Abstract
A sequential one-pot four-component reaction for the efficient synthesis of novel 16-(aryl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one derivatives has been developed. The synthesis was achieved by reacting 2-hydroxynaphthalene-1,4-dione, benzene-1,2-diamine, aromatic aldehydes, and 1,3-indandione in the presence of oxalic acid as a reusable and homogenous organocatalyst in EtOH/H2O (1:1) under reflux. The present approach of this methodology offers several advantages such as high yields, clean reaction profiles, operational simplicity, simple work-up procedures, and green aspects by avoiding toxic catalysts and solvents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, interest in green chemistry [1–3] has developed, and a major challenge to organic chemists is that of reducing the use of organic solvents and toxic reagents for facile, efficient, and nonpolluting synthetic procedures. In order to have economic savings and pollution prevention, multi-component reactions (MCRs) [4–7] have considerable ecological interest as a powerful strategy in the synthesis of complex heterocyclic molecules, drug design, and drug discovery, arising from minimization of time, waste, energy, and cost.
Phenazine-based compounds are nitrogen-containing heterocycles that are the main core of many natural and synthetic organic materials [8–10]. Phenazines exhibit important biological activities and wide applications in pharmaceutical use such as trypanocidal [11], fungicidal [12, 13], antimalarial [14, 15], antiplatelet [16], and antitumour [17] activities.
Furthermore, pyran annulated heterocyle derivatives are an important class of oxygen-containing heterocycles and are usually structural subunits in a variety of important natural compounds, including carbohydrates, alkaloids, polyether antibiotics, pheromones, and iridoids [18]. Pyarans are widely employed as cosmetics, pigments [19] and potential biodegradable agrochemicals [20] and have various biological properties such as being anti-leishmanial [21], anti-HIV [22], antioxidant [23], anti-tumor [24] and central nervous system (CNS) activities and effects [25]; they are also used for treatment of Alzheimer’s disease [26] and schizophrenia [27].
Oxalic acid is the only possible compound in which two carboxylic groups are joined directly, and; hence, is supposed to be one of the strongest organic acids. It is abundantly present in many plants so it can be used in green chemistry. The use of organic catalysts as a catalyst is increasing because of their significant advantages, such as the possibility of performing reactions in the presence of acid-sensitive substrates, performing reactions in milder reaction conditions, and selectivity [28]. In the past years, oxalic acid was efficiently used as a catalyst in a variety of chemical reactions like the imino Diels–Alder reaction [29], Beckmann rearrangement [30], protection of carbonyl to thioacetal changes,and deprotection of thioacetal to carbonyls as well [31].
Considering the importance of phenazine, pyran, and quinoxaline derivatives and in continuation of our ongoing program for the synthesis of complex organic compounds based on green chemistry protocols [32–36], herein, we have presented a novel and efficient method for synthesis of 16-(aryl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one derivatives 6a–j. This reaction was achieved via a sequential, one-pot, four-component condensation reaction between 2-hydroxynaphthalene-1,4-dione 1, benzene-1,2-diamine 2, aromatic aldehydes 4 and 1,3-indandione 5 using oxalic acid as a green, inexpensive, and easily available catalyst (Scheme 1).
Experimental
General
All melting points were determined on an Electrothermal 9100 apparatus and are uncorrected. IR spectra were recorded on a Shimadzu IR-470 spectrometer. Elemental analyses for C, H, and N were performed using a Heraeus CHN-O-Rapid analyzer at the Iranian Central Research of Petroleum Company. Mass spectra were recorded on a FINNIGAN-MAT 8430 mass spectrometer operating at an ionization potential of 70 eV. The 1H nuclear magnetic resonance (NMR) and 13C NMR spectra were recorded on a Bruker DRX-400 Avance instrument with deuterated dimethyl sulfoxide (DMSO-d 6) as solvent. Thin-layer chromatography (TLC) was performed on silica-gel Polygram SILG/UV 254 plates. All reagents and solvents were purchased from Merck and Aldrich and used without further purification.
General experimental procedure for the synthesis of 16-(aryl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one derivatives (6a–j)
Initially, 2-hydroxynaphthalene-1,4-dione 1 (1 mmol) and benzene-1,2-diamine 2 (1 mmol) were mixed at 75 °C (solvent free) until in less than 5 min an orange solid of benzo[a]phenazine 3 was formed. Then, aryl aldehyde 4 (1 mmol), 1,3-indandione 5 (1 mmol), and oxalic acid (20 mol%) were added, and this mixture was heated to reflux under stirring in EtOH/H2O [1/1 (v/v), 30 mL] for the specific time (2–2.5 h). Upon completion of the reaction, monitored by TLC, then, the reaction mixture was cooled to room temperature, and H2O (5 mL) was added to this mixture. The precipitate formed was collected by filtration, then, the separated product was washed twice with water (2 × 5 mL). The resulting product subsequently recrystallized from hot ethanol to give the pure solid. After isolation of the product, the filtrate was extracted with CHCl3 (2 × 20 mL). The aqueous layer (including oxalic acid) was separated, and its solvent was evaporated, and oxalic acid was recovered and reused. The spectral and analytical data are presented below.
16-(4-Nitrophenyl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one (6a)
Yellow solid; yield 92 %, 0.466 g; mp 250–252 °C; IR (KBr) (ν max, cm−1): 2930, 1697, 1628, 1592, 1516, 1456, 1404, 1342, 1238, 1126, 973, 760; 1H NMR (400 MHz, DMSO-d 6): δH 6.03 (s, 1H, CH), 7.56–7.61 (m, 1H, Ar–H), 7.80–7.84 (m, 2H, Ar–H), 7.85–7.92 (m, 5H, Ar–H), 8.13 (d, 2H, J = 8.8 Hz, Ar–H), 8.19–8.23 (m, 1H, Ar–H), 8.24–8.32 (m, 3H, Ar–H), 8.79 (d, 1H, J = 7.6 Hz, Ar–H), 9.21–9.25 (m, 1H, Ar–H) ppm; 13C NMR (100 MHz, DMSO-d 6): δC 36.1, 110.3, 113.9, 119.0, 120.9, 121.0, 121.8, 122.5, 123.4, 124.7, 125.2, 128.0, 128.9, 129.2, 129.3, 129.7, 130.6, 130.8, 131.3, 131.9, 139.4, 139.7, 139.9, 142.4, 145.4, 147.1, 148.1, 150.8, 151.1, 190.4 ppm; MS (m/z, %): 507 (M+, 4); Anal. Calcd for C32H17N3O4: C, 75.73; H, 3.38; N, 8.28 %. Found: C, 75.86; H, 3.45; N, 8.13 %.
16-(4-Chlorophenyl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one (6b)
Yellow solid; yield 89 %, 0.441 g; mp 261–263 °C; IR (KBr) (ν max, cm−1): 2900, 1716, 1660, 1587, 1537, 1448, 1327, 1262, 1123, 935, 757; 1H NMR (400 MHz, DMSO-d 6): δH 5.95 (s, 1H, CH), 7.40–7.45 (m, 1H, Ar–H), 7.83–7.86 (m, 2H, Ar–H), 7.88–7.91 (m, 5H, Ar–H), 8.14 (d, 2H, J = 7.6 Hz, Ar–H), 8.25–8.27 (m, 2H, Ar–H), 8.28–8.32 (m, 2H, Ar–H), 8.74 (d, 1H, J = 7.6 Hz, Ar–H), 9.23–9.26 (m, 1H, Ar–H) ppm; 13C NMR (100 MHz, DMSO-d 6): δC 34.5, 106.6, 114.0, 122.5, 122.8, 123.4, 124.1, 124.5, 124.8, 124.9, 126.3, 127.0, 127.4, 127.6, 127.9, 129.2, 129.4, 129.9, 130.1, 130.5, 131.8, 132.4, 140.1, 146.0, 147.1, 148.9, 150.3, 152.3, 188.6 ppm; MS (m/z, %): 496 (M+, 6); Anal. Calcd for C32H17ClN2O2: C, 77.34; H, 3.45; N, 5.64 %. Found: C, 77.48; H, 3.58; N, 5.80 %.
16-(3-Nitrophenyl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one (6c)
Yellow solid; yield 90 %, 0.456 g; mp 234–235 °C; IR (KBr) (ν max, cm−1): 2905, 1720, 1678, 1589, 1525, 1448, 1399, 1338, 1241, 1179, 960, 754; 1H NMR (400 MHz, DMSO-d 6): δH 6.03 (s, 1H, CH), 7.57–7.62 (m, 2H, Ar–H), 7.79–8.03 (m, 6H, Ar–H), 8.19–8.26 (m, 3H, Ar–H), 8.35 (d, 1H, J = 7.6 Hz, Ar–H), 8.40 (dd, 1H, J 1 = 8.0 Hz, J 2 = 2.0 Hz, Ar–H), 8.59 (s, 1H, Ar–H), 8.64 (d, 1H, J = 8.0 Hz, Ar–H), 9.12 (d, 1H, J = 7.6 Hz, Ar–H) ppm; 13C NMR (100 MHz, DMSO-d 6): δC 35.5, 113.9, 121.5, 122.0, 122.3, 123.0, 123.2, 123.3, 123.7, 124.8, 126.6, 126.9, 128.7, 129.0, 129.1, 129.7, 129.8, 130.1, 130.4, 130.7, 130.8, 135.4, 136.1, 136.2, 136.6, 139.6, 142.1, 144.9, 147.8, 150.1, 152.2, 188.8 ppm; MS (m/z, %): 507 (M+, 7); Anal. Calcd for C32H17N3O4: C, 75.73; H, 3.38; N, 8.28 %. Found: C, 75.85; H, 3.46; N, 8.41 %.
16-(2-Chlorophenyl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one (6d)
Brown solid; yield 88 %, 0.436 g; mp 296–298 °C; IR (KBr) (ν max, cm−1): 2900, 1703, 1618, 1591, 1526, 1447, 1390, 1338, 1218, 1130, 999, 754; 1H NMR (400 MHz, DMSO-d 6): δH 6.05 (s, 1H, CH), 7.05–7.07 (m, 1H, Ar–H), 7.56–7.60 (m, 1H, Ar–H), 7.82–7.93 (m, 8H, Ar–H), 8.07–8.09 (m, 1H, Ar–H), 8.14 (d, 1H, J = 8.4 Hz, Ar–H), 8.23–8.33 (m, 3H, Ar–H), 9.24–9.27 (m, 1H, Ar–H) ppm; 13C NMR (100 MHz, DMSO-d 6): δC 32.2, 109.3, 113.8, 118.7, 122.1, 122.3, 122.9, 123.4, 124.7, 124.8, 126.9, 127.7, 128.2, 128.4, 128.7, 129.0, 129.1, 129.2, 129.5, 129.6, 130.0, 130.2, 130.4, 130.7, 130.9, 136.4, 139.3, 141.7, 148.5, 150.3, 152.2, 190.8 ppm; MS (m/z, %): 496 (M+, 3); Anal. Calcd for C32H17ClN2O2: C, 77.34; H, 3.45; N, 5.64 %. Found: C, 77.25; H, 3.58; N, 5.77 %.
16-(4-Chloro-3-nitrophenyl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one (6e)
Yellow solid; yield 91 %, 0.492 g; mp 243–245 °C; IR (KBr) (ν max, cm−1): 2910, 1719, 1674, 1589, 1530, 1476, 1401, 1344, 1201, 1135, 951, 754; 1H NMR (400 MHz, DMSO-d 6): δH 5.96 (s, 1H, CH), 7.57–7.59 (m, 1H, Ar–H), 7.67 (d, 1H, J = 8.4 Hz, Ar–H), 7.84–7.87 (m, 2H, Ar–H), 7.91 (d, 3H, J = 8.0 Hz, Ar–H), 7.97 (t, 2H, J = 8.0 Hz, Ar–H), 8.14 (d, 1H, J = 7.2 Hz, Ar–H), 8.22–8.26 (m, 2H, Ar–H), 8.33 (d, 1H, J = 7.6 Hz, Ar–H), 8.36 (s, 1H, Ar–H), 9.16 (d, 1H, J = 7.2 Hz, Ar–H) ppm; 13C NMR (100 MHz, DMSO-d 6): δC 34.8, 113.2, 122.1, 122.3, 122.9, 123.6, 124.8, 125.4, 127.4, 128.7, 129.0, 129.7, 130.3, 130.4, 130.7, 130.8, 131.3, 132.0, 133.5, 134.0, 136.3, 136.5, 138.1, 139.3, 139.9, 140.7, 141.5, 143.8, 147.3, 150.2, 152.1, 191.5 ppm; MS (m/z, %): 541 (M+, 9); Anal. Calcd for C32H16ClN3O4: C, 70.92; H, 2.98; N, 7.75 %. Found: C, 71.09; H, 3.14; N, 7.90 %.
16-(3-Methoxyphenyl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one (6f)
Brown solid; yield 86 %, 0.423 g; mp 290–292 °C; IR (KBr) (ν max, cm−1): 2985, 1709, 1670, 1589, 1526, 1446, 1396, 1337, 1219, 1135, 996, 755; 1H NMR (400 MHz, DMSO-d 6): δH 3.69 (s, 3H, OCH3), 5.91 (s, 1H, CH), 7.17 (t, 1H, J = 8.0 Hz, Ar–H), 7.25 (d, 1H, J = 8.0 Hz, Ar–H), 7.29 (s, 1H, Ar–H), 7.52–7.54 (m, 1H, Ar–H), 7.84–7.93 (m, 7H, Ar–H), 8.15 (d, 1H, J = 8.4 Hz, Ar–H), 8.27–8.34 (m, 3H, Ar–H), 9.25–9.27 (m, 1H, Ar–H) ppm; 13C NMR (100 MHz, DMSO-d 6): δC 35.8, 54.8, 110.8, 117.7, 122.2, 122.7, 123.3, 124.7, 124.8, 125.3, 125.4, 126.7, 128.8, 129.3, 130.0, 130.2, 130.4, 130.9, 131.0, 133.7, 135.8, 139.3, 139.4, 139.6, 140.3, 144.7, 146.6, 148.0, 150.3, 152.4, 190.3 ppm; MS (m/z, %): 492 (M+, 6); Anal. Calcd for C33H20N2O3: C, 80.47; H, 4.09; N, 5.69 %. Found: C, 80.58; H, 4.26; N, 5.82 %.
3-(15-Oxo-15,16-dihydrobenzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-16-yl)benzonitrile (6g)
Brown solid; yield 90 %, 0.438 g; mp 230–232 °C; IR (KBr) (ν max, cm−1): 2895, 2200, 1709, 1679, 1590, 1529, 1454, 1410, 1335, 1252, 1124, 959, 767; 1H NMR (400 MHz, DMSO-d 6): δH 5.96 (s, 1H, CH), 7.50 (t, 1H, J = 8.0 Hz, Ar–H), 7.55–7.57 (m, 1H, Ar–H), 7.65 (d, 1H, J = 7.6 Hz, Ar–H), 7.67 (t, 1H, J = 8.0 Hz, Ar–H), 7.83–7.90 (m, 3H, Ar–H), 7.91–8.04 (m, 3H, Ar–H), 8.10 (s, 1H, Ar–H), 8.25 (t, 2H, J = 8.0 Hz, Ar–H), 8.31 (d, 1H, J = 7.6 Hz, Ar–H), 8.65 (d, 1H, J = 8.0 Hz, Ar–H), 9.15–9.17 (m, 1H, Ar–H) ppm; 13C NMR (100 MHz, DMSO-d 6): δC 35.3, 111.2, 114.2, 118.9, 122.0, 122.2, 123.2, 123.3, 123.6, 124.8, 128.7, 128.9, 129.1, 129.5, 129.7, 129.8, 130.2, 130.4, 130.7, 130.8, 131.7, 133.4, 135.4, 136.1, 136.2, 136.5, 137.5, 141.7, 142.2, 144.2, 150.2, 152.3, 191.0 ppm; MS (m/z, %): 487 (M+, 10); Anal. Calcd for C33H17N3O2: C, 81.30; H, 3.51; N, 8.62 %. Found: C, 81.46; H, 3.45; N, 8.77 %.
16-(3,4-Dimethoxyphenyl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one (6h)
Yellow solid; yield 85 %, 0.444 g; mp 246–247 °C; IR (KBr) (ν max, cm−1): 2935, 1701, 1664, 1563, 1496, 1444, 1415, 1336, 1264, 1135, 994, 754; 1H NMR (400 MHz, DMSO-d 6): δH 3.89, 3.90 (s, 6H, 2OCH3), 5.88 (s, 1H, CH), 7.14 (d, 1H, J = 8.4 Hz, Ar–H), 7.77 (s, 1H, Ar–H), 7.82–7.86 (m, 1H, Ar–H), 7.89–7.92 (m, 5H, Ar–H), 7.95–7.96 (m, 1H, Ar–H), 8.00 (dd, 1H, J 1 = 8.4 Hz, J 2 = 2.0 Hz, Ar–H), 8.14 (d, 1H, J = 7.6 Hz, Ar–H), 8.27 (d, 1H, J = 8.0 Hz, Ar–H), 8.30–8.32 (m, 1H, Ar–H), 8.66 (d, 1H, J = 2.0 Hz, Ar–H), 9.24–9.26 (m, 1H, Ar–H) ppm; 13C NMR (100 MHz, DMSO-d 6): δC 34.8, 55.4, 55.8, 111.4, 111.6, 112.4, 115.7, 122.7, 122.8, 124.7, 126.0, 126.1, 128.0, 128.4, 128.7, 129.2, 129.6, 130.0, 130.2, 130.7, 130.9, 131.0, 135.5, 135.6, 139.2, 141.7, 146.3, 148.2, 149.1, 150.8, 189.8 ppm; MS (m/z, %): 522 (M+, 5); Anal. Calcd for C34H22N2O4: C, 78.15; H, 4.24; N, 5.36 %. Found: C, 78.28; H, 4.43; N, 5.19 %.
16-(2-Hydroxy-3-methoxyphenyl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one (6i)
Yellow solid; yield 87 %, 0.442 g; mp 248–250 °C; IR (KBr) (ν max, cm−1): 2935, 1708, 1669, 1591, 1505, 1446, 1394, 1338, 1254, 1120, 960, 754; 1H NMR (400 MHz, DMSO-d 6): δH 3.93 (s, 3H, OCH3), 6.02 (s, 1H, CH), 6.96 (d, 1H, J = 8.4 Hz, Ar–H), 7.76 (s, 1H, OH), 7.83–7.88 (m, 2H, Ar–H), 7.90–7.95 (m, 7H, Ar–H), 8.16 (d, 1H, J = 7.2 Hz, Ar–H), 8.28 (d, 1H, J = 8.4 Hz, Ar–H), 8.31–8.33 (m, 1H, Ar–H), 8.71 (d, 1H, J = 1.6 Hz, Ar–H), 9.25–9.27 (m, 1H, Ar–H) ppm; 13C NMR (100 MHz, DMSO-d 6): δC 34.9, 55.5, 111.4, 111.6, 112.4, 115.6, 116.8, 122.6, 122.7, 122.8, 124.7, 125.1, 128.0, 128.5, 128.7, 129.2, 130.0, 130.2, 130.9, 131.6, 135.3, 135.5, 139.1, 141.6, 146.8, 147.7, 147.9, 153.2, 189.9 ppm; MS (m/z, %): 508 (M+, 4); Anal. Calcd for C33H20N2O4: C, 77.94; H, 3.96; N, 5.51 %. Found: C, 78.12; H, 3.81; N, 5.68 %.
16-(2-Hydroxy-5-nitrophenyl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one (6j)
Yellow solid; yield 88 %, 0.460 g; mp 227–229 °C; IR (KBr) (ν max, cm−1): 2890, 1716, 1678, 1586, 1525, 1453, 1402, 1338, 1248, 1198, 960, 730; 1H NMR (400 MHz, DMSO-d 6): δH 6.03 (s, 1H, CH), 7.57–7.60 (m, 1H, Ar–H), 7.83 (t, 1H, J = 8.0 Hz, Ar–H), 7.86–7.95 (m, 3H, Ar–H), 7.97–8.05 (m, 4H, Ar–H), 8.20 (d, 1H, J = 7.6 Hz, Ar–H), 8.24–8.29 (m, 1H, Ar–H), 8.35 (d, 1H, J = 8.0 Hz, Ar–H), 8.41 (dd, 1H, J 1 = 8.0 Hz, J 2 = 1.6 Hz, Ar–H), 8.66 (d, 1H, J = 8.0 Hz, Ar–H), 9.14–9.18 (m, 1H, Ar–H), 9.55 (s, 1H, OH) ppm; 13C NMR (100 MHz, DMSO-d 6): δC 34.9, 108.4, 108.5, 109.3, 112.4, 121.5, 122.9, 123.2, 123.4, 126.6, 126.9, 128.7, 129.0, 129.7, 130.1, 130.5, 130.9, 133.9, 135.4, 136.1, 136.2, 136.6, 139.7, 142.1, 146.3, 151.0, 152.8, 153.9, 191.3 ppm; MS (m/z, %): 523 (M+, 7); Anal. Calcd for C32H17N3O5: C, 73.42; H, 3.27; N, 8.03 %. Found: C, 73.60; H, 3.43; N, 8.11 %.
Results and discussion
In this article, we began this study by subjecting 2-hydroxynaphthalene-1,4-dione, benzene-1,2-diamine and 4-nitrobenzaldehyde to reactions with 1,3-indandione in the presence of oxalic acid as catalyst in EtOH under reflux. Unfortunately, complex mixtures were observed. To minimize the formation of byproducts, the 2-hydroxynaphthalene-1,4-dione and benzene-1,2-diamine were mixed at 75 °C until in less than 5 min an orange solid of benzo[a]phenazine was formed without using any catalyst under solvent-free conditions. Next, 4-nitrobenzaldehyde, 1,3-indandione, and oxalic acid as catalyst were added and the mixture was heated to reflux under stirring in EtOH for 3 h. The desired product was obtained as a yellow solid in 82 % yield. This two-step procedure allows the one-pot four-component reaction to be controlled, avoiding the separation of intermediates, as well as time-consuming and costly purification processes.
Then, to find optimized reaction conditions, our initial efforts focused on the search for a catalyst for the one-pot, four-component condensation reaction between 2-hydroxynaphthalene-1,4-dione, benzene-1,2-diamine, aromatic aldehydes, and 1,3-indandione. For this purpose, the condensation reaction between 2-hydroxynaphthalene-1,4-dione, benzene-1,2-diamine, 4-nitrobenzaldehyde and 1,3-indandione for the synthesis of compound 6a was selected as a model reaction in the presence of different catalytic systems. So, 2-hydroxynaphthalene-1,4-dione (1 mmol) and benzene-1,2-diamine (1 mmol) were mixed at 75 °C until in less than 5 min an orange solid of benzo[a]phenazine was formed without using any catalyst under solvent-free conditions. Then, 4-nitrobenzaldehyde (1 mmol), 1,3-indandione (1 mmol), and catalyst were added, and this mixture was heated to reflux under stirring in EtOH, and the results are summarized in Table 1.
To select the best solvent for the reaction, the synthesis of compound 6a was examined in different solvents (Table 1). As Table 1 indicates, the examined solvents were not efficient separately. Higher yields and shorter reaction times were obtained when the reaction was carried out in EtOH/H2O (1:1), due to its strong hydrogen bonding ability, hydrophobic effects, and high polarity. Thus, EtOH/H2O (1:1) was used as reaction media for all reactions.
After extensive screening, we found that the optimized best yields and time profiles were obtained with 20 mol% of oxalic acid in EtOH/H2O (1:1) under reflux conditions, which furnished the corresponding 16-(4-nitrophenyl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one 6a in 92 % yield within 2 h (Table 1, entry 12). Increasing the amount of oxalic acid to more than 30 mol%, showed no substantial improvement in the yield, whereas the yield decreased by decreasing the amount of the catalyst to 10 mol%. Moreover, it was observed that the reaction did not proceed efficiently in the absence of oxalic acid after a long time (4 h). As shown in Table 1, oxalic acid as an organic catalyst afforded the better results with respect to the inorganic catalysts.
To realize the generality and versatility of the catalyst, using these optimized conditions, the reaction scope was evaluated by using different aromatic aldehydes. All the reactions were complete in 2–2.5 h and resulted in the formation of the corresponding novel 16-(aryl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one derivatives (Scheme 1, Table 2, entries 1–10) in high yields as identified by spectral data. Also, the reaction was examined in the presence of aliphatic aldehydes such as n-heptanal and n-octanal but the product expected was not obtained in these reaction conditions even after 8 h, because the activity of aliphatic aldehydes are less than aromatic aldehydes (Table 2, entries 11, 12).
The influence of electron-withdrawing and electron-donating substituents on the aromatic ring of aldehydes upon the reaction yields was investigated. The results showed that both electron-withdrawing and electron-donating substituents had no significant effect on the reaction yields. Moreover, the presence of halogen on the aromatic ring of aldehydes had negligible effect on the reaction results.
Structural assignments of these new compounds have been made on the basis of IR, 1H NMR, 13C NMR, and elemental analysis. The mass spectra of these compounds displayed molecular ion peaks at the appropriate m/z values.
Recovery of the catalysts is important in green organic synthesis. Thus, we also, for recyclability of the catalysts, investigated the recycling of the oxalic acid in EtOH/H2O (1:1) under reflux conditions using a selected model reaction of 2-hydroxynaphthalene-1,4-dione, benzene-1,2-diamine, 4-nitrobenzaldehyde and 1,3-indandione in the presence of oxalic acid as homogeneous catalyst (Table 2, entry 1).
After completion of the reaction, the reaction mixture was cooled to room temperature and 5 mL of water was added to this mixture. The oxalic acid was dissolved in water and filtered for separation of the crude product. The separated product was washed twice with water (2 × 5 mL). The resulting product subsequently recrystallized from hot ethanol to give the pure solid. After isolation of the product, the filtrate was extracted with CHCl3 (2 × 20 mL). The aqueous layer (including oxalic acid) was separated, and its solvent was evaporated to obtain pure oxalic acid.
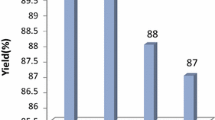
As shown in Fig. 1, the recycled catalyst was used for the next run under identical reaction conditions. It was observed that the recovered catalyst works with the same performance up to the 2nd run, while in the 3rd, 4th and 5th runs product yield gets reduced slightly that may be due to little with loss of catalyst during each recovery process.
The reusability of the catalyst (0.018 g, 0.2 mmol) in the synthesis of 16-(4-nitrophenyl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one from 2-hydroxynaphthalene-1,4-dione (1 mmol), benzene-1,2-diamine (1 mmol), 4-nitrobenzaldehyde (1 mmol) and 1,3-indandione (1 mmol) in EtOH/H2O (1:1) under reflux conditions (2 h)
A reaction mechanism consistent with the above results is shown in Scheme 2. Oxalic acid plays a key role as a Brønsted–Lowry acid catalyst in this reaction. The formation of 6 proceeds via initial condensation of 2-hydroxynaphthalene-1,4-dione 1 and benzene-1,2-diamine 2 to afford benzo[a]phenazin-5-ol 3 as reported, which in situ generates an ortho-quinone methide (o-QM) intermediate 7 upon nucleophilic addition to aldehyde. Subsequent Michael addition of the o-QM with 1,3-indandione 5, followed by cyclization and dehydration, leads to the formation of product 6.
Conclusions
In summary, we have demonstrated an eco-friendly and efficient route for the one-pot, four-component synthesis of 16-(aryl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one derivatives via the reaction of 2-hydroxynaphthalene-1,4-dione, benzene-1,2-diamine, aromatic aldehydes and 1,3-indandione by using a catalytic amount of oxalic acid in EtOH/H2O (1:1) under reflux conditions. The advantages of this synthesis are the use of an inexpensive, readily available and reusable catalyst, a convenient one-pot operation, short reaction times, easy work-up, excellent yields of products, and reduced waste production without the use of toxic catalysts and hazardous organic solvents. In all these cases, they make our work a useful protocol for a green and economically cost-effective synthesis. Moreover, this robust sequential reaction is expected to find immense application in organic synthesis, medicinal chemistry, and material sciences.
References
P. Anastas, N. Eghbali, Chem. Soc. Rev. 39, 301 (2010)
P.T. Anastas, J.C. Warner, In Green Chemistry: Theory and Practice (Oxford University Press, New York, 1998), p. 30
W. Leitner, Green Chem. 11, 603 (2009)
J. Zhu, H. Bienayme, Multicomponent Reactions (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005)
B.B. Toure, D.G. Hall, Chem. Rev. 109, 4439 (2009)
J.D. Sunderhaus, S.F. Martin, Chem. Eur. J. 15, 1300 (2009)
B. Ganem, Acc. Chem. Res. 42, 463 (2009)
J.B. Laursen, J. Nielsen, Chem. Rev. 104, 1663 (2004)
J. Bloxham, C.P. Dell, C. Smith, Heterocycles 38, 399 (1994)
S.B. Ferreira, K. Salomão, F.C. da Silva, A.V. Pinto, C.R. Kaiser, A.C. Pinto, V.F. Ferreira, S.L. de Castro, Eur. J. Med. Chem. 46, 3071 (2011)
C. Neves-Pinto, V. Malta, M. Pinto, R. Santos, S. Castro, A. Pinto, J. Med. Chem. 45, 740 (2002)
D. Cartwright, W. Chilton, D. Benson, Appl. Microbiol. Biotechnol. 43, 211 (1995)
J. Ligon, S. Dwight, P. Hammer, N. Torkewitz, D. Hofmann, H. Kempf, K. Pee, Pest. Manag. Sci. 56, 688 (2000)
M. Makgatho, R. Anderson, J. O’Sullivan, T. Egan, J. Freese, N. Cornelius, C. Van Rensburg, Drug Dev. Res. 50, 195 (2000)
V. Andrade-Nieto, M. Goulart, J.F. da Silva, M.J. da Silva, M. Pinto, A. Pinto, M. Zalis, L. Carvalho, A. Krettli, Bioorg. Med. Chem. Lett. 14, 1145 (2004)
M. Muller, T. Sorrell, Prostaglandins 50, 301 (1995)
G.W. Rewcastle, W.A. Denny, B.C. Baguley, J. Med. Chem. 30, 843 (1987)
L.F. Tietze, G. Kettschau, Top. Curr. Chem. 189, 12 (1997)
G.P. Ellis, in: A. Weissberger, E.C. Taylor (Eds.) (Wiley, New York, NY, 1977), pp. 11–139 (Chapter II)
E.A. Hafez, M.H. Elnagdi, A.G.A. Elagemey, F.M.A.A. El-Taweel, Heterocycles 26, 903 (1987)
T. Narender, S. Gupta, Bioorg. Med. Chem. Lett. 14, 3913 (2004)
K. Asres, A. Seyoum, C. Veeresham, F. Bucar, S. Gibbons, Phytother. Res. 19, 557 (2005)
A.J. Johnson, R.A. Kumar, S.A. Rasheed, S.P. Chandrika, A. Chandrasekhar, S. Baby, A. Subramoniam, J. Ethnopharmacol. 130, 267 (2010)
H. Quan-Bin, Y. Nian-Yun, T. Hong-Lei, Q. Chun-Feng, S. Jing-Zheng, C.C. Donald, C. Shi-Lin, Q.L. Kathy, X. Hong-Xi, Phytochemistry 69, 2187 (2008)
F. Eiden, F. Denk, Arch. Pharm. 324, 353 (1991)
C. Bruhlmann, F. Ooms, P.A. Carrupt, B. Testa, M. Catto, F. Leonetti, C. Altomare, A. Carotti, J. Med. Chem. 44, 3195 (2001)
S.R. Kesten, T.G. Heffner, S.J. Johnson, T.A. Pugsley, J.L. Wright, D.L. Wise, J. Med. Chem. 42, 3718 (1999)
A. Khalafi-Nezhad, A. Parhami, A. Zare, A.R. Zare, A. Hasaninejad, F. Panahi, Synthesis 4, 617 (2008)
R. Nagarajan, P.T. Perumal, Synth. Commun. 31, 1733 (2001)
S. Chandrasekhar, K. Gopalaiah, Tetrahedron Lett. 44, 7437 (2003)
H. Miyake, Y. Nakao, M. Sasaki, Chem. Lett. 36, 104 (2007)
A.Y.E. Abadi, M.T. Maghsoodlou, R. Heydari, R. Mohebat, Res. Chem. Intermed. (2015). doi:10.1007/s11164-015-2083-5
M.T. Maghsoodlou, N. Hazeri, M. Lashkari, F. Nejad Shahrokhabadi, B. Naghshbandi, M.S. Kazemi-doost, M. Rashidi, F. Mir, M. Kangani, S. Salahi, Res. Chem. Intermed. (2014). doi:10.1007/s11164-014-1793-4
F. Noori Sadeh, M.T. Maghsoodlou, N. Hazeri, M. Kangani, Res. Chem. Intermed. (2014). doi:10.1007/s11164-014-1710-x
N. Hazeri, M.T. Maghsoodlou, F. Mir, M. Kangani, H. Saravani, E. Molashahi, Chin. J. Catal. 35, 391 (2014)
S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-khorasani, A.C. Willis, Chin. Chem. Lett. 23, 569 (2012)
Acknowledgments
We gratefully acknowledge financial support from the Research Council of the Islamic Azad University of Yazd and University of Sistan and Baluchestan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohebat, R., Abadi, A.Y.E., Maghsoodlou, MT. et al. A green and efficient four-component sequential protocol for the synthesis of novel 16-(aryl)benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazin-15(16H)-one derivatives using oxalic acid as a reusable and cost-effective organic catalyst. Res Chem Intermed 42, 7121–7132 (2016). https://doi.org/10.1007/s11164-016-2522-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2522-y