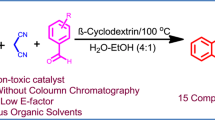
Abstract
A one-pot, multi-component, green, and highly efficient procedure has been developed for synthesis of 4-thiazolidinones. Use of β-cyclodextrin-SO3H as an eco-friendly and recyclable catalyst resulted in excellent yields under solvent-free conditions. This procedure has the advantages of readily available starting materials, short reaction times, high yields, easy workup, broad substrate scope, and use of an environment-friendly catalyst. The catalyst can be recycled with slight loss of its catalytic activity.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
4-Thiazolidinones are among the most important nitrogen and sulfur-containing heterocycles. Among diverse, medicinally relevant structures, 4-thiazolidinone derivatives are known for their wide range of pharmacological activity, for example antibacterial [1], antimalarial [2], antitubercular [3–5], anticancer [6], anti-inflammatory [7], antiproliferative [8, 9], anticonvulsant [10, 11], and antidiabetic [12] activity, and as COX-1 and COX-2 inhibitors [13, 14].
The most common approach for synthesis of the thiazolidinone ring involves three-component reaction of an aldehyde, an amine, and thioglycolic acid in one or two steps. Researchers have dedicated much effort to synthesis of 4-thiazolidinones using benzene or toluene [15, 16], ionic liquids [17, 18], PEG [19], ZnCl2 [20], Bi(SCH2COOH)3 [21], protic acid [22], zeolite [23], silica gel [24], and Saccharomyces cerevisiae [25] as catalysts. However, some of these procedures have major disadvantages, for example prolonged reaction time, use of special apparatus (Dean and Stark assembly), use of expensive and environmentally toxic catalysts and solvents, and low or moderate yields. Therefore, because of recent interest in green chemistry, there is a need to develop a simple and highly efficient procedure using a reusable and environment-friendly catalyst.
Green chemistry efficiently utilizes renewable raw materials, eliminates waste, and avoids the use of toxic and/or hazardous solvents and reagents in the manufacture of chemical products [26]. In this regard, inexpensive, biodegradable, renewable, and widely abundant biopolymers have attracted attention as solid supports for catalytic reactions [27, 28]. During last decade, a variety of biopolymers, for example chitosan [29–31], alginate [32], starch [33–35], gelatin [36], and cellulose derivatives [37–40], have been used as supports for catalytic applications. Recently, β-cyclodextrin-SO3H has emerged as a promising biopolymeric solid support acid catalyst and one of the best choices for the design and development of green methods for synthesis of pharmacologically valuable heterocycles that include 3,4-dihydropyrimidine-2(1H)-ones [41] and 2,3-dihydroquinazolin-4(1H)-ones [42].
Encouraged by this and as a part of our continual efforts toward the development of efficient, economical, and new methods [43–45], we investigated the activity of the readily available, renewable, recyclable, and environmentally benign β-cyclodextrin-SO3H as catalyst for synthesis of 4-thiazolidinone derivatives.
General experimental procedure for synthesis of 4-thiazolidinone derivatives (4 a–q)
Aromatic aldehyde (4 mmol), aromatic amine (4 mmol), thioglycolic acid (4.5 mmol), and β-cyclodextrin-SO3H catalyst (10 mol %) were mixed then heated at 90 °C. The progress of the reaction was monitored by TLC on silica with n-hexane–ethyl acetate (8:2) as mobile phase. After completion of the reaction, the mixture was cooled to room temperature, poured into cold water, and quenched with sat. NaHCO3 solution. Thus precipitate was isolated by filtration to furnish the crude product; this was then dried and crystallized from ethanol to obtain the pure compound.
Spectral data for representative compound
2-(4-Chlorophenyl)-3-phenylthiazolidin-4-one (4a): 1H NMR (300 MHz, acetone-d6): 7.49–7.45 (m, 2H, ArH), 7.35–7.25 (m, 6H, ArH), 7.17–7.11 (m, 1H, ArH), 6.47 (s, 1H, methine), 4.00–3.95 (dd, J = 1.5 Hz and 15.6 Hz, 1H, methylene), 3.87–3.81 (d, J = 1.5 Hz and 15.6 Hz, 1H, methylene); 13C NMR (75 MHz, acetone-d6): 171.22, 140.34, 139.04, 134.66, 130.53, 129.94, 129.67, 129.64, 129.31, 127.36, 126.54, 126.11, 64.51, 33.57; MS (ESI) m/z 290.1 [M + H]+; Anal. Calcd for C15H12ClNOS: C 62.17, H 4.17, N 4.83, S 11.07 %; found C 61.96, H 4.24, N 4.75, S 11.11 %.
Results and discussion
In search of the best experimental reaction conditions for preparation of 4-thiazolidinones, reaction of p-chlorobenzaldehyde (1a), aniline (2a), and thioglycolic acid (3) was selected as model reaction (Scheme 1).
In the context of green chemistry, it was decided to prefer water as solvent in our initial study for optimization of the catalyst. During this study, the model reaction was performed using water as a reaction medium at different temperatures. The desired product 4a was obtained in traces only, even after reaction for 10 h at room temperature and in water under reflux (Table 1, entries 1, 2).
In a subsequent experimental study, different acid catalysts bearing sulfonated functionality, for example sulfamic acid, p-toluenesulfonic acid (p-TSA), sulfanilic acid, camphor sulfonic acid (CSA), and β-cyclodextrin-SO3H were screened under reflux conditions with water as a solvent. Reactions conducted in the presence of sulfamic acid, p-TSA, sulfanilic acid, and CSA were sluggish, and incomplete even after 10 h with 20, 24, 30, and 32 % yields respectively (Table 1, entries 3–6). When β-cyclodextrin-SO3H was used, the reaction afforded 4a in 74 % yield within 10 h (Table 1, entry 8). The effect of β-cyclodextrin on the reaction was also studied, but 4a was obtained in 12 % yield only (Table 1, entry 7).
To investigate the effect of solvent, the model reaction was further performed with β-cyclodextrin-SO3H in different solvents (ethanol, methanol, DCM, THF, benzene and toluene; Table 2, entries 1–6). Use of these solvents failed to improve the yield and rate of the reaction, however. Considering the increasing importance of solvent-free reactions in organic synthesis, we examined the catalytic efficiency of β-cyclodextrin-SO3H in the absence of the solvent. The rate of the reaction increased substantially and the desired product 4a was obtained in higher yield 92 % (Table 2, entry 7). When the model reaction was conducted under solvent-free and catalyst-free conditions at 100 °C it afforded 4a in 16 % yield.
Subsequently, a variety of acid catalysts were tested for synthesis of 4a under solvent-free conditions; the results are listed in Table 3. β-cyclodextrin-SO3H proved superior, producing the best yield of 4a. In the absence of catalyst the product was formed in 16 % yield only (Table 3, entry 1). The order of the reactivity of the different catalysts in the reaction was: β-cyclodextrin-SO3H > silica sulfuric acid > HClO4–SiO2 > citric acid > xanthan sulfuric acid > CSA > carbon sulfuric acid > sulfanilic acid > boric acid > p-TSA > phosphotungstic acid > sulfamic acid. Thus, β-cyclodextrin-SO3H proved the most efficient acidic catalyst in terms of time and yield.
Different reaction temperatures were also investigated; the results showed that 90 °C was a suitable temperature for the reaction (Table 4, entry 5). To determine the appropriate concentration of β-cyclodextrin-SO3H, the model reaction was investigated with different concentrations (1, 5, and 10 mol %). This study indicated that 10 mol % β-cyclodextrin-SO3H was sufficient to perform the reaction smoothly (Table 4, entry 8).
To assess the generality of this reaction, substrate scope was investigated under the optimized conditions; the results are listed in Table 5. Different aldehydes and anilines were cyclocondensed with thioglycolic acid to afford a variety of 4-thiazolidinones (Table 5, entries 1–16). Aromatic aldehydes with electron-withdrawing or donating substituents reacted very well, affording good to excellent yields of 4-thiazolidinones. Yields were not significantly different, which indicated that the nature or position of the substituent had a negligible effect on the rate of the reaction. Similarly, heterocyclic aldehydes gave the expected product in excellent yields (Table 5, entry 17).
For reasons of economy, the stability and sustained activity of the catalysts are extremely important. Thus, recovery and reuse of β-cyclodextrin-SO3H in the model reaction were also studied. After completion of the reaction, the mixture was cooled to room temperature and ethyl acetate was added. The solid catalyst was isolated by filtration of the reaction mixture, washed with ethyl acetate, dried in an oven at 60 °C, then reused for further catalytic cycles. The reusability of the β-cyclodextrin-SO3H was established in up to five successive runs without significant loss of activity, as shown in Fig. 1.
A plausible mechanism for the reaction is proposed as shown in Fig. 2. β-Cyclodextrin-SO3H activates the aldehyde by increasing the electrophilicity of its carbonyl group to facilitate the formation of the imine intermediate [A]. The anion of β-cyclodextrin-SO3H might enhance the nucleophilicity of the mercapto group of thioglycolic acid, causing its facile addition to the imine intermediate generated in situ. The β-cyclodextrin-SO3H then activates the carbonyl group of acid of intermediate [B], leading to intramolecular cyclization then dehydration to afford the thiazolidinone.
To show the merits of this work over the methods reported in the literature, we compared the results obtained by use of β-cyclodextrin-SO3H with those obtained by use of [bmim][BF4] [17], [MOEMIM]TFA [17], [bmim][PF6] [18], PEG-400 [19], Bi(SCH2COOH)3 [21], HClO4–SiO2 [22], Silica gel [24], and Saccharomyces cerevisiae [25] as catalysts for synthesis of 4-thiazolidinones. As shown in Table 6, β-cyclodextrin-SO3H acts as an effective catalyst in respect of reaction time and yield of the obtained products. Thus, this procedure with β-cyclodextrin-SO3H as catalyst seems superior to other recently reported synthetic methods.
Conclusion
In conclusion, we have successfully developed a β-cyclodextrin-SO3H-catalyzed, solvent-free method for synthesis of 4-thiazolidinones. The catalyst is highly active and the corresponding products are obtained in excellent yield. The β-cyclodextrin-SO3H catalyst was, moreover, easily recovered and reused without significant loss of activity. This procedure has several advantages over other methods, for example shorter reaction times, recycling and reuse of a heterogeneous biopolymer-based solid-acid catalyst, convenient one-pot operation, and simple experimental procedure.
References
K.G. Desai, K.R. Desai, J. Sulfur Chem. 27, 315 (2006)
V.R. Solomon, W. Haq, K. Srivastava, S.K. Puri, S.B. Katti, J. Med. Chem. 50, 394 (2007)
V.P. Trivedi, N.K. Undavia, P.B. Trivadi, J. Indian Chem. Soc. 81, 506 (2004)
G.C. Kucukguzel, J.R. Shchullek, A. Kaocatepe, E. Clercq, F. Sahinv, M. Gulluce, Eur. J. Med. Chem. 41, 353 (2006)
S. Tumul, K.G. Anil, H. Wahajul, S. Sudhir, B.K. Setu, Arkivoc ii, 120 (2005)
Z. Hongyu, S. Wu, S. Zhai, A. Liu, Y. Sun, R. Li, Y. Zhang, S. Ekins, P.W. Swaan, B. Fang, B. Zhangand, B. Yan, J. Med. Chem. 51, 1242 (2008)
M.G. Vigorita, R. Ottana, F. Monforte, R. Maccari, A. Trovato, M.T. Monforte, M.F. Taviano, Bioorg. Med. Chem. Lett. 11, 2791 (2001)
R. Ottana, S. Carotti, R. Maccari, I. Landini, G. Chiricosta, B. Caciagli, M.G. Vigorita, E. Mini, Bioorg. Med. Chem. Lett. 15, 3930 (2005)
V. Gududuru, E. Hurh, J.T. Dalton, D.D. Miller, Bioorg. Med. Chem. Lett. 14, 5289 (2004)
Archana, V.K. Srivastava, A. Kumar, Eur. J. Med. Chem. 37, 873 (2002)
C. Dwivedi, S.S. Gupta, S.S. Parmar, J. Med. Chem. 15, 553 (1972)
A.C. Tripathi, S.J. Gupta, G.N. Fatima, P.K. Sonar, A. Verma, S.K. Saraf, Eur. J. Med. Chem. 72, 52 (2014)
G.C. Look, J.R. Schullek, C.P. Homes, J.P. Chinn, E.M. Gordon, M.A. Gallop, Bioorg. Med. Chem. Lett. 6, 707 (1996)
A. Zarghi, L. Najafnia, B. Daraee, O.G. Dadrass, M. Hedayati, Bioorg. Med. Chem. Lett. 17, 5634 (2007)
P.G. Belardi, D. Simonoi, F. Moroder, S. Manferdini, L. Muchhi, F.D. Vecchia, J. Heterocyl. Chem. 19, 557 (1982)
C.P. Holmes, J.P. Chinn, C.G. Look, E.M. Gorden, M.A. Gallop, J. Org. Chem. 60, 7328 (1995)
A.K. Yadav, M. Kumar, T. Yadav, R. Jain, Tetrahedron Lett. 50, 5031 (2009)
X. Zhang, X. Li, D. Li, G. Qu, J. Wang, P.M. Loiseau, X. Fan, Bioorg. Med. Chem. Lett. 19, 6280 (2009)
M.R. Bhosle, J.R. Mali, A.A. Mulay, R.A. Mane, Heteroatom Chem. 23, 166 (2012)
S.K. Srivastava, S.L. Srivastava, J. Ind. Chem. Soc. 77, 104 (2002)
N. Foroughifar, S. Ebrahimi, Chin. Chem. Lett. 24, 389 (2013)
D. Kumar, M. Sonawane, B. Pujala, V.K. Jain, S. Bhagat, A.K. Chakraborti, Green Chem. 15, 2872 (2013)
V. Tiwari, J. Meshram, P. Ali, Der Pharma Chemica 2, 187 (2010)
M.P. Thakare, P. Kumar, N. Kumar, S.K. Pandey, Tetrahedron Lett. 55, 2463 (2014)
U.R. Pratap, D.V. Jawale, M.R. Bhosle, R.A. Mane, Tetrahedron Lett. 52, 1689 (2011)
R.A. Sheldon, Green Chem. 16, 950 (2014)
R. Breslow, Acc. Chem. Res. 13, 170 (1980)
J.H. Clark, D.J. Macquarrie, Green Chemistry and Technology (Blackwell, Abingdon, 2002)
M.G. Dekamin, M. Azimoshan, L. Ramezani, Green Chem. 15, 811 (2013)
B.C.E. Makhubela, A. Jardine, G.S. Smith, Green Chem. 14, 338 (2012)
E. Guibal, Prog. Polym. Sci. 30, 71 (2005)
W.L. Wei, H.W. Zhu, C.L. Zhao, React. Funct. Polym. 59, 33 (2004)
H. Gliemann, U. Nickel, S. Schneider, J. Raman Spectosc. 29, 89 (1998)
K. Huang, L. Xue, Y.C. Hu, M.Y. Huang, Y.Y. Jiang, React. Funct. Polym. 50, 199 (2002)
M.J. Gronnow, R. Luque, D.J. Macquarrie, J.H. Clark, Green Chem. 7, 552 (2005)
C. Creechio, P. Ruggiero, M.D.R. Pizzigallo, Biotechnol. Bioeng. 48, 585 (1995)
S. Ahmad, M. Ali, Appl. Catal. A: Gen. 331, 149 (2007)
S. Ahmad, M. Ali, R. Jafar, S. Ebrahim, Chem. Pharm. Bull. 55, 957 (2007)
S. Ahmad, R. Abbas, B. Zahra, Catal. Commun. 9, 13 (2008)
J.V. Madhav, Y.T. Reddy, P.N. Reddy, M.N. Reddy, S. Kuarm, P.A. Crooks, B. Rajitha, J. Mol. Catal. A: Chem. 304, 85 (2009)
S. Asghari, M. Tajbakhsh, B.J. Kenari, S. Khaksar, Chin. Chem. Lett. 22, 127 (2011)
J. Wu, X. Du, J. Ma, Y. Zhang, Q. Shi, L. Luo, B. Song, S. Yanga, D. Hua, Green Chem. 16, 3210 (2014)
J.B. Gujar, M.A. Chaudhari, D.S. Kawade, M.S. Shingare, Tetrahedron Lett. 55, 6939 (2014)
P.V. Shinde, V.B. Labade, B.B. Shingate, M.S. Shingare, Tetrahedron Lett. 53, 1523 (2012)
K.F. Shelke, S.B. Sapkal, K.S. Niralwad, B.B. Shingate, M.S. Shingare, Cent. Euro. J. Chem. 8, 12 (2010)
Acknowledgments
An Emeritus Scientist Fellowship awarded to M.S.S. by the Council of Scientific and Industrial Research, New Delhi, is gratefully acknowledged. We are grateful to the Head, Department of Chemistry, Dr B.A.M. University, Aurangabad, for providing the laboratory facilities. M.A.C. is grateful to University authorities for financial assistance in the form of University Scholar Research Fellowship. We also thank SAIF Division, CDRI, Lucknow, for providing analytical data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaudhari, M.A., Gujar, J.B., Kawade, D.S. et al. β-Cyclodextrin-SO3H-catalyzed facile and highly efficient synthesis of 4-thiazolidinones under solvent free conditions. Res Chem Intermed 41, 10027–10035 (2015). https://doi.org/10.1007/s11164-015-2010-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2010-9