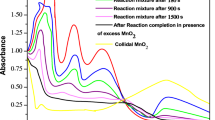
Abstract
The kinetics of propanol oxidation by quinquivalent vanadium in the presence of H2SO4 has been followed by UV-spectrophotometer at 30 °C temperature. It has been found that sodium dodecyl sulfate (SDS) micelles produce a large catalytic effect on this oxidation process. The pseudo-first-order rate constants for the reaction increases with SDS concentrations. The kinetic results have been explained by considering the preferential partitioning of reactants between the micellar and aqueous phase and also local the concentration effect. The oxidized product propionaldehyde is identified by 2,4-DNP test and 1H-NMR spectral measurement. The formation of aggregate in reaction condition was confirmed by measuring the CMC value of SDS in aqueous medium and in the presence of propanol by conductivity measurements. The change in the size of aggregate, from DLS measurement, confirms the localization of reactant species towards the aggregate, which is the consequence of rate enhancement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oxidative transformation of alcohols to their corresponding aldehydes plays a key role in the field of organic chemistry and also in the development of industrial processes. Such transformation occurs in various fields of chemistry and biology. Propionaldehyde is one of the important reactive aldehydes, which is highly inflammable in nature. There is a wide range of applications of propionaldehyde in chemical industry, e.g., in the manufacture of plastics, pesticides, perfumes, in the synthesis of rubber chemicals, and as a disinfectant and preservative. From the literature, it was found that propionaldehyde produces through the oxidation of propanol by different way [1–5]. Our main target is to develop such transformation on an industrial scale in an environmentally friendly solvent, water.
The presence of oxidants and reductants in solution usually leads to redox chemistry. Transition-metal compounds at higher oxidation states, e.g., chromium (VI) [6, 7], iron (III) [8, 9], ruthenium (III) [10], cerium (IV) [11, 12], and manganese (VII) [13, 14], have been widely used for the conversion of alcohols into carbonyl compounds. However, traditional oxidants are often toxic and release considerable amounts of by-products [15].
Vanadium is an important element in biology, inorganic chemistry, and organic synthesis [16]. The toxicity of vanadium has been found to be too much less and there is no information that vanadium compounds have embryotoxic, teratogenic, and carcinogenic effects. Therefore, we select NH4VO3 (where vanadium is in +5 state) as one of the promising oxidants for our oxidative transformation of propanol to propionaldehyde. The literature provides many examples where rapid oxidation of numerous biological compounds takes place by vanadium (V) compounds under physiological conditions [17]. Before this report, several researchers [18–20] and also our laboratory recently accounted [21–27] several kinetic studies on the oxidation of different important chemical compounds where vanadium (V) was used as an oxidant.
Here we reported the oxidation kinetics of propanol by ammonium metavanadate in aqueous media as well as aggregated surfactant media. The use of a surfactant system in this oxidation process is to speed up the time-consuming reaction in aqueous media. Surfactant is one of the important classes of amphiphilic molecules consisting of hydrophilic and hydrophobic domains. They are widely used and have an impact on almost every sector of modern industry [28].
Micellar systems and microemulsions are macroscopically homogeneous systems that act as highly efficient microscopically heterogeneous media for several reactions. Surfactant systems are used in different fields of research due to their high capability for the solubilization of reactants regardless of their type: organic, inorganic, polar, and nonpolar [29]. Several factors may account for the rate enhancement of an organic reaction in aqueous solution when the reactants are incorporated into or onto a micelle: electrostatic effects, approximation effects, and medium effects. Due to these factors, a significant amount of systematic kinetic results have been reported on the effect of micelles on various organic reactions during the last few decades [6, 7, 11–13, 21–34].
This work concentrates on the systematic kinetic study of the oxidation of propanol to propionaldehyde by quinquivalent vanadium in an environmentally friendly aqueous solvent rather than any other hazardous carcinogenic organic solvent (like acetic acid, DMSO, CHCl3, THF, toluene, etc.). The present study aims at the use of surfactant systems as a catalytic nano-reactor for the oxidation of propanol, in aqueous sulfuric acid media. Replacement of the above-mentioned organic solvents by aqueous micellar media has drawn special attention to the reaction kinetics. Surfactant medium allows for dissolving sufficient amounts of reactant (substrate and oxidant) in the interior of it surface that’s why many of the organic reaction are preferably occurs in the surfactant medium.
Experimental
Materials
The ammonium metavanadate (Fisher Scientific), propanol (Merck), sodium dodecyl sulfate (SDS) (SRL), sulfuric acid (Fisher Scientific) of AR grade were used as received. Double-distilled water was used to prepare reaction mixtures in each case, for other studies unless other systems are mentioned specifically.
It is difficult to solubilize ammonium metavanadate (NH4VO3) in aqueous medium, due to its insoluble character in hot or cold water. On heating, ammonium metavanadate was dissolved in concentrated sulfuric acid, which gives a yellow solution.
Kinetic measurement
The reactions were performed in the absence and presence of surfactant solution of different concentrations under pseudo-first-order conditions at constant sulfuric acid concentration. The reaction was initiated by the addition of ammonium metavanadate solution to the thermally equilibrated (303.0 ± 0.1 K) reaction mixture. A UV–Vis spectrophotometer (SHIMADZU-3600) of 1-cm path length cuvettes are used to monitor the progress of the reaction. For every kinetic measurement, the temperature (303.0 ± 0.1 K) of the reaction mixture was kept constant using a SHIMADZU Temperature Control System TCC 240A. The pseudo-first-order rate constants were calculated from the slopes of −ln(A285) against time (t) plot. We repeated each experiment at least twice. Rate constants have been found to be reproducible within a precision of about 3 %.
Critical micelle concentration
The critical micelle concentrations of the SDS surfactant alone and in the presence of substrate (propanol) were determined by means of conductivity studies as well. All solutions were prepared in doubly distilled water with a specific conductivity of 1–3 μS cm−1. Specific conductivity data were taken by using an EUTECH Instrument of model PCD 6500 conductometer. The conductivity cell was calibrated with KCl solutions. During measurement of conductivity, all solutions were immersed in a water bath, controlling the temperature variation at ±0.1 °C. Trials were repeated three times for reproducibility.
Dynamic light scattering
The size of the aggregated surfactant was determined by dynamic light scattering method using a Zetasizers Nano-ZS apparatus from Malvern Instruments Ltd. The beam of a 4-mW He–Ne laser (operating at a wavelength of 633 nm) was focused into the cuvette containing the surfactant solution. The intensity of scattered light was recorded in backscattering geometry at an angle of 90°. All the results were achieved in triplicate.
Product analysis
The product analysis was made under kinetic conditions. The reaction mixture with the oxidant was kept in the dark for 8 h until completion of oxidation. The solution was then treated overnight with an excess of a freshly filtered saturated solution of 2,4-dinitrophenylhydrazine in 2 M hydrochloric acid. The precipitated 2,4-dinitrophenylhydrazone (DNP) was collected by filtration. It was weighed both before and after being recrystallized from ethanol and the yield ≈85–90 %. This slightly decreased yield is probably due to the formation of hemiacetal between the carbonyl compound and the corresponding unreacted propanol. No positive test [35] for the carboxylic acids due to further oxidation of the respective aldehydes was noticed. This indicates no further oxidation of the aldehyde under the experimental conditions.
Melting point of the DNP derivative of the product aldehyde and that of an authentic sample confirmed that the product was propionaldehyde. The 1H-NMR spectra of separated oxidized product were recorded on a Bruker DPX 400-MHz spectrometer, calibrated using TMS as the internal standard in CDCl3 solvent.
Results and discussion
Spectrophotometric analysis
The stock solution of ammonium metavanadate, i.e., vanadium (V) is light yellow in color as usual. This light yellow color slowly changes to green and under experimental conditions the observed final color of the solution is electric blue, which is due to the formation of vanadium (IV). The appearance of the short-lived green color is due to the mixture of yellow vanadium (V) and blue vanadium (IV) species. The blue color of vanadium (IV) species is due to the charge transfer transition from oxygen to vanadium as well as two d–d transitions: (a) 2B2g → 2Eg, (b) 2B2g → 2B1g.
Product analysis and spectroscopic results indicate that propionaldehyde was the main oxidized product. Figure S1 represents the 1H-NMR spectra of oxidized product, separated from the reaction mixture. In the 1H-NMR spectrum, one sharp triplet (3H, δ value 1.2 ppm) was found, which is due to the –CH3 proton, another doublet of quartet (2H, δ value 2.5 ppm) for –CH2– and last one is, δ 9.8 ppm (triplet, 1H) for –CHO proton. Therefore, this redox process involves the oxidation of propanol to propionaldehyde and subsequent reduction of vanadium (V) to vanadium (IV).
Kinetics and micellar effect
We found that all solutions show UV-absorption maxima (λ) at 285 nm [36]. The pseudo-first-order rate constant value is calculated from linear straight line plot of −ln(A)285 versus time (Fig. 1 is the representative plot for determination of k obs in s−1). The vanadium (V) oxidation of propanol in aqueous medium in the presence of sulfuric acid was found to be an very time-consuming reaction and the observed pseudo-first-order rate constant value is 18.6 × 10−6 s−1. In acidic solutions (at pH 1.0) of vanadate, the major vanadium (V) species remains in the form of cationic dioxovanadium, VO2 + ion (Eq. 1).
A wide separation between alcohol and the cationic vanadium species in solution is the important factor behind the slowness of the reaction. It was earlier stated that the micellar medium, i.e., surfactant solution is one of the best choices for chemists for the solubilization of different reactants inside it. We are also interested in the beauty of the surfactant and in our study we choice an anionic surfactant, SDS. It has been found that SDS influences the rate of the oxidation route.
In the SDS-catalyzed path, the observed pseudo-first-order rate constant increases to 123.1 × 10−6 s−1 (SDS = 14 mol dm−3) and consequently the half-life (t 1/2) value decreases from 10.34 to 1.56 h (Table 1) compared to the uncatalyzed reaction. This rate enhancement is due to different reasons.
We performed the conductivity measurement for the determination of CMC value of SDS in presence of substrate and propanol of fixed concentration. It has been found that the normal CMC value of the SDS surfactant reduces from 8.02 to 6.15 mol dm−3 (Fig. 2). This diminishes in CMC value indicates that in presence of propanol (1 × 10−2 mol dm−3), the repulsion among head group reduces and the hydrophobic interaction increase to some extent. In this kinetic report, the CMC determination were performed to confirm the formation of aggregate in reaction condition.
In this oxidation process, SDS plays a catalytic role. From Fig. 3 it has been found that the k obs value increases straight way from beginning when increasing the SDS. This increase in the k obs value is due to the presence of an increasing amount of reactant molecules towards the micellar surface, i.e., local concentration effect (Scheme 1) and the reactions occurs in both region, aqueous, and micellar pseudophase (Scheme 2).
The micellar surface of SDS is anionic nature where as vanadium presence inside the solution as a cationic species. Therefore, electrostatic attractions take place between these two opposite-charged species. DLS measurements also agree with this elucidation. The size of the micelle-like aggregate formed by SDS (12 × 10−3 mol dm−3) has been found to be 18 nm (Fig. 4a). When ammonium metavanadate (vanadium (V)) presence in that SDS (12 × 10−3 mol dm−3) medium, the size of the aggregate enhances to 38 nm (Fig. 4b). This increase in size of the micellar aggregate is due to the replacement of counter ions (Na+ ion) by the positive vanadium (V) species in the Stern layer region (Scheme 1). Again, in the presence of sulfuric acid, the –OH group of propanol obtains a more positive character. Therefore, propanol also preferably choice to stay in micellar stern layer region (Scheme 1). So micelle like aggregate assists the reactant molecule to come close to each other. In the presence of SDS, this enhancement in the oxidation process is known as micellar catalysis. In SDS medium, the increment in the observed rate constant value stops at the concentration 10 × 10−3 mol dm−3 and after this, any increase in the concentration of SDS no longer influences the oxidation. This kind of behavior of micellar aggregate in the redox process was also shown by different research groups [28–31].
In the presence of propanol, the aggregation process starts at 6.15 mol dm−3. During the addition of SDS of concentration range 6.0–10.0 mol dm−3, the k obs value increases much more as the number of micelle-like aggregate increases with an average constant size in the solution. Thus, so many number of reactant molecule localized in several number of micelle like aggregate, causes enhancement in the oxidation process. Therefore, we can say that each micelle-like aggregate seems to be a micro-heterogeneous nano-reactor. Further addition of SDS (>10 mol dm−3) causes micelle–micelle repulsion, and as a result, the k obs value does not increase as earlier.
Conclusions
It has been found that the CMC value determined from conductivity measurements is well matched to the kinetically determined CMC under reaction conditions. Anionic surfactant SDS supervises this particular oxidative transformation thoroughly. The micellar catalysis is due to the ability of micelles to concentrate both reactants at their surface. By analyzing the kinetic results, CMC data, and DLS measurements, we can conclude that the SDS catalyzed redox reaction between ammonium metavanadate and propanol occurs mainly micellar pseudophase. The reaction was carried out in an environmentally friendly aqueous environment and no drastic conditions as well as no toxic chemicals (towards human health) were used as a whole.
References
R.A. Basson, H.J. van der Linde, J. Chem. Soc. A. 662–665 (1968)
R.A. Basson, H.J. van der Linde, Chem. Commun. (Lond.) 91–92 (1967)
A.R. Day, A. Eisner, J. Phys. Chem. 36, 1912–1915 (1932)
J. Schnaidt, M. Heinen, Z. Jusys, R.J. Behm, Electrochim. Acta 104, 505–517 (2013)
P. Ocon, C. Alonso, R. Celdran, J.G. Velasco, J. Electroanal. Chem. Interfacial Electrochem. 206, 179–196 (1986)
A. Ghosh, R. Saha, B. Saha, J. Ind. Eng. Chem. 20, 345–350 (2014)
A. Ghosh, R. Saha, K. Mukherjee, S.K. Ghosh, S.S. Bhattacharyya, S. Laskar, B. Saha, Int. J. Chem. Kinet. 45, 175–179 (2013)
V.V. Namboodiri, V. Polshettiwar, R.S. Varma, Tetrahedron Lett. 48, 8839–8842 (2007)
S. Rani, B.R. Bhat, Tetrahedron Lett. 51, 6403–6405 (2010)
M.S. Thompson, T.J. Meyer, J. Am. Chem. Soc. 104, 4106–4115 (1982)
A. Ghosh, R. Saha, K. Mukherjee, P. Sar, S.K. Ghosh, S. Malik, S.S. Bhattacharyya, B. Saha, J. Mol. Liq. 190, 81–86 (2014)
A. Ghosh, R. Saha, P. Sar, B. Saha, J. Mol. Liq. 186, 122–130 (2013)
M.A. Malika, S.A. AL-Thabaitib, Z. Khan, Eng. Asp. 337, 9–14 (2009)
S. Dash, S. Patel, B.K. Mishra, Tetrahedron 65, 707–739 (2009)
B. Saha, C. Orvig, Coord. Chem. Rev. 254, 2959–2972 (2010)
T. Hirao, Chem. Rev. 97, 2707–2724 (1997)
D.C. Crans, J.J. Smee, E. Gaidamauskas, L. Yang, Chem. Rev. 104, 849–902 (2004)
E.O. Odebunmi, A.S. Ogunlaja, S.O. Owalude, J. Chil. Chem. Soc. 55, 293–297 (2010)
P.C. Wilkins, M.D. Johnson, A.A. Holder, D.C. Crans, Inorg. Chem. 45, 1471–1479 (2006)
S. Malik, B. Jain, S. Ghosh, Asian J. Exp. Sci. 21, 87–92 (2007)
P. Sar, A. Ghosh, R. Saha, B. Saha, Res. Chem. Intermed. (2014). doi:10.1007/s11164-014-1635-4
P. Sar, A. Ghosh, D. Ghosh, B. Saha, Res. Chem. Intermed. (2014). doi:10.1007/s11164-014-1682-x
S.K. Ghosh, A. Basu, K.K. Paul, B. Saha, Mol. Phys. 107, 615–619 (2009)
S.K. Ghosh, R. Saha, A. Ghosh, K. Mukherjee, B. Saha, Tenside Surf. Det. 49, 296–299 (2012)
B. Saha, K.M. Chowdhury, J. Mandal, J. Solut. Chem. 37, 1321–1328 (2008)
B. Saha, S. Sarkar, K.M. Chowdhury, Int. J. Chem. Kinet. 40, 282–286 (2008)
B. Saha, Inorg. React. Mech. 6, 287–291 (2008)
K.R. Lange (ed.), Surfactants: A Practical Handbook (Hanser Publishers, Munich, 1999)
M.J. Schwuger, K. Stickdorn, R. Schomacker, Chem. Rev. 95, 849–864 (1995)
Kabir-ud-Din, M.S. Ali, Z. Khan, Colloid Polym. Sci. 284, 627–633 (2006)
C.A. Bunton, Arkivoc. 7, 490–504 (2011)
B. Kumar, M.L. Satnami, K.K. Ghosh, K. Kuca, J. Phys. Org. Chem. 25, 864–871 (2012)
M.A. Malik, F. Nabi, Z. Khan, J. Dispers Sci Technol. 29, 1396–1400 (2008)
M. Arias, L.G. Rı´O, J.C. Mejuto, P.R. Dafonte, J.S. Gaandara, J. Agric. Food Chem. 53, 7172–7178 (2005)
F. Feigl, Spot Tests in Organic Analysis, vol. 342 (Elsevier, Amsterdam, 1956), p. 121
T.S. Smith, V.L. Pecoraro, Inorg. Chem. 41, 6754–6760 (2002)
Acknowledgments
We are grateful to the UGC and CSIR, New Delhi, for providing financial help in the form of a research grant and fellowship. The authors would also like to recognize IACS, Kolkata, India, for providing SEM and DLS measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sar, P., Ghosh, A. & Saha, B. The influence of SDS micelle on the oxidative transformation of propanol to propionaldehyde by quinquivalent vanadium in aqueous medium at room temperature. Res Chem Intermed 41, 7775–7784 (2015). https://doi.org/10.1007/s11164-014-1858-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1858-4