Abstract
A variety of 4H-chromenes, benzochromenes, 4,5-dihydropyrano[3,2-c]chromenes, 4H-pyran-3-carboxylates, and 3,4-disubstituted isoxazol-5(4H)-ones have been synthesized in high yields by using potassium hydrogen phthalate (KHP) as an inexpensive, commercially available catalyst. It was found that the three-component tandem reaction enabled synthesis of pyran-annulated heterocycles in water at 50 °C. 3,4-Disubstituted isoxazol-5(4H)-ones were synthesized by use of 10 mol% KHP in water at room temperature. Also, treatment of methylene-containing compounds (malononitrile or ethyl cyanoacetate) with aromatic aldehydes in the presence of 5 mol% KHP resulted in α,β-unsaturated nitriles. The procedure is an easily performed, straightforward method for synthesis of a variety of pyran-annulated compounds, isoxazol-5(4H)-one-containing heterocycles, and Knoevenagel adducts. The reaction is safe, uses mild conditions, and is environmentally benign. Other notable advantages are reuse of the catalyst, no use of hazardous organic solvents, and ease of work-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, multicomponent reactions (MCRs) have become essential, efficient, bond-forming methods for expedient synthesis of a wide range of active organic compounds and natural products without separation and purification of intermediates. The MCRs, which are important classes of chemical transformations, have recently attracted much attention owing to their high efficacy, shorter reaction times, mild conditions, simplicity, and environmental friendliness [1–3]. These effective and attractive processes also eliminate waste production and costly purification processes. They are also simple strategies for synthesis of heterocyclic structures in a single vessel from three or more components with high atom and structural economy [4–7]. Implementation of MCRs in water as reaction medium is one of the most suitable methods, and is significant in the context of green chemistry [8–12].
Pyran-annulated frameworks, for example 4H-chromene or 4H-pyran-containing heterocyclic rings are important structures with potent biological and pharmaceutical activity. Over the past few years, both these six-membered oxygen-containing heterocycles and their derivatives have attracted much attention, owing to their wide range of biological activity, for example spasmolytic, diuretic, anticoagulant, anti-anaphylactic [13], antimicrobial [14], antifungal [15], antibacterial [16], antioxidant [17], antileishmanial [18], antitumor [19], local anesthetic [20], antihistaminic [21], and antiallergenic [22] activity and because of their use for treatment of Alzheimer’s disease [23] and schizophrenia [24]. Moreover, several chromenes are widely used as pigments [25], cosmetics [26], and laser dyes [27]. Some diversely functionalized 4H-chromenes with strong biological activity are shown in Fig. 1. Compound MX58151 (A) might have potential for treatment of drug-resistant cancers [28, 29]. Chromene pyrazole derivative B is an inhibitor of human Chk1 kinase [30]. Compound EPC2407 (C) is currently in phase I/II clinical trials as vascular anticancer drug and apoptosis inducer for treatment of patients with advanced solid tumors [31]. Benzochromene LY290181 (D) is an inhibitor of diabetes-induced vascular dysfunction [26, 32–35]. 2-Amino-5-oxo-5,6,7,8-tetrahydro-4H-chromenes (E) act as antibacterial agents [36]. HA14-1 (F) is an antagonist for antiapoptotic Bcl-2 proteins which can effectively induce apoptosis of human acute myeloid leukemia cells [37]. Compounds G and H have a benzopyran core and are non-peptidic insulin-regulated aminopeptidases (IRAP) that can enhance memory in two memory models [38]. Chromene I and structurally similar compounds act as selective inhibitors of excitatory amino acid transporter 1 (EAAT1) [39].
Taking into consideration these characteristics of derivatives of highly functionalized 4H-chromenes and 4H-pyrans, much attention has been focused on the development of ecologically benign methods for synthesis of such useful heterocyclic structures by multicomponent cyclization of a variety of aldehydes, C–H enolizable molecules, and methylene-containing compounds.
Several synthetic methods have been widely applied for synthesis of pyran-based heterocyclic compounds, using many homogeneous or heterogeneous organic, inorganic, and nanomaterial catalysts, for example hexamethylenetetramine (HMT) [40], heteropolyacid [41], diammonium hydrogen phosphate [42], DBU [43], piperidine [44–47], tetrabutylammonium bromide (TBAB) [48], ionic liquids [49], morpholine [50], 4-(dimethylamino)pyridine (DMAP) [51], urea [52], 3-hydroxypropanaminium acetate (HPAA) [53], N-propylpiperazine sodium n-propionate (SBPPSP) [54], silica gel [55], sulfonic acid-functionalized silica [56], 2-hydroxyethanaminium acetate [57], α-Fe2O3 [58], cellulose-SO3H [59], potassium sodium tartrate (KNaC4H4O6.4H2O) [60], meglumine [61], I2/K2CO3 [62], cetyltrimethylammonium bromide (CTABr) [63], nano-sized MgO [64], nano-structured Na2CaP2O7 [65], NaHCO3 [66], Na2CO3 [67], triazine-functionalized ordered mesoporous organosilica [68], potassium phosphate tribasic trihydrate [69], Mg/Al hydrotalcite [70], Amberlyst A21 [71], DABCO [72], CeO2/CaO nanocomposite oxide [73], triton B [74], tetrabutylammonium chloride (TBAC) [75], nano-eggshell powder [76], basic alumina [77], [bmim]OH [78, 79], LiBr [80], glycine [81], silica nanoparticles [82], ionic liquid choline chloride-urea [83], borax [84], natural clinoptilolite (CP) zeolite [85], SBA-DABCO [86], imidazole [87], tungstic acid-functionalized SBA-15 [88], multi-walled carbon nanotube-supported Fe3O4 nanoparticles [89], CoFe2O4 nanoparticles [90], tendon hydrolysate (TH) [91], aminosilane-modified Fe3O4 nanoparticles (MNPs-NH2) [92], starch solution [93], ammonium acetate [94], aminopropylated SiO2 [95], surfactant-modified bentonite (CTMAB-bentonite) [96], and amino-functionalized MCM-41 [97].
Functionalized isoxazoles have long been regarded as “privileged substructures” in pharmaceutical chemistry and have been widely used as frameworks for drug development, salient targets in bioorganic research, and building blocks in synthetic organic chemistry [98–100]. Many isoxazole-containing heterocycles have diverse biological activity, including antimicrobial, fungicidal, anticonvulsant, HDAC inhibitory, protein-tyrosine phosphatase 1B (PTP1B) inhibitory, analgesic, antioxidant, anti-apoptotic, anti-obesity, COX-2 inhibitory, nematicidal, anti-nociceptive, anti-inflammatory, antiviral, anti-tubercular, herbicidal, protein kinase C (PKC) inhibitory, and antineoplastic [101–107] activity. The isoxazole nucleus is also found in inhibitory agents [108], sulfisoxazoles [109], antibiotics [110, 111], and anti-androgens [112, 113]. Isoxazoles are, thus, very attractive synthetic targets for investigation of efficient and green synthetic methods.
3,4-Disubstituted isoxazol-5(4H)-ones have been prepared by one-pot, three-component reaction (3CR) of β-oxoesters, hydroxylamine hydrochloride, and aryl aldehydes in the presence of organic bases [114–122] and nanomaterials [123]. Catalyst-free grinding or heating has also been reported [124]. More recently, we have used sodium ascorbate [125], sodium citrate [126], sodium saccharin [127], sodium tetraborate [128], sodium azide [129], boric acid [130], and potassium phthalimide (PPI) [131] as catalysts for synthesis of isoxazol-5(4H)-ones.
Since the discovery of the Knoevenagel condensation reaction [132], it has been shown to be a convenient method for synthesis of numerous chemical compounds including substituted olefins, coumarin derivatives [133], hetero Diels–Alder products [134], cosmetics [135], perfumes [136], pharmaceutical chemicals [137], and materials for organic solar cells [138, 139]. The Knoevenagel condensation of aldehydes with active methylene compounds gives rise to the corresponding alkene derivatives. This condensation can be easily conducted in the presence of a variety of catalysts including Lewis acids [140], zeolites [141], amine-functionalized materials [142–144], ionic liquids [145, 146], and nanomaterials [147, 148], each of which gives variable yields of the Knoevenagel adducts. In addition, solid bases [149, 150] and organic bases, for example piperidine, pyridine, urea, DABCO, and guanidine [151–153], have been used for Knoevenagel condensations.
Although the procedures listed above have their own merits, some require use of a hazardous amine-based catalyst; others use such organic solvents as DMSO and harsh reaction conditions. Some of the methods involve complex steps, furnish low yields, and require tedious work-up procedures, expensive catalysts, and special apparatus (ultrasound or microwaves). Therefore, development of efficient, simple, mild, and eco-friendly procedures for preparation of densely functionalized 4H-chromenes, 4H-pyrans, isoxazol-5(4H)-ones, and Knoevenagel products is of substantial interest. Water is one of the best solvents for many organic transformations because of its intrinsic features such as safety, nontoxicity, and nonflammability. It is also inexpensive, environmentally benign, and more readily available than organic solvents. Isolation of products from an aqueous environment is usually easy. The rate and chemoselectivity of many chemical reactions have also been improved by use of aqueous media [154–156].
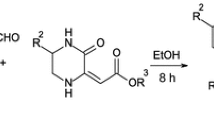
Development of solid-phase, inexpensive, green, and commercially available catalysts is an interesting topic in organic synthesis which is attracting much attention [157, 158]. Potassium hydrogen phthalate (KHP), a traditionally used primary solid standard for volumetric titrations [159] and the reference pH standards [160], can be used as a surface modifier [161], as a crystal analyzer for long-wave X-ray spectrometry, and as substrate for the growth of highly oriented films of conjugated polymers [162, 163]. Our literature survey revealed there is no report on use of KHP as a catalyst in the synthesis of 4H-chromenes, 4H-pyrans, isoxazol-5(4H)-ones, and α,β-unsaturated nitriles. To the best of our knowledge, this is the first report of the use of KHP for synthesis of these structures (Schemes 1, 2).
Experimental
General
Unless otherwise specified, all chemicals were purchased from commercial sources and were used without further purification, with the exception of liquid aldehydes, which were distilled before use. The products were characterized by comparison of their physical data with those of known samples or from their spectral data. Melting points were measured on a Buchi 510 melting point apparatus and are uncorrected. 1H NMR and 13C NMR spectra were recorded at ambient temperature on Bruker Avance DRX 500 and 400-MHz instruments with DMSO-d 6 as solvent. FT-IR spectra were recorded on a Perkin–Elmer RXI spectrometer. The progress of reactions was monitored by thin-layer chromatography (TLC) on Merck pre-coated silica gel 60 F254 aluminum sheets, visualized by use of UV light.
General procedure for synthesis of chromene derivatives (4a–n) 4H-chromene-3-carbonitriles (4a–j) benzochromenes (6a–h and 7a–l), 4,5-dihydropyrano[3,2-c]chromenes (9a–n), 5-oxo-4-aryl-5,6,7,8-tetrahydro-4H-chromenes (12a–r, 13a–e), and ethyl 6-amino-4-aryl-5-cyano-2-methyl-4H-pyran-3-carboxylates (15a–g)
A mixture of aromatic aldehyde 2 (1 mmol), C–H enolizable compounds 1, 5, 8, 10, 11, or 14 (1 mmol), malononitrile or ethyl cyanoacetate 3 (1 mmol), and KHP (25 mol%) in distilled water (5 mL) was heated at 50 °C. The progress of the reaction was monitored by TLC analysis. After completion of the reaction, the reaction mixture was cooled to room temperature and solid which precipitated was filtered, washed with cold distilled water (4 mL), and air-dried to obtain the pure products. If necessary, the solid products can be recrystallized from suitable solvent. After removal of water from the filtered solution, the catalyst was recovered and then used for subsequent reaction. The identity of the known products was confirmed by comparison of their spectroscopic data and physical properties with those available in recent papers [164–166]. Spectral data for 4b, 6e, and 7e were:
2-Amino-7-hydroxy-4-(4-nitrophenyl)-4H-chromene-3-carbonitrile (4b)
1H NMR (400 MHz, DMSO-d 6): δ = 9.83 (s, 1H), 8.22 (d, J = 8.8 Hz, 2H), 7.44 (d, J = 8.8 Hz, 2H), 7.02 (s, 2H), 6.82 (d, J = 8.4 Hz, 1H), 6.52 (dd, J = 8.4, 2.5 Hz, 1H), 6.45 (d, J = 2.5 Hz, 1H), 4.87 (s, 1H); 13C NMR (100 MHz, DMSO-d 6): δ = 161.3, 158.1, 154.4,147.0, 130.5, 129.2, 125.5, 121.3, 118.6, 113.3, 112.8, 102.9, 79.5, 55.6.
2-Amino-4-(p-tolyl)-4H-benzo[h]chromene-3-carbonitrile (6e)
1H NMR (400 MHz, DMSO-d 6): δ = 8.03 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.33–7.42 (m, 3H, ArH), 7.15–7.25 (m, 4H), 7.08 (d, J = 8.0 Hz, 1H), 6.90 (s, 2H), 4.56 (s, 1H), 2.25 (s, 3H); 13C NMR (100 MHz, DMSO-d 6): δ = 161.0, 145.3, 141.5, 133.6, 132.8, 130.4, 128.7, 127.5, 126.1, 125.7, 124.2, 123.9, 122.8, 122.1, 120.4, 119.6, 56.0, 43.8, 31.5.
3-Amino-1-(4-bromophenyl)-1H-benzo[f]chromene-2-carbonitrile (7e)
1H NMR (400 MHz, DMSO-d 6): δ = 7.84 (d, J = 8.5 Hz, 2H), 7.61-7.64 (m, 1H), 7.39–7.44 (m, 4H), 7.28 (d, J = 6.3 Hz, 2H), 7.07 (d, J = 8.4 Hz, 2H), 5.24 (s, 1H), 4.64 (s, 2H); 13C NMR (100 MHz, DMSO-d 6): δ = 160.2, 147.3, 145.6, 132.1, 131.3, 130.5, 130.2, 129.7, 128.9127.7, 125.5, 124.0, 120.9, 120.2, 117.3, 115.5, 57.7, 37.9.
General procedure for preparation of 3,4-disubstituted isoxazol-5(4H)-ones (18a–t)
Equimolar quantities of aryl aldehyde 2 (1 mmol), hydroxylamine hydrochloride 16 (0.07 g, 1 mmol), β-ketoesters 14 or 17 (0.130 g, 1 mmol), 10 mol% KHP, and distilled water (5 mL) were mixed at room temperature. After completion of the reaction, the solid product was isolated by simple filtration and washed with water (4 mL). The filtrate was evaporated to remove the water and leave the catalyst. The same catalyst was used for synthesis of further derivatives. If necessary, further purification was performed by recrystallization from hot ethanol to give the desired compounds in high yields. The identities of known products were confirmed by comparison of their spectroscopic data and physical properties with those available in recent papers [114–116, 120–131].
Spectral data for 3-(chloromethyl)-4-(4-(diethylamino)-2-hydroxybenzylidene)isoxazol-5(4H)-one (18t)
1H NMR (400 MHz, DMSO‐d 6): δ = 11.08 (s, 1H), 9.14 (d, J = 9.6 Hz, 1H), 8.07 (s, 1H), 6.52 (dd, J = 2.0, 9.6 Hz, 1H), 6.18 (d, J = 2.4 Hz, 1H), 3.50 (q, J = 6.8, 7.2 Hz, 4H), 1.17 (t, J = 6.8, 7.2 Hz, 6H); 13C NMR (100 MHz, DMSO‐d 6): δ = 170.9, 164.2, 162.2, 156.4, 143.1, 136.1, 111.9, 106.9, 101.2, 95.8, 45.2, 36.4, 13.1.
General procedure for preparation of α,β-unsaturated nitriles (19a–w)
A mixture of aryl aldehyde 2 (1 mmol), malononitrile or ethyl cyanoacetate 3 (1 mmol), and 5 mol% KHP in distilled water (5 mL) was stirred at room temperature. After completion of the reaction, the solid product was isolated by simple filtration and washed with water (4 mL). The filtrate was evaporated to remove water and leave the catalyst. The same catalyst was used for subsequent reactions. The identities of known products were confirmed by comparison of their spectroscopic data and physical properties with those available in recent papers [144, 146, 150, 153]. Spectral data for 19a and 19n were:
2-Benzylidenemalononitrile (19a)
1H NMR (400 MHz, CDCl3): δ = 7.93 (d, J = 7.6 Hz, 2H), 7.80 (s, 1H), 7.63 (t, J = 7.5 Hz, 1H), 7.55 (t, J = 7.5 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ = 160.0, 134.6, 131.0, 130.7, 129.8, 113.7, 82.9.
Ethyl 2-cyano-3-(4-nitrophenyl)acrylate (19n)
1H NMR (400 MHz, CDCl3): δ = 8.34 (d, J = 8.6 Hz, 2H), 8.32 (s, 1H), 8.15 (d, J = 8.6 Hz, 2H), 4.45 (q, J = 7.2 Hz, 2H), 1.42 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ = 161.4, 151.6, 149.8, 136.9, 131.6, 124.2, 114.5, 107.6, 63.2, 14.1.
Results and discussion
Initially, the best reaction conditions were investigated by using the reaction between equimolar quantities (1 mmol) of resorcinol (1), 4-methylbenzaldehyde (2e), and malononitrile (3a) as model reaction. The results are listed in Table 1.
It was observed that in the absence of the catalyst, reaction of resorcinol (1) with 4-methylbenzaldehyde (2e) and malononitrile (3a) led to only trace amounts of 2-amino-7-hydroxy-4-(p-tolyl)-4H-chromene-3-carbonitrile (4e) after 10 h at room temperature (RT) and 50 °C (entries 1 and 2). When the reaction was performed using 2.5 mol% KHP in water, product formation was observed after 4 h at 50 °C (entry 3), demonstrating the effectiveness of the catalyst in the reaction. When the reaction was conducted in the presence of 5 mol% KHP, the yield improved slightly (entry 4). Increasing the amount of catalyst from 5 to 10 mol% and 15 mol% did not result in significant improvement in the yields and reaction times at the same temperature (entries 5 and 6). Treating the starting materials with 20 mol% catalyst in water led to 70 % yield of expected product (4e) after 3.5 h at 50 °C (entry 7). These results encouraged us to investigate use of a larger quantity of catalyst. Performing the reaction with 25 mol% KHP in water led to the formation of 4e in 92 % yield after 3 h at 50 °C (entry 8). A larger amount of catalyst (30 mol%) neither increased the yield nor shortened the reaction time (entry 9). The product was obtained in 60 % yield when the substrates and 25 mol% KHP were stirred at RT (entry 10). It was also observed that higher reaction temperatures led to lower yields (entries 11 and 12). Solvent effects are also screened. Performing the reaction in MeCN, 1,4-dioxane, CH2Cl2, EtOAc, or CHCl3 led to a trace amount of product (4e) (entries 13–17). EtOH and a 1:1 (v/v) mixture of EtOH and H2O also afforded lower yields of the desired product (4e) under similar conditions (entries 18 and 19). Hence, use of 25 mol% catalyst and heating at 50 °C in water were selected as the best conditions for this reaction.
Using these optimized reaction conditions the scope of the reaction was investigated by use of a variety of aryl and heteroaryl aldehydes (2a–j), and 2-amino-4-aryl-7-hydroxy-4H-chromene-3-carbonitriles (4a–j) were isolated in high yields by use of this simple 3CR (Scheme 3). The results are summarized in Table 2.
These results indicated that this KHP-catalyzed 3CR worked well for a wide variety of heteroaryl aldehydes and substituted benzaldehydes with numerous functional groups including electron-deficient (for example –NO2 and –Cl) and electron-rich (for example –CH3, –OCH3, –OH, and –N(CH3)2) substituents. In all cases, the reaction proceeded smoothly and the expected products (4a–j) were obtained with high purity. If further purification was required, they could be recrystallized from ethanol. The electronic nature of the functional groups on the phenyl ring at the C-4 position has no significant effect on the yield of desired products; however the reaction times are affected. Substrates with electron-deficient functional groups (entries 2–4) reacted rapidly whereas electron-rich substituents (entries 5-8) decreased the reactivity, so longer reaction time was required. The reaction also proceeded smoothly with electron-rich aromatic heterocyclic aldehydes, for example furan-2-carbaldehyde (2i) and thiophene-2-carbaldehyde (2j), and high yields were obtained (entries 9 and 10). According to the results listed in Table 2, because of steric crowding between the two hydroxyl groups in resorcinol, reaction occurs at the C-4 position.
Inspired by these results, the optimized reaction conditions were also applied to 3CR of aryl and heteroaryl aldehydes (2) with cyano-containing compounds (3a, b), and naphthols (α-naphthol (5) and β-naphthol (6)) at 50 °C (Scheme 4).
When aryl aldehydes, malononitrile (3a) or ethyl cyanoacetate (3b), and 1-naphthol (5) were treated with 25 mol% KHP in water at 50 °C, the 2-amino-4-aryl-4H-benzo[h]chromenes (6a–h) were obtained in high yields (Table 3, entries 1–8). On replacing 1-naphthol (5) with 2-naphthol (6), the reaction was successful and 3-amino-1-aryl-1H-benzo[f]chromene derivatives (7a–l) were also isolated in high yields (Table 3, entries 9–20).
Reaction with compounds with a variety of functional groups on the phenyl ring proceeded smoothly to give high yields of the products. On the basis of the yields of the reaction, we concluded that the electronic nature of the substituent had no effect on reaction yield. However, when aromatic aldehydes with electron-deficient groups were used as substrates, the reaction time was shorter, because the reaction proceeded faster than with aromatic aldehydes with electron-rich groups. It was found that this reaction involving less reactive ethyl cyanoacetate (3b) gave the corresponding products in lower yield and required longer reaction time than malononitrile (3a) (Table 3, entries 8 and 18–20); this may be because the cyanide group stabilized the reaction intermediates better than the ester group.
Among the different attempts to extend the generality of the procedure, the aryl aldehydes (2) reacted with active methylene-containing compounds (3a, b) and 4-hydroxycoumarin (8), as an activated C–H enolizable substrate in the presence of 25 mol% KHP to give the corresponding 2-amino-5-oxo-4-aryl-4,5-dihydropyrano[3,2-c]chromenes (9a–n) in high yields (Scheme 5).
Representative results are listed in Table 4. Under the optimized reaction conditions, a wide variety of substituted benzaldehydes, including those containing electron-deficient (for example, –NO2 and –Cl; entries 2–4, 10, 12 and 13) and electron-rich (for example, –OCH3, –CH3, –N(CH3)2, –OH; entries 5–7, 9 and 14) functional groups, and a π-excessive heterocyclic aldehyde (entry 8) were investigated. The 3CR was almost equally facile with aryl aldehydes; as expected, reaction of substituted benzaldehydes bearing electron-deficient groups occurred in higher yields and with shorter reaction times than for the electron-rich counterparts.
In other attempts, the optimized reaction conditions were also applied to synthesis of 4H-chromene and 4H-pyran derivatives. Use of KHP as catalyst was investigated for preparation of 4H-chromene and 4H-pyran heterocycles from aldehydes (2), active nitrile-containing compounds (3a, b), and three enolizable 1,3-dicarbonyl compounds, 5,5-dimethylcyclohexane-1,3-dione (dimedone) (10), 1,3-cyclohexanedione (11), and ethyl acetoacetate (14) (Scheme 6).
A wide range of aryl aldehydes with electron-donating and electron-withdrawing functional groups and π-excessive heterocyclic aldehydes (i.e., furan-2-carbaldehyde and thiophene-2-carbaldehyde), were used in these 3CRs, affording the corresponding 5-oxo-4-aryl-5,6,7,8-tetrahydro-4H-chromene derivatives (12a–r, 13a–e). The products were obtained in high yields, as summarized in Table 5.
The results obtained showed that the electronic nature of the substituent on the aromatic moiety had little effect on yield. In addition, sterically hindered aldehydes, for example 2,4-dichlorobenzaldehyde (2n) and 2-nitrobenzaldehyde (2p), also reacted with malononitrile (3a) and dimedone (10) to give the corresponding products (12l and 12n) in high yields (entries 12 and 14), although the reactions proceeded rather slowly. Hence, steric factors had no remarkable effect on the rate and yield of this 3CR. In all cases, the reaction was clean, and no chromatographic separation was necessary, because no impurities were observed. It was found that the rate of reaction of ethyl cyanoacetate (3b) with cyclic 1,3-dicarbonyl compounds (10 and 11) was slower than that of malononitrile (3a), possibly because of the lower reactivity of the cyanoacetates. When ethyl acetoacetate (14) was used as enolizable substrate, the reaction proceeded efficiently, and ethyl 6-amino-5-cyano-2-methyl-4-aryl-4H-pyran-3-carboxylate derivatives (15a–g) were formed in high yields (Table 5, entries 24–30).
After successful synthesis of chromenes and 4H-pyran derivatives (described above), we decided to investigate other 3CR to the synthesis of 3,4-disubstituted isoxazol-5(4H)-ones (Scheme 2). As usual, the optimum reaction conditions were studied. Therefore, we investigated three-component synthesis of 4-(4-hydroxybenzylidene)-3-methylisoxazol-5(4H)-one (18d) as a model to find the best reaction conditions. Table 6 shows the results of these studies. The best reaction conditions were: 10 mol% KHP, reaction time 30 min, water as solvent, and RT (bold values in Table 6).
After finding the best conditions, the scope of the procedure was studied by reaction of different substituted aryl/heteroaryl aldehydes with ethyl acetoacetate (14) and hydroxylamine hydrochloride (16) in the presence of 10 mol% KHP in water at RT (entries 1–13). When ethyl 4-chloroacetoacetate (17) was used instead of ethyl acetoacetate (14) the 3CR proceeded furnishing another type of 3,4-disubstituted isoxazol-5(4H)-one derivative (entries 15–23). The results for this 3CR are presented in Table 7. The reactions are clean and afford 3,4-disubstituted isoxazol-5(4H)-ones (18a–t) in excellent yields (88–97 %) in reaction times ranging time from 30 to 120 min.
It was found that substituted benzaldehydes with electron-rich functional groups afforded the corresponding products. In addition, reaction with π-excessive heterocyclic aldehydes also proceeded smoothly with high yields in short reaction times (entries 6–7, 12, and 18). Sterically hindered aryl aldehydes (2q and 2r) also afforded the desired products (entries 10 and 20). Interestingly, when 4-hydroxy-3-nitrobezaldehyde (2s), which contains both electron-accepting (–NO2) and electron-donating (–OH) functional groups, was used, the corresponding product (18k) was obtained in a shorter reaction time and with higher yield (entry 11). Accordingly, it is concluded that this reaction is affected by electron-donor functional groups and their position on the phenyl ring. Attempts to react aromatic aldehydes bearing electron-deficient groups and π-deficient heterocyclic aldehydes were unsuccessful, even after 12 h under the optimized reaction conditions.
Although the exact mechanism of these transformations is not completely clear, on the basis of our previous work [164–166] a feasible reaction mechanism for formation of the final chromene and pyran products (4, 6, 7, 9, 12, 13, and 15) is proposed in Scheme 7. Dissociation of KHP in water gave the potassium cation (K+) and the hydrogen phthalate anion (HP−). Hydrogen phthalate reacts reversibly with water to furnish hydronium (H3O+) and phthalate anions (P2−). Removal of the acidic hydrogen from active methylene nitriles (3a, b) by P2− leads to generation of arylidenenitrile anions A. The arylidene nitrile intermediates F (Knoevenagel adducts) are then formed by Knoevenagel condensation of activated aldehydes B with intermediate arylidenenitrile anions A. The intermediates F then participate in Michael-type addition with the enolizable compounds, giving rise to in-situ formation of intermediates G (Michael adducts), which subsequently undergo intramolecular nucleophilic cyclization (Thorpe–Ziegler type reaction) and tautomerization to afford the desired pyran-based heterocyclic compounds.
To validate the suggested mechanism, we synthesized 4b in two steps under similar conditions (Scheme 8). In the first step, the Knoevenagel condensation product 19b was prepared. Measurement of the melting point of solid 19b and comparison with that of the known compound (prepared in accordance with Ref. [153]), confirmed formation of the arylidene malononitrile intermediate 19b in this step. Also, the FT-IR spectrum of intermediate 19b contained a nitrile peak at 2,220 cm−1, suggesting formation of a malononitrile. Reaction of intermediate 19b with resorcinol (1) gave product 4b, similar to the one-pot reaction. Additional evidence was obtained by TLC analysis of the model reaction. TLC analysis 7 min after initiation of the reaction showed that the spots of 3-methylbenzaldehyde and malononitrile had disappeared and a new spot had appeared. The new spot is that of the condensation product 19b, which is evidence in support of the suggested pathway.
Another pathway could be proposed for this reaction (Scheme 9). Under the optimum reaction conditions, the reaction between resorcinol (1) and 4-nitrobenzaldehyde (2b) was investigated. Increasing the reaction time to 3 h did not lead exclusively to formation of 4b. It can be concluded that the reaction proceeds via the mechanism suggested in Scheme 7.
Encouraged by the results above and the importance of α,β-unsaturated nitriles as valuable intermediates in the preparation of fine chemicals, we decided to perform the Knoevenagel reaction with wide ranges of aryl aldehydes and cyano-containing compounds (3a, b) (Scheme 10). In this reaction, α,β-unsaturated nitriles (19a–w) were obtained in excellent yields and short reaction times.
In this case, condensation of benzaldehyde (2a) with malononitrile (3a) was chosen as model reaction to find the best reaction conditions. It was found that the maximum product yield was obtained after 5 min when the reaction was conducted in the presence of 5 mol% KHP at room temperature (Table 8, entry 1). On the basis of these optimum reaction conditions, a variety of aryl aldehydes were reacted with malononitrile (3a) in the presence of KHP in water (Table 8). Both electron-rich and electron-deficient substituted benzaldehydes reacted well and gave excellent product yields in short reaction times. Substituted benzaldehydes bearing electron-rich substituents needed more time than their electron-deficient counterparts (entries 1–8 and 10–12), however. Furan-2-carbaldehyde as electron-rich heterocyclic aldehyde also afforded high product yield (entry 9). Treatment of aldehydes with ethyl cyanoacetate (3b) also produced α,β-unsaturated nitriles in excellent yields under similar reaction conditions. Compared with malononitrile (3a), reaction of ethyl cyanoacetate (3b) with the same aromatic aldehydes required longer reaction times (entries 13–23).
On the basis of our previous work [129, 131] and other research work reported in the literature [114–124], we propose the mechanism in Scheme 11 for formation of 18a–t under the reaction conditions in which KHP acts as catalyst. Initially, the oxime intermediates J are probably formed. The Knoevenagel adducts L were formed by condensation of oxime intermediates J with activated aldehydes B. In the next step, intramolecular cyclization of L leads to formation of cyclic intermediates M that subsequently undergoes proton exchange and elimination of ethanol to generate the corresponding 3,4-disubstituted isoxazol-5(4H)-ones (18a–t).
In all reactions the catalyst is recoverable by evaporation of the solvent from filtrate solution after each run. The recycled catalyst was used, untreated, for consecutive runs in four series of the same model reactions under the optimized conditions for up to five runs (Table 9). The decrease in yield is probably related to slight reduction in the activity of the catalyst or decreasing amount of the catalyst recycled, which is attributed to handling.
Conclusions
In summary, we report, for the first time, the applicability of KHP as a readily available, efficient, and solid catalyst for synthesis of diverse pyran-annulated compounds and 3,4-disubstituted isoxazol-5(4H)-ones by means of one-pot 3CRs in water. In addition, Knoevenagel condensation products were achieved in excellent yields after 5–18 min at RT. The procedure is promising from the perspectives of ecological and practical chemistry, and is applicable to a variety of substrates including aryl and heteroary aldehydes, active methylene-containing molecules, and C–H enolizable compounds; the corresponding products are obtained in excellent yields. This environmentally benign procedure has many benefits, for example clean reaction profiles, absence of hazardous organic solvents, small amounts of waste, ease of isolation of the products, efficiency, low-cost, mild conditions, and economical catalyst compared with other catalysts. The KHP catalyst is also recyclable and can be reused up to five times.
References
Y.M. Litvinov, V.Y. Mortikov, A.M. Shestopalov, J. Comb. Chem. 10, 741 (2008)
M.S. Singh, S. Chowdhury, RSC Adv. 2, 4547 (2012)
K. Wang, K. Nguyen, Y. Huang, A. Dömling, J. Comb. Chem. 11, 920 (2009)
J.-P. Wan, Y. Liu, RSC Adv. 2, 9763 (2012)
A. Dömling, W. Wang, K. Wang, Chem. Rev. 112, 3083 (2012)
C. Graaff, E. Ruijter, R.V.A. Orru, Chem. Soc. Rev. 41, 3969 (2012)
B.H. Rotstein, S. Zaretsky, V. Rai, A.K. Yudin, Chem. Rev. (2014). doi:10.1021/cr400615v
N.R. Candeias, P.M.S.D. Cal, V. Andre, M.T. Duarte, L.F. Veiros, P.M.P. Gois, Tetrahedron 66, 2736 (2010)
K. Kumaravel, G. Vasuki, Curr. Org. Chem. 13, 1820 (2009)
A. Chanda, V.V. Fokin, Chem. Rev. 109, 725 (2009)
Y. Gu, Green Chem. 14, 2091 (2012)
R.N. Butler, A.G. Coyne, Chem. Rev. 110, 6302 (2010)
J. Poupaert, P. Carato, E. Colacino, Curr. Med. Chem. 12, 877 (2005)
H.G. Kathrotiya, M.P. Patel, Med. Chem. Res. 21, 3406 (2012)
L. Alvey, S. Prado, B. Saint-Joanis, S. Michel, M. Koch, S.T. Cole, F. Tillequin, Y.L. Janin, Eur. J. Med. Chem. 44, 2497 (2009)
M. Kidwai, S. Saxena, M.K.R. Khan, S.S. Thukral, Bioorg. Med. Chem. Lett. 15, 4295 (2005)
T. Symeonidis, M. Chamilos, D.J. Hadjipavlou-Litina, M. Kallitsakis, K.E. Litinas, Bioorg. Med. Chem. Lett. 19, 1139 (2009)
T. Narender, Shweta, S. Gupta, Bioorg. Med. Chem. Lett. 14, 3913 (2004)
K. Mansouri, R. Khodarahmi, A. Foroumadi, A. Mostafaie, H. Mohammadi Motlagh, Med. Chem. Res. 20, 920 (2011)
M. Longobardi, A. Bargagna, E. Mariani, P. Schenone, E. Marmo, IL Farm. 45, 399 (1990)
K. Görlitzer, A. Dehne, E. Engler, Arch. Pharm. Weinh. Ger. 317, 526 (1983)
P. Coudert, J.M. Coyquelet, J. Bastide, Y. Marion, J. Fialip, Ann. Pharm. Fr. 46, 91 (1988)
C. Bruhlmann, F. Ooms, P. Carrupt, B. Testa, M. Catto, F. Leonetti, C. Altomare, A. Cartti, J. Med. Chem. 44, 3195 (2001)
S.R. Kesten, T.G. Heffner, S.J. Johnson, T.A. Pugsley, J.L. Wright, D.L. Wise, J. Med. Chem. 42, 3718 (1999)
G.P. Ellis, in The Chemistry of Heterocyclic of Compounds. Chromenes, Harmones and Chromones, ed. by A. Weissberger, EC Taylor (Wiley, New York, 1977), Chapter II, pp. 11–13
E.A.A. Hafez, M.H. Elnagdi, A.G.A. Elagamey, F.M.A.A. El-Taweel, Heterocycles 26, 903 (1987)
G.A. Reynolds, K.H. Drexhage, Opt. Commun. 13, 222 (1975)
W. Kemnitzer, S. Kasibhatla, S. Jiang, H. Zhang, J. Zhao, S. Jia, L. Xu, C. Crogan-Grundy, R. Denis, N. Barriault, L. Vaillancourt, S. Charron, J. Dodd, G. Attardo, D. Labrecque, S. Lamothe, H. Gourdeau, B. Tseng, J. Drewe, S.X. Cai, Bioorg. Med. Chem. Lett. 15, 4745 (2005)
W. Kemnitzer, J. Drewe, S. Jiang, H. Zhang, Y. Wang, J. Zhao, S. Jia, J. Herich, D. Labreque, R. Storer, K. Meerovitch, D. Bouffard, R. Rej, R. Denis, C. Blais, S. Lamothe, G. Attardo, H. Gourdeau, B. Tseng, S. Kasibhatla, S.X. Cai, J. Med. Chem. 47, 6299 (2004)
N. Foloppe, M. Fisher, R. Howes, A. Potter, A.G.S. Robertson, A.E. Surgenor, Bioorg. Med. Chem. 14, 4792 (2006)
S.X. Cai, J. Drewe, W. Kemnitzer, Anti-Cancer Agents Med. Chem. 9, 437 (2009)
G.P. Ellis, The Chemistry of Heterocyclic Compounds. in Chromenes, Chromanes, and Chromones, ed. by A. Taylor, EC Weissberger (Wiley, New York, 1977), Chapter II
M.A. Sofan, M.A. El-Taweel, M.H. Elnagdi, Liebigs Ann. Chem. 1989, 935 (1989)
F.M. Abdel Galil, B.Y. Riad, S.M. Sherif, M.H. Elnagdi, Chem. Lett. 11, 1123 (1982)
R.S. Varma, R. Dahiya, J. Org. Chem. 63, 8038 (1998)
D. Kumar, V.B. Reddy, S. Sharad, U. Dube, S. Kapur, Eur. J. Med. Chem. 44, 3805 (2009)
J.L. Wang, D.X. Liu, Z.J. Zhang, S.M. Shan, X.B. Han, S.M. Srinivasula, C.M. Croce, E.S. Alnemri, Z.W. Huang, Proc. Natl. Acad. Sci. USA 97, 7124 (2000)
S.J. Mountford, A.L. Albiston, W.N. Charman, L. Ng, J.K. Holien, M.W. Parker, J.A. Nicolazzo, P.E. Thompson, S.Y. Chai, J. Med. Chem. 57, 1368 (2014)
T.H.V. Huynh, B. Abrahamsen, K.K. Madsen, A. Gonzalez-Franquesa, A.A. Jensen, L. Bunch, Bioorg. Med. Chem. 20, 6831 (2012)
H.J. Wang, J. Lu, Z.H. Zhang, Monatsh. Chem. 141, 1107 (2010)
M.M. Heravi, K. Bakhtiari, V. Zadsirjan, F.F. Bamoharram, O.M. Heravi, Bioorg. Med. Chem. Lett. 17, 4262 (2007)
S. Abdolmohammadi, S. Balalaie, Tetrahedron Lett. 48, 3299 (2007)
J.M. Khurana, B. Nand, P. Saluja, Tetrahedron 66, 5637 (2010)
C.B. Sangani, D.C. Mungra, M.P. Patel, R.G. Patel, Cent. Eur. J. Chem. 9, 635 (2011)
A.M. El-Agrody, H.S.M. Abd-Rabboh, A.M. Al-Ghamdi, Med. Chem. Res. 22, 1339 (2013)
A.M. El-Agrody, A.M. Fouda, E.S.A.E.H. Khattab, Med. Chem. Res. 22, 6105–6120 (2013). doi:10.1007/s00044-013-0602-8
A.M. El-Agrody, A.M. Fouda, A.A.M. Al-Dies, Med. Chem. Res. (2014). doi:10.1007/s00044-013-0904-x
J.M. Khurana, S. Kumar, Tetrahedron Lett. 50, 4125 (2009)
A. Shaabani, S. Samadi, Z. Badri, A. Rahmati, Catal. Lett. 104, 39 (2005)
M.M. Heravi, M. Zakeri, N. Mohammadi, Chin. J. Chem. 29, 1163 (2011)
A.T. Khan, M. Lal, S. Ali, M.D. Khan, Tetrahedron Lett. 52, 5327 (2011)
G. Brahmachari, B. Banerjee, ACS Sustain Chem. Eng. 2, 411–422 (2014). doi:10.1021/sc400312n
H.R. Shaterian, A.R. Oveisi, J. Iran. Chem. Soc. 8, 545 (2011)
K. Niknam, A. Jamali, Chin. J. Catal. 33, 1840 (2012)
T.S.R. Prasanna, K.M. Raju, J. Korean Chem. Soc. 55, 662 (2011)
G. Mohammadi Ziarani, A. Badiei, M. Azizi, P. Zarabadi, Iran. J. Chem. Chem. Eng. 30, 59 (2011)
H.R. Shaterian, M. Honarmand, Synth. Commun. 41, 3573 (2011)
H. Nagabhushan, S.S. Saundalkar, L. Muralidhar, B.M. Nagabhushana, C.R. Girija, D. Nagaraja, M.A. Pasha, V.P. Jayashankara, Chin. Chem. Lett. 22, 143 (2011)
H.R. Shaterian, F. Rigi, Res. Chem. Intermed. (2013). doi:10.1007/s11164-013-1145-9
N. Hazeri, M.T. Maghsoodlou, M.R. Mousavi, J. Aboonajmi, M. Safarzaei, Res. Chem. Intermed. (2013). doi:10.1007/s11164-013-1179-z
R.-Y. Guo, Z.-M. An, L.-P. Mo, R.-Z. Wang, H.-X. Liu, S.-X. Wang, Z.-H. Zhang, ACS Comb. Sci. 15, 557 (2013)
Y.M. Ren, C. Cai, Catal. Commun. 9, 1017 (2008)
T.S. Jin, J.C. Xiao, S.J. Wang, T.S. Li, Ultrason. Sonochem. 11, 393 (2004)
J. Safari, Z. Zarnegar, M. Heydarian, J. Taibah Univ. Sci. 7, 17 (2013)
A. Solhy, A. Elmakssoudi, R. Tahir, M. Karkouri, M. Larzek, M. Bousmina, M. Zahouily, Green Chem. 12, 2261 (2010)
D. Zhou, Z. Ren, J. Chen, W. Cao, H. Deng, J. Heterocycl. Chem. 45, 1865 (2008)
M.R. Naimi-Jamal, S. Mashkouri, A. Sharifi, Mol. Divers. 14, 473 (2010)
J. Mondal, A. Modak, M. Nandi, H. Uyama, A. Bhaumik, RSC Adv. 2, 11306 (2012)
Z.Q. Zhou, F. Yang, L. Wu, A. Zhang, Chem. Sci. Trans. 1, 57 (2012)
S.R. Kale, S.S. Kahandal, A.S. Burange, M.B. Gawande, R.V. Jayaram, Catal. Sci. Technol. 3, 2050 (2009)
M. Bihani, P.P. Bora, G. Bez, H. Askari, C. R. Chim. 16, 419 (2013)
S. Shinde, G. Rashinkar, R. Salunkhe, J. Mol. Liq. 178, 122 (2013)
S. Samantaray, D.K. Pradhan, G. Hota, B.G. Mishra, Chem. Eng. J. 193–194, 1 (2012)
G. Sabitha, M. Bhikshapathi, S. Nayak, R. Srinivas, J.S. Yadav, J. Heterocycl. Chem. 48, 267 (2011)
H. Mehrabi, N. Kamali, J. Iran. Chem. Soc. 9, 599 (2012)
E. Mosaddegh, Ultrason. Sonochem. 20, 1436 (2013)
R. Maggi, R. Ballini, G. Sartori, R. Sartorio, Tetrahedron Lett. 45, 2297 (2004)
K. Gong, H.-L. Wang, J. Luo, Z.-L. Liu, J. Heterocycl. Chem. 46, 1145 (2009)
J.M. Khurana, A. Chaudhary, Green Chem. Lett. Rev. 5, 633 (2012)
W.B. Sun, P. Zhang, J. Fan, S.H. Chen, Z.H. Zhang, Synth. Commun. 40, 587 (2010)
B. Datta, M.A. Pasha, Ultrason. Sonochem. 19, 725 (2012)
S. Banerjee, A. Horn, A. Khatri, G. Sereda, Tetrahedron Lett. 52, 1878 (2011)
C.N. Revanna, T.R. Swaroop, G.M. Raghavendra, D.G. Bhadregowda, K. Mantelingu, K.S. Rangappa, J. Heterocycl. Chem. 49, 851 (2012)
A. Molla, E. Hossain, S. Hussain, RSC Adv. 3, 21517 (2013)
S.M. Baghbanian, N. Rezaei, H. Tashakkorian, Green Chem. 15, 3446 (2013)
J. Davarpanah, A.R. Kiasat, RSC Adv. 4, 4403 (2014)
M.N. Khan, S. Pal, S. Karamthulla, L.H. Choudhury, RSC Adv. 4, 3732 (2014)
S.K. Kundu, J. Mondal, A. Bhaumik, Dalton Trans. 42, 10515 (2013)
A. Fallah-Shojaei, K. Tabatabaeian, F. Shirini, S.Z. Hejazi, RSC Adv. 4, 9509 (2014)
J.K. Rajput, G. Kaur, Catal. Sci. Technol. 4, 142 (2014)
R. Sangsuwan, S. Sangher, T. Aree, C. Mahidol, S. Ruchirawatabd, P. Kittakoop, RSC Adv. 4, 13708 (2014)
J. Safari, Z. Zarnegar, J. Mol. Struct. 1072, 53 (2014)
N. Hazeri, M.Ta Maghsoodlou, F. Mir, M. Kangani, H. Saravani, E. Molashahi, Chin. J. Catal. 35, 391 (2014)
S. Kanakaraju, B. Prasanna, S. Basavoju, G.V.P. Chandramouli, Arab. J. Chem. (2013). doi: 10.1016/j.arabjc.2013.10.014
V.M. Joshi, R.L. Magar, P.B. Throat, S.U. Tekale, B.R. Patil, M.P. Kale, R.P. Pawar, Chin. Chem. Lett. 25, 455 (2014)
M.E. Sedaghat, M. Rajabpour Booshehri, M.R. Nazarifar, F. Farhadi, Appl. Clay Sci. 95, 55 (2014)
M. Mirza-Aghayan, S. Nazmdeh, R. Boukherroub, M. Rahimifard, A.A. Tarlani, M. Abolghasemi-Malakshah, Synth. Commun. 43, 1499 (2013)
T.M.V.D. Pinho e Melo, Curr. Org. Chem. 9, 925 (2005)
L. Carlsen, D. Dopp, H. Dopp, F. Duus, H. Hartmann, S. Lang-Fugmann, B. Schulze, R.K. Smalley, B.J. Wakefield, in Houben-Weyl, Methods in Organic Chemistry; ed. by E Schaumann, Georg Thieme Verlag: Stuttgart, Germany (1992), E8a, pp. 45–204
B. Frolund, A.T. Jorgensen, L. Tagmose, T.B. Stensbol, H.T. Vestergaad, C. Engblom, U. Kristiansen, C. Sanchez, P. Krogsgaard-Larsen, T. Liljefors, J. Med. Chem. 45, 2454 (2002)
W.S. Hamama, M.E. Ibrahim, H.H. Zooro, Synth. Commun. 43, 2393 (2013)
A. Babulreddy, R.V. Hymavathi, M.M. Hussain, G.N. Swamy, J. Heterocycl. Chem. 50, 727 (2013)
M. Brahmayya, B. Venkateswararao, D. Krishnarao, S. Durgarao, U.V. Prasad, T. Damodharam, R. Mishra, J. Pharm. Res. 7, 516 (2013)
H. Song, W.B. Feng, F. Cheng, D.Q. Shi, J. Heterocycl. Chem. 47, 1310 (2010)
J.P. Demers, W.E. Hageman, S.G. Johnson, D.H. Klaubert, R.A. Look, J.B. Moore, Bioorg. Med. Chem. Lett. 4, 2451 (1994)
W.M. Abdou, R.F. Barghash, R.E. Khidre, Monatsh. Chem. 144, 1233 (2013)
C. Changtam, P. Hongmanee, A. Suksamrarn, Eur. J. Med. Chem. 45, 4446 (2010)
S.K. Laughlin, M.P. Clark, J.F. Djung, A. Golebiowski, T.A. Brugel, M. Sabat, R.G. Bookland, M.J. Laufersweiler, J.C. VanRens, J.A. Townes, B. De, L.C. Hsieh, S.C. Xu, R.L. Walter, M.J. Mekel, M.J. Janusz, Bioorg. Med. Chem. Lett. 15, 2399 (2005)
V.K. Sharma, S.K. Mishra, N. Nesnas, Environ. Sci. Technol. 40, 7222 (2006)
S.A. Lawrence, V. Roth, R. Slinger, B. Toye, I. Gaboury, B. Lemyre, BMC Pediatr. 5, 49 (2005)
D.V. Vorobyeva, N.M. Karimova, I.L. Odinets, G.V. Roschenthaler, S.N. Osipov, Org. Biomol. Chem. 9, 7335 (2011)
T. Ishioka, A. Kubo, Y. Koiso, K. Nagasawa, A. Itaib, Y. Hashimotol, Bioorg. Med. Chem. 10, 1555 (2002)
T. Ishioka, A. Tanatani, K. Nagasawa, Y. Hashimoto, Bioorg. Med. Chem. Lett. 13, 2655 (2003)
Q. Liu, Y.N. Zhang, Bull. Korean Chem. Soc. 32, 3559 (2011)
Q. Liu, X. Hou, Phosphorus, Sulfur Silicon Relat. Elem. 187, 448 (2012)
Q. Liu, R.T. Wu, J. Chem. Res. 35, 598 (2011)
M. Mirzadeh, G.H. Mahdavinia, EJ Chem. 9, 425 (2012)
F. Saikh, J. Das, S. Ghosh, Terahedron Lett. 54, 4679 (2013)
K. Ablajan, H. Xiamuxi, Synth. Commun. 42, 1128 (2012)
Q.F. Cheng, X.Y. Liu, Q.F. Wang, L.S. Liu, W.J. Liu, Q. Lin, X.J. Yang, Chin. J. Org. Chem. 29, 1267 (2009)
K. Ablajan, H. Xiamuxi, Chin. Chem. Lett. 22, 151 (2011)
Y.-Q. Zhang, J.-J. Ma, C. Wang, J.-C. Li, D.-N. Zhang, X.-H. Zang, J. Li, Chin. J. Org. Chem. 28, 141 (2008)
S. Fozooni, N.G. Hosseinzadeh, H. Hamidian, M.R. Akhgar, J. Braz. Chem. Soc. 24, 1649 (2013)
Y.Q. Zhang, C. Wang, M.Y. Zhang, P.L. Cui, Y.M. Li, X. Zhou, J.C. Li, Chin. J. Org. Chem. 28, 914 (2008)
H. Kiyani, Org. Chem. Indian J. 13, 97 (2013)
H. Kiyani, F. Ghorbani, Heterocycl. Lett. 2, 145 (2013)
H. Kiyani, F. Ghorbani, Heterocycl. Lett. 3, 359 (2013)
H. Kiyani, F. Ghorbani, Open. J. Org. Chem. 1, 5 (2013)
H. Kiyani, F. Ghorbani, Elixir Org. Chem. 58A, 14948 (2013)
H. Kiyani, F. Ghorbani, Res. Chem. Intermed. (2013). doi: 10.1007/s11164-013-1411-x
H. Kiyani, F. Ghorbani, J. Saudi Chem. Soc. (2013). doi:10.1016/j.jscs.2013.11.002
E. Knoevenagel, Ber. Dtsch. Chem. Ges. 29, 172 (1896)
A. Song, X. Wang, K.S. Lam, Tetrahedron Lett. 44, 1755 (2003)
I. Devi, P.J. Bhuyan, Tetrahedron Lett. 45, 7727 (2004)
L.F. Tietze, Pure Appl. Chem. 76, 1967 (2004)
Y. Zhang, Q. Dou, L. Dai, X. Wang, Y. Chen, RSC Adv. 2, 8979 (2012)
S.M. Lai, R. Martin-Aranda, K.L. Yeung, Chem. Commun. 218−219 (2003). doi:10.1039/B209297B
A. Yassin, T. Rousseau, P. Leriche, A. Cravino, J. Roncali, Sol. Energy Mater. Sol. Cells 95, 462 (2011)
Y.D. Lin, T.J. Chow, J. Photochem, Photobiol. A: Chem. 230, 47 (2012)
P. Leelavathi, S.R. Kumar, J. Mol. Catal. A: Chem. 240, 99 (2004)
T.I. Reddy, R.S. Varma, Tetrahedron Lett. 38, 1721 (1997)
G. Li, J. Xiao, W. Zhang, Green Chem. 13, 1828 (2011)
J. Luo, T. Xin, Y. Wang, New J. Chem. 37, 269 (2013)
S. Liu, Y. Ni, J. Yang, H. Hu, A. Ying, S. Xu, Chin. J. Chem. 32, 343 (2014)
H. Valizadeh, H. Gholipour, Synth. Commun. 40, 1477 (2010)
S. Zhao, X. Wang, L. Zhang, RSC Adv. 3, 11691 (2013)
J.M. Khurana, K. Vij, Catal. Lett. 138, 104 (2010)
B.V. Kumar, H.S. Bhojyan Naik, D. Girija, B.V.J. Kumar, Chem. Sci. 123, 615 (2011)
Y. Goa, P. Wu, T. Tatsumi, J. Catal. 224, 107 (2004)
H. Kiyani, F. Ghorbani, Org. Chem. Indian J. 9, 367 (2013)
S. Ghosh, J. Das, S. Chattopadhyay, Tetrahedron Lett. 52, 2869 (2011)
J. Han, Y. Xu, Y. Su, X. She, X. Pan, Catal. Commun. 9, 2077 (2008)
Y.Q. Yu, Z.L. Wang, J. Chin. Chem. Soc. 60, 288 (2013)
M.C. Pirrung, Chem. Eur. J. 12, 1312 (2006)
S. Chitra, N. Paul, S. Muthusubramanian, P. Manisankar, Green Chem. 13, 2777 (2011)
Organic Reactions in Water: Principles, Strategies and Applications, ed. by U.M. Lindström (Blackwell, Oxford, 2007)
D.P. Debecker, E.M. Gaigneaux, G. Busca, Chem. Eur. J. 5, 3920 (2009)
K. Tanabe, W.F. Holderich, Appl. Catal. A 181, 399 (1999)
L. McMills, F. Nyasulu, R. Barlag, J. Chem. Educ. 89, 958 (2012)
D.R. White, N.A. Harris, J.P. Rife, J. Chem. Eng. Data 34, 347 (1989)
J. Li, H.D.H. Stöver, Langmuir 24, 13237 (2008)
S. Parthiban, S. Murali, G. Madhurambal, S.P. Meenakshisundaram, S.C. Mojumdar, J. Therm. Anal. Calorim. 100, 751 (2010)
K. Muthu, G. Bhagavannarayana, C. Chandrasekaran, S. Parthiban, S.P. Meenakshisundaram, S.C. Mojumdar, J. Therm. Anal. Calorim. 100, 793 (2010)
H. Kiyani, F. Ghorbani, Chem. Pap. 68, 1104 (2014)
H. Kiyani, F. Ghorbani, J. Saudi Chem. Soc. 18, 689 (2014)
H. Kiyani, F. Ghorbani, Res. Chem. Intermed. (2013). doi: 10.1007/s11164-013-1508-2
Acknowledgment
The authors are grateful to the Damghan University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiyani, H., Ghorbani, F. Efficient tandem synthesis of a variety of pyran-annulated heterocycles, 3,4-disubstituted isoxazol-5(4H)-ones, and α,β-unsaturated nitriles catalyzed by potassium hydrogen phthalate in water. Res Chem Intermed 41, 7847–7882 (2015). https://doi.org/10.1007/s11164-014-1863-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1863-7