 12.5, 52.9, 94.4, 96.3, 118.4, 118.8, 119.4, 123.0, 123.6, 123.8, 124.4, 127.0, 127.7, 127.8, 128.9, 129.0, 129.1, 130.0, 130.2, 132.4, 146.3, 152.2, 153.3, 164.8, 168.0, 169.8; MS m/z (%): 43.2 (17), 115.2 (50), 144.2 (60), 202.2 (39), 230.2 (100), 2454.2 (32), 291.2 (52), 375.3 (M+, 1).
1-[(5-Methylisoxazol-3-ylamino)-(pyridin-4-yl)-methyl]-naphthalen-2-ol ( 4 k )
White powder, IR (KBr νmax, cm−1): 3375, 3140, 2924, 1629, 1604, 1576, 1560, 1512; 1H NMR (300 MHz, DMSO-d6): 2.24 (3H, s, CH3), 5.89 (1H, s, CHNH), 6.70–6.93 (2H, dd, Haromat), 7.25–7.30 (4H, m, Haromat), 7.38–7.43 (1H, t, Haromat), 7.79–7.84 (2H, t, Haromat), 7.92–7.95 (1H, d, NH), 8.44–8.46 (2H, d, Haromat), 10.2 (1H, s, OH); 13C NMR (75 MHz, DMSO-d6): 12.5, 52.3, 94.4, 118.8, 119.2, 121.9, 122.9, 123.8, 126.9, 129.0, 129.0, 130.1, 132.4, 149.6, 153.0, 153.4, 164.8, 168.0; MS m/z (%): 83.0 (9), 121.0 (9), 188.0 (13), 234.0 (15), 256.0 (30), 304.2 (20), 332.1 (100), 333.1 (14), 334.1 (M++ H, 3).
1-[(5-Methylisoxazol-3-ylamino)-(thiophen-2-yl)methyl]-naphthalen-2-ol ( 4 l )
White powder, IR (KBr νmax, cm−1): 3394, 3113, 1624, 1582, 1537, 1530, 1516; 1H NMR (300 MHz, DMSO-d6): 2.21 (3H, s, CH3), 5.90 (1H, s, CHNH), 6.79–6.80 (1H, d, Haromat), 6.89–6.91 (2H, t, Haromat), 6.97 (1H, d, Haromat), 7.25–7.33 (3H, m, Haromat), 7.40–7.45 (1H, t, Haromat), 7.77–7.84 (2H, m, Haromat),8.09–8.12 (1H, d, NH), 10.2 (1H, s, OH); 13C NMR (75 MHz, DMSO-d6): 12.5, 50.5, 94.4, 118.9, 119.6, 122.9, 123.7, 124.3, 124.7, 126.7, 126.9, 128.9, 129.0, 129.8, 132.5,148.2, 153.3, 164.5, 168.0; MS m/z (%):59.0 (3), 96.9 (100), 118.9 (10), 224.0 (11), 236.9 (7), 235.0 (M+–H, 96).
Results and discussion
We compared the catalytic activity of oxalic acid with some other catalysts reported in the literature for the three-component condensation reaction of benzaldehyde, 2-naphthol and 3-amino-5-methylisoxazole in solvent-free conditions after 40 min at 80 °C (Table 1). In the absence of the catalyst, no product was formed (Table 1, entry 1). After several experiments, oxalic acid was found to the most efficient catalyst among all the other examined catalysts under these conditions.
To achieve an optimal temperature, solvent and amounts of catalyst, we used different solvents such as ethanol, tetrahydrofuran (THF), dimethylformamide (DMF), AcOH:H2O(1:1) and chloroform in 70 °C, but no superior results were obtained as compared to solvent-free condition (Table 2, entries 1–8). Also, we studied the influence of temperature on the reaction time and percentage yield. Table 2 displays that 90 °C was optimum temperature for maximum conversion. Increasing the temperature above 90 °C neither improved the yield nor decreased the reaction time (Table 2, entries 9–13). In contrast, reducing the temperature is detrimental to the reaction, resulting in lower yields. We also studied the model reaction catalyzed by oxalic acid with different catalyst loading (Table 2, entries 14–16). The optimal quantity of catalyst was found to be 10 mol%. Excess amount of catalyst did not increase the yields.
The reactions of 2-naphthol with various aromatic aldehydes and 3-amino-5-methylisoxazole were carried out in the presence of 10 mol% oxalic acid at 90 °C. The results are summarized in Table 3 and showed an excellent tolerance toward electron-withdrawing and electron-donating groups with similar yields and reaction times. However, aromatic aldehydes containing electron-withdrawing groups gave shorter times and higher yields than that with electron-donating groups. A suggested mechanism for this transformation is shown in Scheme 2. According to the literature [28, 32, 33], it is suggested that the reaction is performed through o-QMs. All the products 4a–l are new compounds identified by IR, 1H NMR, 13C NMR and MS spectral data.
Conclusion
In this research, we have demonstrated a green and efficient method for the synthesis of 3-aminoisoxazolmethylnaphthols via condensation of an aldehyde, 2-naphthol and 3-amino-5-methylisoxazole using oxalic acid as the catalyst in solvent-free conditions. The catalyst is green, inexpensive and readily commercially available. Simple experimental procedure, excellent yields, shorter reaction time, easy workup, product purity, a nonhazardous nature and environmentally friendly reaction conditions are the key features of this procedure.
References
T.J. Mueller, Beilstein J. Org. Chem. 7, 960 (2011)
L.H. Choudhury, T. Parvin, Tetrahedron Lett. 67, 8213 (2011)
F. DeMoliner, S. Crosignani, A. Galatini, R. Riva, A. Basso, ACS Comb. Sci. 13, 453 (2011)
N. Isambert, M.D.S. Duque, J.C. Plaquevent, Y. Genisson, J. Rodriguez, T. Constantieux, Chem. Soc. Rev. 40, 1347 (2011)
J.C. Menendez, Synthesis 15, 2624 (2006)
L.M. Wang, J. Xia, F. Qin, C. Qian, J. Sun, Synthesis 8, 1241 (2003)
A.K. Bose, S. Pednekar, S.N. Ganguly, G. Chakraborty, M.S. Manhas, Tetrahedron Lett. 45, 8351 (2004)
D. Prajapati, J.S. Sandhu, Synlett 2, 235 (2004)
C. Cardellicchio, M.A.M. Capozzi, F. Naso, Tetrahedron Asymmetry 21, 507 (2010)
K. Kobayashi, T. Matoba, S. Irisawa, T. Matsumoto, O. Morikawa, H. Konishi, Chem. Lett. 27, 551 (1998)
R. Bossio, C.F. Marcos, S. Marcaccini, R. Pepino, Tetrahedron Lett. 38, 2519 (1997)
T. Akiyama, K. Matsuda, K. Fuchibe, Synlett 2005, 322 (2005)
G. Zhao, T. Jiang, H. Gao, B. Han, J. Huang, D. Sun, Green Chem. 6, 75 (2004)
T. Akiyama, J. Takaya, H. Kagoshima, Tetrahedron Lett. 40, 7831 (1999)
M. Zhang, H.F. Jiang, H.L. Liu, Q.H. Zhu, Org. Lett. 9, 4111 (2007)
R.M. Armstrong, A.P. Combs, P.A. Tempest, S.D. Brown, T.A. Keating, Acc. Chem. Res. 29, 123 (1996)
X.X. Zhang, J.S. Bradshaw, R.M. Izatt, Chem. Rev. 97, 3313 (1997)
W. Wang, F. Ma, X. Shen, C. Zhang, Tetrahedron Asymmetry 18, 832 (2007)
S. Maeng, C.A. Zarate, J. Du, R.J. Schloesser, J. McCammon, G. Chen, H.K. Manji, Biol. Psychiatry 63, 349 (2008)
P.A. Bradley, R.J. Carroll, Y.C. Lecouturier, R. Moore, P. Noeureuil, B. Patel, J. Snow, S. Wheeler, Org. Process Res. Dev. 14, 1326 (2010)
M. Rostami, A.R. Khosropour, V. Mirkhani, I. Mohammadpoor-Baltork, M. Moghadam, S. Tangestaninejad, Monatsh. Chem. 142, 1175 (2011)
M. Rostami, A.R. Khosropour, V. Mirkhani, M. Moghadam, S. Tangestaninejad, I. Mohammadpoor-Baltork, Appl. Catal. A: Gen. 397, 27 (2011)
E. Nakayama, M. Miyanchi, K. Fugimoto, J. Idle, J. Antibiot. 43, 1122 (1990)
P.B. Reddy, S.M. Reddy, E. Rajanarendar, A.K. Murthy, Indian Phytopathol. 37, 369 (1984)
R.P. Clausen, E.K. Moltzen, J. Perregaard, S.M. Lenz, C. Sanchez, E. Falch, B. Frolund, T. Bolvig, A. Sarup, O.M. Larsson, A. Schousboe, P. Krogsgaard-Larsen, Bioorg. Med. Chem. 3, 895 (2005)
H. Kano, I. Adachi, R. Kido, K. Hirose, J. Med. Chem. 10, 411 (1967)
M. Fatahpour, N. Hazeri, B. Adrom, M.T. Maghsoodlou, M. Lashkari, Res. Chem. Intermed. 44, 2111 (2018)
B. Adrom, N. Hazeri, M.T. Maghsoodlou, M. Mollamohammadi, Res. Chem. Intermed. 41, 4741 (2015)
M.T. Maghsoodlou, M. Karima, M. Lashkari, B. Adrom, J. Aboonajmi, J. Iran. Chem. Soc. 14, 329 (2017)
M. Kangani, N. Hazeri, M.T. Maghsoodlou, A. Ebrahimi, Organ. Chem. Res. 2, 81 (2016)
M. Fatahpour, F. Noori Sadeh, N. Hazeri, M.T. Maghsoodlou, M. Lashkari, J. Iran. Chem. Soc. 14, 1945 (2017)
R.K. Singh, R. Duvedi, Arab. J. Chem. 11, 91 (2018)
H. Kiyani, H. Darbandi, Bulg. Chem. Commun. 49, 562 (2017)
Acknowledgements
We gratefully acknowledge financial support from the Research Council of the University of Sistan and Baluchestan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Safarzaei, M., Maghsoodlou, M.T., Mollashahi, E. et al. Synthesis of 3-aminoisoxazolmethylnaphthols via one-pot three-component reaction under solvent-free conditions. Res Chem Intermed 44, 7449–7458 (2018). https://doi.org/10.1007/s11164-018-3566-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3566-y