Abstract
The aim of this study was to compare the degradation efficiency (DE%) of imidacloprid as a model pesticide by electro-Fenton (EF) and photoelectro-Fenton processes (PEF) using undivided three-electrode electrochemical cell and UV irradiation in a batch mode. The potential of the working electrode (graphite) was fixed at −1.0 V versus the saturated calomel electrode. The selected operating conditions for treatment of imidacloprid (20 mg/L) were: pH 2.8, Fe2+ concentration of 0.36 mM and Na2SO4 concentration of 0.15 M as the background electrolyte, which produced a DE% of 59.23 and 80.49 % for EF and PEF after 180 min, respectively. Considerable synergistic effect between EF and UV processes was observed due to the regeneration of Fe2+ ions and more production of hydroxyl radicals (·OH). Besides, accumulation of the electro-generated H2O2 in the electrochemical system as the source of ·OH radicals was confirmed. Moreover, total organic carbon measurements under the optimized condition demonstrated that 50.73 and 67.15 % of the organic substrates were mineralized after 300 min of the treatment by the EF and PEF, respectively. Eventually, the experimental results revealed that the degradation and mineralization rates of the pesticide followed pseudo-first-order kinetics; however, the rate constants of the mineralization were lower than the degradation ones owing to the generated intermediates, which required more treatment during the processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extensive kinds of pesticides are discharged into the aquatic environments from various sources such as agricultural runoffs, industrial effluents and chemical spills. These compounds are stable, carcinogenic and toxic in the environment even at their low concentrations and they can affect the aesthetic value of environment; moreover, they often have unfavourable effects on living organisms. For these reasons, strict environmental regulations are used in order to remove them from industrial wastewaters [1, 2]. There are several processes for treatment of organic contaminants in aqueous solutions such as adsorption, chemical coagulation, membrane processes and bioremediation. However, the mentioned methods generate secondary wastes by solely transferring the contaminants from a liquid to solid phase, which requires extensive treatment or partial elimination of the wastes, depending on their chemical nature and the treatment process itself [3–6].
In recent years, electrochemical advanced oxidation processes (EAOPs), including electro-Fenton (EF), photoelectro-Fenton (PEF) and anodic oxidation, have received great interest for degradation and mineralization of organic pollutants in aqueous medium owing to the production of noticeable amounts of reactive species, especially an hydroxyl radical (·OH). This radical is a powerful oxidant (E o = 2.8 V/SHE) and can destroy and mineralize water contaminants, carbon dioxide and inorganic compounds effectively and non-selectively [7, 8]. In these methods, hydrogen peroxide, which is considered as a "green" (environmentally friendly) reagent, is generated by the two-electron reduction of oxygen on the cathode surface in acidic conditions (Eq. 1) [9]. Properties of cathodes with carbon material-like graphite exhibit electrical conductivity, wide utilizable potential and low activity for hydrogen peroxide decomposition (Eq. 2), making them proper for in situ generation of H2O2; therefore, the EF process facilitates H2O2 usage by preventing its risky storage and shipment [10, 11].
Addition of Fe2+ or Fe3+ in a small amount to the acidic solution enhances the oxidation capability of the electro-generated H2O2 considerably by producing ·OH radicals via a Fenton reaction (Eq. 3). The EF process proceeds by the catalytic performance of the Fe3+/Fe2+ system, from the regeneration of Fe2+ by the reduction of Fe3+ on the cathode surface (Eq. 4), which minimizes the iron species concentrations in the solution [12, 13].
The EF process can be promoted by applying UV irradiation simultaneously, which can be explained mainly by photoreduction of Fe(OH)2+ (Eq. 5), which is the predominant form of Fe3+ in an acidic medium, and photodecomposition of stable Fe3+ complexes with generated organic ligand intermediates like carboxylic acids (Eq. 6); these two reactions in the PEF process regenerate Fe2+ and enhance the formation of active species, particularly ·OH radicals and, consequently, the destruction of organic pollutants. Furthermore, the oxidative capacity of the PEF increases due to the photolysis of H2O2 under UV irradiation to form more hydroxyl radicals (Eq. 7) [14–16].
Several researchers have reported the degradation of various organic pollutants such as pesticides or dyes by the EF or PEF processes and the destruction mechanism of the pollutants was explained properly by pseudo-first order kinetics [17–21]. Imidacloprid is a systemic pesticide used extensively in the world; moreover, it is mobile in soil, has high water solubility, is toxic and persistent in nature. It is classified by the US Environmental Protection Agency (USEPA) to be a potential water contaminant and categorized by the World Health Organization (WHO) as moderately hazardous (Class II). Hence, its treatment is essential from an environmental point of view [22]. To the best of our knowledge, there is no report for the degradation and mineralization of imidacloprid by EF and/or PEF processes in a comparative approach.
The primary aim of this research was to compare the degradation efficiency of imidacloprid as a model pesticide pollutant by the EF and PEF processes, utilizing a graphite cathode in a batch mode. Then, the effect of major operational parameters on the degradation of the contaminant, including initial concentration of imidacloprid, pH, concentration of Fe2+ ions and concentration of background electrolyte, was investigated to obtain the desired conditions. Next, the obtained data was used to study the kinetics of imidacloprid removal. Eventually, imidacloprid mineralization was monitored during both treatment processes under the optimal conditions by total organic carbon (TOC) decay.
Experimental
Chemicals
Imidacloprid (1-((6-chloro-3-pyridinyl)methyl)-N-nitro-2-imidazolidinimine) with a purity of 95 % was obtained from Chem-Service (USA) and its chemical structure and properties are presented in Table 1. Other chemicals were provided by Merck, Germany.
Experimental set-up and procedures
Experiments were carried out in an undivided three-electrode electrochemical cell controlled by a DC power supply (ADAK PS808, Iran). The working, counter and reference electrodes (Azar electrode Co., Iran) were a graphite rod (9 cm2), a platinum piece (1 cm2) and a saturated calomel electrode (SCE), respectively. A low pressure mercury lamp (15 W, UV-C, manufactured by Osram, Germany) was placed at the top of the cell with the distance of 15 cm from the solution surface and was switched on in the PEF process. First, the cell was filled with aqueous solution of imidacloprid (150 mL) containing Na2SO4 to maintain conductivity with certain concentrations. Then, H2SO4 was added to adjust the pH, and a catalytic quantity of ferrous ion (FeSO4) was added into the solution just before the beginning of the electrolysis. Prior to the each run, oxygen was bubbled for 20 min to saturate the solution with it, and during the electrolysis, oxygen was continuously sparged on the cathode surface with a flow rate of 20 mL/min. For controlled potential electrolysis, the potential of the working electrode was fixed at −1.0 V versus SCE [23]. Imidacloprid degradation was followed by a decrease in absorbance at the maximum wavelength of the pesticide (270 nm), as per a UV–Vis spectrophotometer (Elmer-Perkin, SE550), and degradation efficiency was calculated using the following equation; where A 0 and A were the absorbance at the initial and distinct time of each processes, respectively.
Hydrogen peroxide concentration was determined spectrophotometrically by the standard iodide method [24].
Results and discussion
Comparison of EF and PEF processes in degradation of imidacloprid, and kinetics study
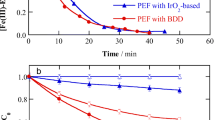
The degradation efficiency of imidacloprid (20 mg/L) at the same operational conditions was 9.14, 59.23, and 80.49 % for photolysis, EF and PEF processes after 180 min of treatment, respectively (Fig. 1). UV irradiation had no considerable effect on imidacloprid removal. However, when the EF process was carried out in the exposure of UV light at λ max = 254 nm (PEF), DE% was enhanced noticeably, owing to the increased production of ·OH radicals (Eqs. 5, 7), and the regeneration of Fe2+ (Eqs. 5, 6); hence, high oxidative capability was observed in the PEF process compared to the EF at the same operational conditions [14–16, 25].
Accumulation of the electro-generated H2O2 in the electrochemical system during the initial 3 h of electrolysis was investigated in the presence and absence of UV irradiation or Fe2+. Figure 2, curve (a) shows a gradual rise in H2O2 concentration in solution during the electrolysis without Fe2+ and UV. When Fe2+ was added to the solution without UV irradiation, less H2O2 was accumulated [Fig. 2, curve (b)] in comparison with Fig. 2, curve (a); this slightly lower concentration can be related to the decomposition of H2O2 by the Fenton reaction (Eq. 3). In the presence of Fe2+ and UV light [Fig. 2, curve (c)] the lowest H2O2 concentration was observed compared to the others; this is owing to not only the Fenton reaction (Eq. 3), but also the greater production of ·OH radicals in UV exposure (Eqs. 5, 7) and their reaction with H2O2 to yield oxygen and water (Eqs. 9, 10). As a consequence, the generation of hydroxyl radicals as the main oxidant was proven by monitoring of the H2O2 concentration [9, 16, 26].
Inset plot of Fig. 1 was depicted based on the pseudo-first order kinetics assumption (Eq. 11), and the apparent reaction rate constant (k app) for each process was determined by the slope of the plot of ln(A 0/A) versus time (t) (Table 2) with a high correlation coefficient (R 2), which confirmed the proposed mechanism [14, 27, 28].
The synergistic effect of UV irradiation and EF for degradation of imidacloprid was remarkable and expressed in the terms of the obtained apparent pseudo first-order rate constants by Eq. (12) as 42 % (Eq. 12) [9, 28].
Effect of operational parameters on EF and PEF processes
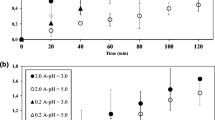
The effects of the major operating conditions, including initial imidacloprid, Fe2+ and electrolyte concentrations and pH on the degradation of the pesticide were studied. Degradation of imidacloprid declined by increasing its concentration (Fig. 3a, b) because the same amounts of active oxidizing species generated in the identical conditions of electrolysis had to degrade more pesticide and degradation intermediates [14, 25]. The desired pH for EF and PEF processes is found to be acidic, around 3, where the maximum production of ·OH radicals by the Fenton reaction is expected (Eq. 3), owing to the adequate amount of H+ for the generation of H2O2. As can be seen from Fig. 4a and b, the optimum pH was selected as 2.8, which is consistent with other research; however, at lower pHs, the reduction of H2O2 and H+ (Eqs. 13, 14) as side reactions decreased hydrogen peroxide production [29, 30].
A higher Fe2+ concentration catalyzed the formation of hydroxyl radicals (Eq. 3). However, if its concentration was more than an optimal amount, which was found to be 0.36 mmol/L (mM) in this study (Fig. 5a, b), DE% was decreased owing to the scavenging effect of extra Fe2+ ions on hydroxyl radicals (Eq. 15); furthermore, more generated Fe3+ ions also reacted with H2O2 to form hydroperoxyl radicals (HO ·2 ) (Eqs. 16, 17), which was a weaker oxidant than were the ·OH radicals [25, 31].
As can be seen in Fig. 6a, b, DE% was increased with enhancing of the electrolyte (Na2SO4) concentration due to greater H2O2 production resulting from the generation of S2O8 2− (Eqs. 18, 19). However, it was decreased when the electrolyte concentration was greater than the desired value (0.15 M), owing to the SO4 2− performance as an hydroxyl radical scavenger (Eq. 20) [32–34].
All the apparent rate constants for degradation of imidacloprid in various operational conditions by the EF and PEF processes were determined from the slope of ln(A 0/A) against process time (t) (inset plots of Figs. 3, 4, 5, 6), and are presented in Table 3.
Mineralization of imidacloprid by EF and PEF processes
The oxidizing power of the EF and PEF processes to mineralize 20 mg/L of imidacloprid in aqueous solution was monitored by TOC decay during 3 h of treatment. Figure 7 demonstrates that approximately 51 and 67 % of TOC were decreased by EF and PEF, respectively. Therefore, the applied electrochemical system can mineralize imidacloprid to inorganic compounds such as H2O and CO2. The mineralization kinetics of the pesticide also obeyed pseudo-first order kinetics (inset plot of Fig. 7) and the apparent mineralization rate constants were presented in Table 2. However, the mineralization rate was less than the degradation rate under the same experimental conditions, owing to the generated intermediates, which needed to be oxidized more to water, carbon dioxide, and inorganic salts [35, 36].
Conclusion
In this study, all of the utilized methods obeyed pseudo-first order kinetics. The synergistic effect of UV irradiation on EF was remarkable, owing to the regeneration of Fe2+ by photoreduction of Fe(OH)2+ and production of extra ·OH radicals by photodecomposition of stable Fe3+ complexes and H2O2. The optimal operational conditions for treatment of imidacloprid (20 mg/L) by EF and PEF processes were a pH of 2.8, an Fe2+ concentration of 0.36 mM and a Na2SO4 concentration of 0.15 M as the electrolyte. Furthermore, the ability of the electrochemical system to produce H2O2 was proven. TOC removal indicated that the EF and PEF processes were also able to mineralize the pesticide; however, the rate of mineralization was lower than the degradation in the same operational conditions, which can be attributed to the generated degradation intermediates.
References
W.K. Lafi, Z. Al-Qodah, J. Hazard. Mater. 137, 489–497 (2006)
V.A. Sakkas, A. Dimou, K. Pitarakis, G. Mantis, T. Albanis, Environ. Chem. Lett. 3, 57–61 (2005)
E. Ayranci, N. Hoda, Chemosphere 60, 1600–1607 (2005)
M.L. Hladik, A.L. Roberts, E.J. Bouwer, Water Res. 39, 5033–5034 (2005)
K.V. Plakas, A.J. Karabelas, Desalination 287, 255–265 (2012)
D. Singh, Indian J. Microbiol. 48, 35–40 (2008)
A. El-Ghenymy, S. Garcia-Segura, R.M. Rodríguez, E. Brillas, M.S. El Begrani, B.A. Abdelouahid, J. Hazard. Mater. 221–222, 288–297 (2012)
E.J. Ruiz, A. Hernández-Ramírez, J.M. Peralta-Hernández, C. Arias, E. Brillas, Chem. Eng. J. 171, 385–392 (2011)
B. Vahid, A.R. Khataee, Electrochim. Acta 88, 614–620 (2013)
E. Brillas, I. Sires, M.A. Oturan, Chem. Rev. 109, 6570–6661 (2009)
X. Zhang, J. Fu, Y. Zhang, L. Lei, Sep. Purif. Technol. 64, 116–123 (2008)
A.K. Abdessalem, N. Bellakhal, N. Oturan, M. Dachraoui, M.A. Oturan, Desalination 250, 450–455 (2010)
E. Brillas, B. Boye, I. Sirés, J.A. Garrido, R.M. Rodríguez, C. Arias, P.L. Cabot, C. Comninellis, Electrochim. Acta 49, 4487–4496 (2004)
A.R. Khataee, B. Vahid, B. Behjati, M. Safarpour, S.W. Joo, Chem. Eng. Res. Des. 92, 362–367 (2014)
H. Xu, W. Xu, J. Wang, Environ. Prog. Sustain. Energy 30, 208–215 (2011)
A.R. Khataee, B. Vahid, B. Behjati, M. Safarpour, Environ. Prog. Sustain. Energy 32, 557–563 (2013)
S. Irmak, H.I. Yavuz, O. Erbatur, Appl. Catal. B: Environ. 63, 243–248 (2006)
I. Sires, C. Arias, P.L. Cabot, F. Centellas, R.M. Rodriguez, J.A. Garrido, E. Brillas, Environ. Chem. 1, 26–28 (2004)
E. Brillas, B. Boye, M.A. Banos, J.C. Calpe, J.A. Garrido, Chemosphere 51, 227–235 (2003)
N. Daneshvar, S. Aber, V. Vatanpour, M.H. Rasoulifard, J. Electroanal. Chem. 615, 165–174 (2008)
C. Barrera-Díaza, F. Ureňa-Nuňez, E. Campos, M. Palomar-Pardavé, M. Romero-Romo, Radiat. Phys. Chem. 67, 657–663 (2003)
C. Cox, J. Pest. Reform. 21, 12–20 (2001)
M.A. Oturan, N. Oturan, C. Lahitte, S. Trevin, J. Electroanal. Chem. 507, 96–102 (2001)
N.V. Kiassen, D. Marchington, H.C.E. McGowant, Anal. Chem. 66, 2921–2925 (1994)
M. Zarei, A.R. Khataee, R. Ordikhani-Seyedlar, M. Fathinia, Electrochim. Acta 55, 7259–7265 (2010)
E. Brillas, J.C. Caple, J. Casado, Water Res. 34, 225–2262 (2000)
L.G. Devi, K.E. Rajashekhar, J. Mol. Catal. A: Chem. 334, 65–76 (2011)
A.R. Khataee, A. Akbarpour, B. Vahid, J. Taiwan Inst. Chem. Eng. 45, 930–936 (2014)
W.P. Ting, M.C. Lu, Y.H. Huang, J. Hazard. Mater. 161, 1484–1490 (2009)
B. Boye, M.M. Dieng, E. Brillas, J. Electroanal. Chem. 557, 135–146 (2003)
E. Neyens, J. Baeyens, J. Hazard. Mater. 98, 33–50 (2003)
G. Saracco, L. Solarino, R. Aigotti, V. Specchia, M. Maja, Electrochim. Acta 46, 373–380 (2000)
M. Zhang, T. An, X. Hu, C. Wang, G. Sheng, J. Fu, Appl. Catal. A: General 260, 215–222 (2004)
M. Diagne, N. Oturan, M.A. Oturan, Chemosphere 66, 841–848 (2007)
K. Rajeshwar, M. Osugi, W. Chanmanee, C. Chenthamarakshan, M. Zanoni, P. Kajitvichyanukul, R. Krishnan-Ayer, J. Photochem. Photobiol. C 9, 171–192 (2008)
B. Vahid, T. Mousanejad, A.R. Khataee, Res. Chem. Intermed. In press. doi: 10.1007/s11164-014-1796-1
Acknowledgments
The authors thank the University of Tabriz, Iran for financial and other supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sedaghat, M., Vahid, B., Aber, S. et al. Electrochemical and photo-assisted electrochemical treatment of the pesticide imidacloprid in aqueous solution by the Fenton process: effect of operational parameters. Res Chem Intermed 42, 855–868 (2016). https://doi.org/10.1007/s11164-015-2059-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2059-5