Abstract
2-Hydroxy-5-sulfobenzoic acid (2-HSBA) efficiently catalyzed the one-pot three-component synthesis of a wide variety of 1-amidoalkyl-2-naphthols and 3,4-disubstituted isoxazol-5(4H)-ones. The three-component process of substituted benzaldehydes, 2-naphthol, and amides (benzamide and acetamide) or urea occur using 10 mol% of 2-HSBA as an organocatalyst under solvent-free reaction conditions (SFRCs) at 100 °C. It was also found that the best results for the preparation of 3,4-disubstituted isoxazol-5(4H)-ones were achieved using 15 mol% of 2-HSBA under aqueous conditions at room temperature. The reactions are easy to do and were completed within 3–25 min (for amidoalkyl naphthols), and 70–120 min (for 3,4-disubstituted isoxazol-5(4H)-ones), while the expected products were obtained in 82–97 % yields. The catalyst can be recovered and reused several times in the template reactions. This procedure offers the advantages of convenience, simple operational procedure, cost-effectiveness, no use of hazardous organic solvents, and the commercial availability of the catalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The one-pot multicomponent reaction (MCR) states that a process implemented from three or more various substrates in a single vessel reaction. This attractive approach is very successful in the preparation of various chemical libraries of eye-catching organic compounds [1]. A one-pot three-component reaction (3CR) in the organic synthetic chemistry that generates 1-aminoalkyl-2-naphthols via a MCR is called the Betti 3-component reaction (Betti-3CR). Betti-3CR, a very well-known process, was introduced by Italian chemist Mario Betti in 1900 [2]. The Betti-3CR represents an expedient protocol to achieve amidoalkyl naphthols, which are also considered as the Betti base analogous (Fig. 1). Betti bases and related molecules have attracted a lot of attention due to their applications in asymmetric synthesis [1], catalytic organic transformations (for example, Mizoroki–Heck and Ullmann coupling reactions) [3], and chiral shift reagents for carboxylic acids or chiral auxiliaries for the synthesis of α-aminophosphonic acids [4].
1-Amidoalkyl-2-naphthols are very important precursors for the preparation of significant bioactive 1-aminomalkyl-2-naphthols via amide hydrolysis, which exhibit depressor, hypotensive and bradycardia effects in humans [5]. Amidoalkyl naphthols can be used in pharmaceutical chemistry as anti-inflammatory, anthelmintic, antibacterial, and antiviral agents [6, 7]. On the other hand, 1-amidoalkyl-2-naphthols and their N-substituted derivatives can be also transformed to 1,3-oxazine-containing compounds as an special class of bioactive heterocycles that present in many biologically important natural products and drug candidates [8–10], which possess many biological activities such as antibiotic [11], antihypertensive [12], analgesic [13], antimalarial [14], antitumor [15], antrheumatic [16], antianginal [17], and anticonvulsant [18]. Consequently, the synthesis of derivatives of the amidoalkyl naphthols is of much current importance for the research groups working in the organic synthesis and medicinal chemistry fields. Numerous approaches have been reported for the synthesis of these compounds. In these methods Lewis or Brönsted acids [19], nanomaterials [20–25], and carbohydrates [26] have been applied to catalyze this transformation.
Metal-free organocatalytic multicomponent reactions (OMCRs) are one of the effective green catalytic synthetic protocols for the synthesis of 1-amidoalkyl-2-naphthols. Recently, newer metal-free organocatalytic versions, including sulfanilic acid [27], pyridinium-based ionic liquid [28], cellulose-SO3H [29], 1-methyl-3-(2-(sulfooxy)ethyl)-1H-imidazol-3-ium chloride [30], poly(4-vinylpyridinium butane sulfonic acid) hydrogen sulfate [31], sulfamic acid [32], polyethylene glycol (PEG)-based dicationic acidic ionic liquid (PEG1000-DAIL) [33], polymer-supported sulfonic acid NKC-9 [34], 1,3-disulfonic acid imidazolium hydrogen sulfate{[Dsim]HSO4} [35], N-(4-sulfonic acid)butyl triethylammonium hydrogen sulfate ([TEBSA][HSO4]) [36], heteropolyanion-based SO3H [37, 38], saccharin sulfonic acid [39], dodecylphosphonic acid [40], 2-methylpyridinium trifluoromethanesulfonate ([2-MPyH]OTf) [41], 2,4,6-trichloro-1,3,5-triazine (TCT) [42], melamine-Br3 [43], succinic acid [44], N-bromophthalimide (NBPI) [45], and 1,3-dibromo-5,5-dimethylhydantoin (DBH) [46] have been reported toward the synthesis of amidoalkyl naphthols. Despite these procedures, newer methodologies for the synthesis of amidoalkyl naphthol derivatives are still in demand.
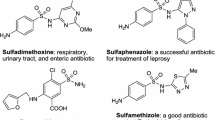
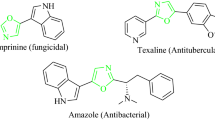
The isoxazol nucleus, on the other hand, is a key five-membered cyclic oxime ester, bearing one oxygen and one nitrogen atoms at adjacent positions, making an attractive target in bioorganic, synthetic organic chemistry, and the pharmaceutical industry, as well as also being building blocks in organic synthetic chemistry [47–49]. Isoxazol-containing heterocycles exhibit a number of applications in the wide variety of fields including merocyanine dyes [50, 51] and liquid crystalline materials [52]. In addition, many compounds containing the isoxazol ring moiety are commonly found to be associated with diverse biological activities [53–59], such as antimicrobial, fungicidal, anticonvulsant, HDAC inhibitory, protein-tyrosine phosphatase 1B (PTP1B) inhibitory, analgesic, antioxidant, anti-apoptotic, anti-obesity, COX-2 inhibitory, nematicidal, anti-nociceptive, anti-inflammatory, antiviral, anti-tubercular, herbicidal, inhibitor of protein kinase C (PKC), and antineoplastic, as well as being known to possess anti-mycobacterial effects. Furthermore, the isoxazol core is also a structural component of many drugs, for example, as inhibitor of tumor necrosis factor-alpha (TNF-α) [60], sulfisoxazoles [61], and antibiotics [62, 63], and has been used as an anti-androgen agent [64, 65]. Isoxazol-5(4H)-ones are powerful proarmoatic acceptors and can also be used for the development of optical storage, nonlinear optical research [50, 51], light-conversion molecular devices [66], and filter dyes in photographic films [67]. A number of interesting compounds with isoxazol nucleus are shown in Fig. 2.
During recent years, 3,4-disubstituted isoxazol-5(4H)-ones have been prepared by using catalytic amounts of sodium benzoate [68], sodium sulfide [69], sodium silicate [70], DABCO [71], nano-Fe2O3, clinoptilolite and H3PW12O40 [72]. The 3CR of β-oxoesters, hydroxylamine hydrochloride, and aryl aldehydes using different conditions and techniques, such as sodium acetate and visible light [73], pyridine under ultrasonic irradiation [74, 75], pyridine under reflux [76, 77], and catalyst-free/grinding or heating [78], also provides isoxazol-5(4H)-ones. Also, we synthesized the same arylmethylene isoxazol-5(4H)-ones by using catalysts including sodium ascorbate [78], sodium citrate [80], sodium saccharin [81], sodium tetraborate [82], sodium azide [83], boric acid [84], and potassium phthalimide (PPI) [85].
It is well understood that organocatalysts are simple organic molecules able to promote a wide range of chemical transformations via various activation modes and the mildness of the reaction conditions required. They also typically have prominent characteristics including metal-free environment, relatively low toxicity, simple functionality, air stability, low-cost, and biological friendliness [86–89]. Moreover, the elimination of solvents, particularly toxic solvents in chemical processes, is one of the most important aspects of green chemistry, and there has been much attention in the implementation of organic reactions under SFRCs. The solvent-free procedures often exhibit significant rate enhancements due to increased reactant concentrations. High efficiency, mild conditions, clean, cost-effectiveness, handling, and economical friendliness are the other striking features of the reactions in solvent-less conditions [90, 91]. Growing interest in the conducting organic transformations in SF presents an interesting field for this avenue of chemistry.
Water has a special place as the most attractive solvent in the synthetic chemistry. Also, the use of water as the solvent not only decreases the risk of the organic solvents but also increases the rate of chemical reactions. Also, MCR in water can be visualized as a well-designed synthetic method to attain a wide range of diverse molecular frameworks [92–95].
2-Hydroxy-5-sulfobenzoic acid (2-HSBA, 5-sulfosalicylic acid, SSA) was employed as the fixation agent in the blood proteins [96], measuring urine protein [97], evaluating the photo-reactivity of photo-catalysts [98], controlling the morphology of nanocrystals [99], an extraction agent for vitamin B6 in food [100], ligands for the formation of 3D coordination polymers with metal ions [101], the dopant in polymerization [102], as an analytical reagent for iron [103] and beryllium [104], and is effective in inducing tolerance to heat, drought and chilling stress in plants [105]. This acid is also used as a catalyst in the preparation of dihydropyrimidine-2(1H)-ones under microwave irradiation [106] and bis(indolyl)methanes in methanol [107]. In this study, a simple one-vessel 3CR condensation has been applied to the synthesis of a number of 1-amidoalkyl-2-naphthols (4a–4ad) and 3,4-disubstituted isoxazol-5(4H)-ones (7a–s) in the presence of 2-HSBA as the catalyst under solvent-free and aqueous conditions, respectively (Schemes 1, 2).
Experimental
General
All chemicals were purchased from Alfa Aesar and Aldrich and were used without further purification, with the exception of 4-methylbenzaldehyde, 4-methoxylbenzaldehyde, benzaldehyde, thiophene-2-carbaldehyde, and thiophene-3-carbaldehyde which were distilled before use. All solvents were distilled before use. The products were characterized by comparison of their physical data with those of known samples or by their spectral data. Melting points were measured on a Buchi 510 melting point apparatus and are uncorrected. NMR spectra were recorded at ambient temperature on a BRUKER AVANCE DRX-400 MHz using CDCl3 or DMSO-d 6 as the solvent. FT-IR spectra were recorded on a Perkin-Elmer RXI spectrometer. The development of reactions was monitored by thin layer chromatography (TLC) analysis on Merck pre-coated silica gel 60 F254 aluminum sheets, visualized by UV light.
Typical procedure for the preparation of 1-amidoalkyl-2-naphthols (4a-ad)
A mixture of 2-naphthol 1 (1 mmol), substituted aldehyde 2 (1 mmol), amide 3 (benzamide or acetamide, 1 mmol) or urea and 2-HSBA (10 mol%) was stirred at 100 °C in an oil bath for 3–30 min. After completion of the reaction (using TLC analysis), the reaction mixture was allowed to cool to room temperature. Next, hot ethyl acetate was added to the resulting mixture and then cooled to RT. The resulting solid product was filtered off, washed with distilled water, and dried to afford the targeted compounds. If further purification was needed, 1-amidoalkyl-2-naphthols can be recrystallized from hot ethanol. The HSBA is soluble in water and ethanol. The catalyst was recovered by evaporation of solvent from the filtrate, washed with the small amount of ethyl acetate, dried, and then used for the subsequent reaction. Spectral data for 4c and 4p were as follows:
N-((2-hydroxynaphthalen-1-yl)(3-nitrophenyl)methyl)acetamide (4c)
1H NMR (400 MHz, DMSO‐d 6): δ = 10.14 (s, 1H), 8.62 (d, J = 8.0 Hz, 1H), 8.02–7.99 (m, 2H), 7.84 (br, 1H), 7.78 (t, J = 8.6 Hz, 2H), 7.59–7.51 (m, 2H), 7.40 (t, J = 7.6 Hz, 1H), 7.26 (t, J = 7.4 Hz, 1H), 7.18 (d, J = 8.7 Hz, 1H), 7.16 (t, J = 8.0 Hz, 1H), 2.02 (s, 3H); 13C NMR (100 MHz, DMSO‐d 6): δ = 170.3, 153.9, 148.2, 145.9, 133.4, 132.7, 130.5, 130.1, 129.2, 128.9, 127.3, 123.2, 123.1, 121.8, 120.9, 118.9, 118.3, 48.2, 23.1.
N-((2-hydroxynaphthalen-1-yl)(3-nitrophenyl)methyl)benzamide (4p)
1H NMR (400 MHz, DMSO-d 6): δ = 10.42 (s, 1H), 9.15 (d, J = 8.0 Hz, 1H), 8.11–8.09 (m, 3H), 7.91–7.84 (m, 4H), 7.72 (d, J = 7.5 Hz, 1H), 7.60–7.49 (m, 5H), 7.41 (d, J = 8.0 Hz, 1H), 7.34 (t, J = 7.5 Hz, 1H), 7.25 (d, J = 8.5 Hz, 1H); 13C NMR (400 MHz, DMSO-d 6): δ = 166.8, 153.9, 148.2, 145.1, 134.4, 133.7, 132.8, 132.1, 130.5, 130.3, 129.3, 128.9, 128.8, 127.9, 127.6, 123.4, 122.9, 122.2, 121.3, 119.2, 117.3, 49.5.
Typical procedure for the synthesis of 3,4-disubstituted isoxazol-5(4H)-ones (7a-s)
A mixture of hydroxylamine hydrochloride 5 (0.0695 g, 1 mmol), β-oxoeser 6 (1 mmol), and 2-HSBA (15 mol%) in 4 mL of distilled water was stirred at room temperature for 15 min, then aromatic aldehyde 2 (1 mmol) was added to the mixture. The reaction mixture was stirred at RT until the reaction was completed. The reaction was monitored by TLC analysis up to the starting materials were consumed completely and the final product spot was not changed. After completion of the reaction, the precipitate was separated by simple filtration, and washed with cold distilled water and dried in the air. Crude products were recrystallized from ethanol (95 %) to afford the title pure compounds. The HSBA is soluble in water and ethanol. After removal of the solvent from the filtrate by evaporation, the catalyst is recovered and reused for the subsequent reactions. The identity of the known products was confirmed by comparison of their spectroscopic data and physical properties with those available in recent papers [68–85]. Spectral data for 7i and 7q were as follows:
N-(4-((3-methyl-5-oxoisoxazol-4(5H)-ylidene)methyl)phenyl)acetamide (7i)
1H NMR (400 MHz, DMSO‐d 6): δ = 10.51 (s, 1H), 8.47 (d, J = 9.2 Hz, 2H), 7.85 (s, 1H), 7.78 (d, J = 8.8 Hz, 2H), 2.28 (s, 3H), 2.12 (s, 3H); 13C NMR (100 MHz, DMSO‐d 6): δ = 170.1, 162.9, 151.5, 145.2, 136.2, 127.4, 118.8, 116.5, 24.8, 11.8.
3-(chloromethyl)-4-(4-(diethylamino)-2-hydroxybenzylidene)isoxazol-5(4H)-one (7q)
1H NMR (400 MHz, DMSO‐d 6): δ = 11.08 (s, 1H), 9.14 (d, J = 9.6 Hz, 1H), 8.07 (s, 1H), 6.52 (dd, J = 2.0, 9.6 Hz, 1H), 6.18 (d, J = 2.4 Hz, 1H), 3.50 (q, J = 6.8, 7.2 Hz, 4H), 1.17 (t, J = 6.8, 7.2 Hz, 6H); 13C NMR (100 MHz, DMSO‐d 6): δ = 170.9, 164.2, 162.2, 156.4, 143.1, 136.1, 111.9, 106.9, 101.2, 95.8, 45.2, 36.4, 13.1.
Results and discussion
At a first step, in order to find the best optimal reaction conditions, three-component condensation of 2-naphthol (1a), benzaldehyde (2a) and acetamide (3a) was employed as the template reaction. Different amounts of 2-HSBA as the catalyst as well as reaction temperature were screened using the template reaction under solvent-free reaction conditions (SFRCs). The results are shown in Table 1. By carrying out the template reaction at 100 °C without a catalyst, no product was formed even after heating for 2 h (entry 1). When 5 mol% loading of catalyst was applied for this condensation, it was observed that the corresponding product 4a was formed in 82 % yield (entry 2). The product formation in the presence of the catalyst upon heating indicates that the presence of a catalyst for this reaction is necessary. In addition, when catalyst loading was increased to 10 mol%; the reaction was completed within 8 min and up to 95 % yield of the desired product 4a was obtained (entry 3). The use of higher amounts of catalyst did not improve the results (entries 4 and 5). Hence, this amount of catalyst is sufficient to promote the reaction. The effect of temperature on the synthesis of 4a was also investigated in the presence of 10 mol% 2-HSBA loading (entries 6 and 10). It was found that the best results were obtained at 100 °C. At temperatures lower than 100 °C, the yield of the product sharply decreased even with longer reaction times (entries 6 and 8). The increase in temperature leads to a decrease in the yield of the product (entries 9 and 10). 2-HSBA shows excellent catalytic activity toward this Betti-3CR. It was concluded that implementating the reaction under SFRCs at 100 °C is the optimal condition for the Betti-3CR in the presence of 10 mol% of the catalyst.
Screening of other acidic reagents such as polyphosphoric acid, cinnamic acid, terephthalic acid, isophthalic acid, ascorbic acid, and methanesulfonic acid, was also tested (Table 2). Only trace products were detected in the presence of polyphosphoric acid, cinnamic acid, and terephthalic acid (entries 1–3). In the other cases, the yield and reaction times were not satisfactory compared with the PISA (entries 4–6).
To check the effect of solvent on the yield of the product, the template reaction was also carried out in various solvents (Table 3). Markedly low yields were observed when EtOH, H2O, CH2Cl2, EtOAc, or CH3CN were utilized as the reaction media (entries 1–5).
To study the applicability of the method, the 3CR of 2-naphthol (1) with amides and a series of structurally diverse substituted benzaldehydes (2a–o) were conducted under the optimal reaction conditions. Representative results are listed in Table 4. The reaction of benzaldehyde substituted with the electron-withdrawing group occurred at a higher yield and shorter reaction time than its electron-donating counterpart. Sterically hindered aryl aldehydes (entries 4, 6, 8, 12, 17, 19 and 20), such as 2-nitrobenzaldehyde (2d), 2-chlorobenzaldehyde (2f), 2,4-dichlorobanzaldehyde (2h) and 2,5-dimethoxybenzaldehyde (2l), also reacted with 2-naphthol (1) and amides (3a, b) to give the corresponding 1-amidoalkyl-2-naphthols in excellent yields, although in some cases the reactions proceeded rather slowly. The use of urea (3c) in the synthesis of the title compounds also gave similar results (entries 26–30). The reaction of 2-naphthol (1) and acetamide (3a) was also performed under the same optimal reaction conditions in the presence of an aliphatic aldehyde, n-butyraldehyde; however, no corresponding amidoalkyl naphthol product was observed after 20 h. In addition, a reaction of 2-naphthol (1) and acetamide (3a) with α,β-unsaturated aldehydes (α-methylcinnamaldehyde and cinnamaldehyde) was implemented and did not lead to product formation after 20 h. On the other hand, the reaction of 2-naphthol (1) and acetamide (3a) with hetero-aromatic aldehydes such as furan-2-carbaldehyde and thiophene-2-carbaldehyde led to corresponding products with 60 and 70 % yields, respectively. This approach was highly operative for the preparation of targeted compounds (4a–ad) as well as, in all cases, 1-amidoalkyl-2-naphthols were the sole products and no by-products were observed.
After the successful synthesis of a series of 1-amidoalkyl-2-naphthols, we turned our attention toward the synthesis of 3,4-disubstituted isoxazol-5(4H)-ones in the presence of 2-HSBA (Scheme 2).
Treatment of 2n with hydroxylamine hydrochloride (5), ethyl acetoacetate (6a), and 10 mol% of 2-HSBA at room temperature in H2O led to the 4-(4-hydroxy-3-methoxybenzylidene)-3-methylisoxazol-5(4H)-one (7h) in 90 % yield for 90 min (Table 5, entry 4). This reaction was selected as a template reaction and the results are summarized in Tables 5 and 6. When 15 mol% of 2-HSBA was used, the desired product was obtained in 96 % yield after 70 min. The reaction in the absence of a catalyst afforded 7h in 55 % yield at longer reaction times (Table 5, entry 1). Using lower amounts of 2-HSBA, the reaction proceed in moderate yields (Table 5, entries 2 and 3). The use of higher amounts of catalyst did not improve the yield and reaction times (Table 5, entry 6). Performing the reaction at higher temperatures using 2-HSBA as the catalyst did not afford satisfactory results (Table 5, entries 7–10). The effect of various solvents including EtOH, acetone, 1,4-dioxane, n-hexane, CH2Cl2, and a mixture of ethanol–water (EtOH:H2O, V:V) was also explored (Table 6). In these cases, the use of other solvents rather than water resulted in low reaction yields (entries 1–6). Under solvent-less conditions, no obvious improvement of the reaction was observed (entry 7). Therefore, water was the most effective solvent in this reaction. In terms of catalyst amounts, temperature and solvent effects, the use of 15 mol% of the catalyst, room temperature and water were selected as the optimized reaction conditions for the present reaction.
With the optimized reaction conditions in hand, the generality of this reaction then examined, as shown in Table 7. A range of aryl aldehyde derivatives with different substituents on the benzene ring were reacted with hydroxylamine hydrochloride (5) and β-oxoesters (6a, b) smoothly to give the corresponding 3,4-disubstituted isoxazol-5(4H)-ones (7a–s) in good to high yields. The reaction was clean, and no chromatographic separation was performed because no impurities were observed. These results show that aromatic aldehydes bearing electron-donating functional groups proved to be good substrates and reacted smoothly. It seems that substituents at ortho-position on the phenyl ring had a slight effect on the yields and reaction times (entries 6, 9 and 21). Containing 2-thienyl and 3-thienyl, heterocyclic substrates both can be converted to the desired products in 94, 88 and 89 % yields, respectively (entries 10–11, and 18). Also, a reaction of ethyl acetoacetate (6a) and hydroxylamine hydrochloride (5) with α,β-unsaturated aldehydes such as cinnamaldehyde was implemented and lead to product formation with high yield (entry 19). Not successful, attempts to conduct the reaction with the electron-withdrawing groups on the phenyl ring and aliphatic aldehydes.
The possible reaction mechanism for the green formation of 1-amidoalkyl-2-naphthol products (4a–ad) can be presented in Scheme 3. This process could proceeds via formation of ortho-quinone methides (o-QMs) [19, 35, 39] between 2-naphthol (1) and the substituted benzaldehydes (2a–o), assisted by catalyst. Then, the Michael addition of amides (3a, b or urea, 3c) to the ortho-QMs offers the 1-amidoalkyl-2-naphthols. The acidic functional groups of 2-HSBA could provide acidic site for activating substituted benzaldehydes efficiently, so facilitating this 3-CR.
Based on the literature [68–85], the following mechanism also proposed for the 3C synthesis of 3,4-disubstituted isoxazol-5(4H)-ones (7a–s) (Scheme 4). It is reasonable to assume that oxime derivatives C were formed by the condensation reaction of hydroxylamine hydrochloride with β-ketoester 5. Then the Knoevenagel adducts F were formed through the condensation of intermediate C and protonated aryl aldehyde 2. The next step may involve intramolecular O-attack cyclization of F to cyclic derivatives G, which undergoes a proton exchange, followed by H is deethanolized to target products 7a–s.
The reusability of the 2-HSBA was also studied (Tables 8, 9). The catalyst was recovered after each run and reused for subsequent cycles (Table 8). It showed nearly the same activity but with a slight decrease of yield. The decreasing of the yield of the product is probably related to a slight decrease in the catalytic activity of the catalyst or could be attributed to the loss of catalyst recovery in the course of the reaction. In addition, Table 9 shows that the catalytic was recycled and reused three times.
The benefit of the catalyst and comparison of the efficiency of the 2-HSBA catalyst with other catalysts in the synthesis of 1-amidoalkyl-2-naphthols and 3,4-disubstituted isoxazol-5(4H)-ones are indicated in Tables 10 and 11. It is clear from these data that 2-HSBA is comparable to the formerly reported approaches in terms of reaction times and yields. Contrasting some of the previous reported methods, this procedure does not require any additives, ionic liquids or hazardous solvents such as chloroform in the synthesis of 1-amidoalkyl-2-naphthols. In addition, according to Table 11, there is no need for bases such as pyridine, heating or special devices such as ultrasound.
Conclusions
In summary, the present methodology is a green, simple, and environmentally benign protocol to access a series of 1-amidoalkyl-2-naphthols and 3,4-disubstituted isoxazol-5(4H)-ones in high yields and shorter reaction times. The 3CR are conducted under thermal SFRCs yielding the corresponding Betti analogue compounds. It was also found that 3CR of aryl aldehydes, hydroxylamine hydrochloride, and ethyl acetoacetate (or ethyl 4-chloroacetoacetate) can be significantly performed to achieve the 3,4-disubstituted isoxazol-5(4H)-ones at room temperature. 2-HSBA displays excellent catalytic activity toward these 3CRs. The use of 2-HSBA in these 3CRs has benefits such as clean reaction profiles, lack of side reactions, green, minimization of waste, simple experimental procedure, recyclability of the catalyst, simplicity of operation, and easy work-up.
References
C. Cardellicchio, M.A.M. Capozzi, F. Naso, Tetrahedron Asymmetry 21, 507 (2010)
M. Betti, Gazz. Chim. Ital. 30 (II), 310 (1900)
L. Yang, Q. Yang, J. Shi, Y. Wang, M. Zhang, Synth. Commun. 44, 2468 (2014)
K.E. Metlushka, B.A. Kashemirov, V.F. Zheltukhin, D.N. Sadkova, B. Buchner, C. Hess, O.N. Kataeva, C.E. McKenna, V.A. Alfonsov, Chem. Eur. J. 15, 6718 (2009)
A.Y. Shen, C.T. Tsai, C.L. Chen, Eur. J. Med. Chem. 34, 877 (1999)
I. Mohanram, Meshram. J. Med. Chem. Res. 23, 939 (2014)
W.S.I. Abou-Elmagd, A.I. Hashem, Med. Chem. Res. 22, 2005 (2013)
E.J. Corey, X.M. Cheng, The Logic of Chemical Synthesis (Wiley, New York, 1989), p. 423
J. Safaei Ghomi, S. Zahedi, Monatsh. Chem. 144, 687 (2013)
J. Safaei Ghomi, S. Zahedi, M.A. Ghasemzadeh, Monatsh. Chem. 145, 1191 (2014)
Y. Kusakabe, J. Nagatsu, M. Shibuya, O. Kawaguchi, C. Hirose, S. Shirato, J. Antibiot. 25, 44 (1972)
J.L. Peglion, J. Vian, B. Gourment, N. Despaux, V. Audinot, M. Millan, Bioorg. Med. Chem. Lett. 7, 881 (1997)
G.Y. Lesher, A.R. Surrey, J. Am. Chem. Soc. 77, 636 (1955)
H. Ren, S. Grady, D. Gamenara, H. Heinzen, P. Moyna, S. Croft, H. Kendrick, V. Yardley, G. Moyna, Bioorg. Med. Chem. Lett. 11, 1851 (2001)
S. Remillard, L.I. Rebhun, G.A. Howie, S.M. Kupchan, Science 189, 1002 (1975)
H. Matsuoka, N. Ohi, M. Mihara, H. Suzuki, K. Miyamoto, N. Maruyama, K. Tsuji, N. Kato, T. Akimoto, Y. Takeda, K. Yano, T. Kuroki, J. Med. Chem. 40, 105 (1997)
F. Benedini, G. Bertolini, R. Cereda, G. Donia, G. Gromo, S. Levi, J. Mizrahi, A. Sala, J. Med. Chem. 38, 130 (1995)
H.S. Mosher, M.B. Frankel, M. Gregory, J. Am. Chem. Soc. 75, 5326 (1953)
M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, V. Khakyzadeh, Appl. Catal. A: General 400, 70 (2011)
J. Safari, Z. Zarnegar, J. Indust, Engin. Chem. 20, 2292 (2014)
A.V. Borhade, B.K. Uphade, D.R. Tope, Res. Chem. Intermed. 40, 211 (2014)
H. Moghanian, A. Mobinikhaledi, A.G. Blackmanc, E. Sarough-Farahani, RSC Adv. 4, 28176 (2014)
V.K. Das, M. Borah, A.J. Thakur, J. Org. Chem. 78, 3361 (2013)
A. Bamoniri, B.F. Mirjalili, S. Nazemian, J. Iran. Chem. Soc. 11, 653 (2014)
R. Tayebee, M.M. Amini, H. Rostamian, A. Aliakbari, Dalton Trans. 43, 1550 (2014)
B. Adrom, N. Hazeri, M. T. Maghsoodlou, M. Mollamohammadi, Res. Chem. Intermed. (2014). doi: 10.1007/s11164-014-1564-2
R.K. Singh, B. Singh, R. Duvedi, S. Kumar, Res. Chem. Intermed. (2014). doi: 10.1007/s11164-013-1513-5
F. Dong, Y. Li-fang, Y. Jin-ming, Res. Chem. Intermed. 39, 2505 (2013)
H.R. Shaterian, F. Rigi, Res. Chem. Intermed. (2013). doi: 10.1007/s11164-013-1145-9
C. Wang, Y. Wan, H.-Y. Wang, L.-L. Zhao, J.-J. Shi, X.-X. Zhang, H. Wu, J. Heterocyclic Chem. 50, 496 (2013)
A.R. Kiasat, A. Mouradzadegun, S.J. Saghanezhad, Chin. J. Catal. 34, 1861 (2013)
R.R. Nagawade, D.B. Shinde, Chin. J. Chem. 25, 1710 (2007)
J. Luo, Q. Zhang, Monatsh. Chem. 142, 923 (2011)
L.T. An, X.H. Lu, F.Q. Ding, W.Q. Jiang, J.P. Zou, Chin. J. Chem. 26, 2117 (2008)
A. Zare, T. Yousofi, A.R. Moosavi-Zare, RSC Adv. 2, 7988 (2012)
A.R. Hajipour, Y. Ghayeb, N. Sheikhan, A.E. Ruoho, Tetrahedron Lett. 50, 5649 (2009)
F. Dong, J. Chenning, Z. Ting, Y. Jinming, J. Chem. Sci. 125, 751 (2013)
M.M. Heravi, N. Tavakoli-Hoseini, F.F. Bamoharram, Synth. Commun. 41, 298 (2011)
A. Zare, H. Kaveh, M. Merajoddin, A.R. Moosavi-Zare, A. Hasaninejad, M.A. Zolfigol, Phosphorus, Sulfur Silicon Relat. Elem. 188, 573 (2013)
M. Zandi, A.R. Sardarian, C. R. Chimie 15, 365 (2012)
H. Alinezhad, M. Tajbakhsh, M. Norouzi, S. Baghery, M. Akbari, C. R. Chimie 17, 7 (2014)
P. Zhang, Z.-H. Zhang, Monatsh. Chem. 140, 199 (2009)
A. Ghorbani-Choghamarani, S. Rashidimoghadam, Chin. J. Catal. 35, 1024 (2014)
N. Hazeri, M.T. Maghsoudlou, S.M. Habibi-Khorassani, J. Aboonajmi, M. Safarzaei, Chem. Sci. Trans. 2(S1), S330 (2013)
B. Maleki, F. Taimazi, Org. Prep. Proced. Int. 46, 252 (2014)
A. Ghorbani-Choghamarani, S. Rashidimoghadam, Res. Chem. Intermed. (2014). doi: 10.1007/s11164-014-1738-y
T.M.V.D. Pinho e Melo, Curr. Org. Chem. 9, 925 (2005)
L. Carlsen, D. Dopp, H. Dopp, F. Duus, H. Hartmann, S. Lang-Fugmann, B. Schulze, R.K. Smalley, B.J. Wakefield, in Houben-Weyl, Methods in Organic Chemistry, vol. E8a, ed. by E. Schaumann (Georg Thieme Verlag, Stuttgart, 1992), pp. 45–204
B. Frolund, A.T. Jorgensen, L. Tagmose, T.B. Stensbol, H.T. Vestergaad, C. Engblom, U. Kristiansen, C. Sanchez, P. Krogsgaard-Larsen, T. Liljefors, J. Med. Chem. 45, 2454 (2002)
X.H. Zhang, L.Y. Wang, Y.H. Zhan, Y.L. Fu, G.H. Zhai, Z.Y. Wen, J. Mol. Struct. 994, 371 (2011)
X.-H. Zhang, Y.-H. Zhan, D. Chen, D. Chen, F. Wang, L.-Y. Wang, Dyes Pigments 93, 1408 (2012)
J. Han, H. Guo, X.-G. Wang, M.-L. Pang, J.-B. Meng, Chin. J. Chem. 25, 129 (2007)
W.S. Hamama, M.E. Ibrahim, H.H. Zooro, Synth. Commun. 43, 2393 (2013)
A. Babulreddy, R.V. Hymavathi, M.M. Hussain, G.N. Swamy, J. Heterocycl. Chem. 50, 727 (2013)
M. Brahmayya, B. Venkateswararao, D. Krishnarao, S. Durgarao, U.V. Prasad, T. Damodharam, R. Mishra, J. Pharm. Res. 7, 516 (2013)
H. Song, W.-B. Feng, F. Cheng, D.-Q. Shi, J. Heterocycl. Chem. 47, 1310 (2010)
J.P. Demers, W.E. Hageman, S.G. Johnson, D.H. Klaubert, R.A. Look, J.B. Moore, Bioorg. Med. Chem. Lett. 4, 2451 (1994)
W.M. Abdou, R.F. Barghash, R.E. Khidre, Monatsh. Chem. 144, 1233 (2013)
C. Changtam, P. Hongmanee, A. Suksamrarn, Eur. J. Med. Chem. 45, 4446 (2010)
S.K. Laughlin, M.P. Clark, J.F. Djung, A. Golebiowski, T.A. Brugel, M. Sabat, R.G. Bookland, M.J. Laufersweiler, J.C. VanRens, J.A. Townes, B. De, L.C. Hsieh, S.C. Xu, R.L. Walter, M.J. Mekel, M.J. Janusz, Bioorg. Med. Chem. Lett. 15, 2399 (2005)
V.K. Sharma, S.K. Mishra, N. Nesnas, Environ. Sci. Technol. 40, 7222 (2006)
S.A. Lawrence, V. Roth, R. Slinger, B. Toye, I. Gaboury, B. Lemyre, BMC Pediatr 5, 49 (2005)
D.V. Vorobyeva, N.M. Karimova, I.L. Odinets, G.V. Roschenthaler, S.N. Osipov, Org. Biomol. Chem. 9, 7335 (2011)
T. Ishioka, A. Kubo, Y. Koiso, K. Nagasawa, A. Itaib, Y. hashimoto. Bioorg. Med. Chem. 10, 1555 (2002)
T. Ishioka, A. Tanatani, K. Nagasawa, Y. Hashimoto, Bioorg. Med. Chem. Lett. 13, 2655 (2003)
S. Biju, M.L.P. Reddy, R.O. Freire, Inorg. Chem. Commun. 10, 393 (2007)
E. Aret, H. Meekes, E. Vlieg, G. Deroover, Dyes Pigments 72, 339 (2007)
Q. Liu, Y.-N. Zhang, Bull. Korean Chem. Soc. 32, 3559 (2011)
Q. Liu, X. Hou, Phosphorus, Sulfur Silicon Relat. Elem. 187, 448 (2012)
Q. Liu, R.T. Wu, J. Chem. Res. 598 (2011)
M. Mirzadeh, G.H. Mahdavinia, E. J. Chem. 9, 425 (2012)
S. Fozooni, N.G. Hosseinzadeh, H. Hamidian, M.R. Akhgar, J. Braz. Chem. Soc. 24, 1649 (2013)
F. Saikh, J. Das, S. Ghosh, Terahedron Lett. 54, 4679 (2013)
K. Ablajan, H. Xiamuxi, Synth. Commun. 42, 1128 (2012)
Q.F. Cheng, X.Y. Liu, Q.F. Wang, L.S. Liu, W.J. Liu, Q. Lin, X.J. Yang, Chin. J. Org. Chem. 29, 1267 (2009)
K. Ablajan, H. Xiamuxi, Chin. Chem. Lett. 22, 151 (2011)
Y.-Q. Zhang, J.-J. Ma, C. Wang, J.-C. Li, D.-N. Zhang, X.-H. Zang, J. Li, Chin. J. Org. Chem. 28, 141 (2008)
Y.Q. Zhang, C. Wang, M.Y. Zhang, P.L. Cui, Y.M. Li, X. Zhou, J.C. Li, Chin. J. Org. Chem. 28, 914 (2008)
H. Kiyani, Org. Chem. Indian J. 13, 97 (2013)
H. Kiyani, F. Ghorbani, Heterocycl. Lett. 2, 145 (2013)
H. Kiyani, F. Ghorbani, Heterocycl. Lett. 3, 359 (2013)
H. Kiyani, F. Ghorbani, Open. J. Org. Chem. 1, 5 (2013)
H. Kiyani, F. Ghorbani, Elixir Org. Chem. 58A, 14948 (2013)
H. Kiyani, F. Ghorbani, Res. Chem. Intermed. (2013). doi: 10.1007/s11164-013-1411-x
H. Kiyani, F. Ghorbani, J. Saudi Chem. Soc. (2013). doi:10.1016/j.jscs.2013.11.002
P.I. Dalko, Enantioselective Organocatalysis: Reactions and Experimental Procedures (Wiley-VCH, Weinheim, 2007)
A. Dondoni, A. Massi, Angew. Chem. Int. Ed. 47, 4638 (2008)
C. Zhong, X. Shi, Eur. J. Org. Chem. 2010, 2999 (2010)
J.O. Metzger, Angew. Chem. Int. Ed. 37, 2975 (1998)
K. Tanaka, F. Toda, Chem. Rev. 100, 1025 (2000)
K. Tanaka, Solvent-Free Organic Synthesis, Weinheim (Wiley-VCH, Germany, 2003)
U.M. Lindstrom, Chem. Rev. 102, 2751 (2002)
Organic Reactions in Water: Principles, Strategies and Applications, ed. U.M. Lindström, Blackwell Publishing, Oxford (2007)
Comprehensive Organic Reactions in Aqueous Media, ed. by C.-J. Li, T.-H. Chan, 2nd ed. (Wiley, Hoboken, New Jersey, 2007)
M.C. Pirrung, Chem. Eur. J. 12, 1312 (2006)
Kirk-Othmer Encyclopedia of Chemical Technology. Wiley. All rights reserved vol. 12 (2004), pp. 102
Y. Suzuki, Int. J. Anal. Bio-Sci. 1, 27 (2013)
Y.A. Shen, T. Xiong, J. Shang, K. Yang, Res. Chem. Intermed. 34, 353 (2008)
W.Y. Yin, X. Chen, M.H. Cao, C.W. Hu, B.Q. Wei, J. Phys. Chem. C 113, 15897 (2009)
J.T. Vanderslice, C.E. Maire, R.F. Doherty, G.R. Beecher, J. Agric. Food Chem. 28, 1145 (1980)
R.-S. Zhou, L. Ye, H. Ding, J.-F. Song, X.-Y. Xu, J.-Q. Xu, J. Solid State Chem. 181, 567 (2008)
P.S. Rao, D.N. Sathyanarayana, S. Palaniappan, Macromolecules 35, 4988 (2002)
H. Weiji, W. Song, C. Chen, H. Yuan, W. Ma, J. Zhao, Environ. Sci. Technol. 41, 5103 (2007)
H.V. Meek, C.V. Banks, Anal. Chem. 22, 1512 (1950)
T. Senaratna, D. Merritt, K. Dixon, E. Bunn, D. Touchell, K. Sivasithamparam, Plant Growth Regul. 39, 77 (2003)
N.N.G. Mohammed, M.S. Pandharpatte, Der Chemica Sinica 1, 15 (2010)
V.B. Jadhav, Elixir Appl. Chem. 70, 24010 (2014)
Z.-K. Lei, L. Xiao, X.-Q. Lu, H. Huang, C.-J. Liu, Molecules 18, 1653 (2013)
Acknowledgment
The authors are grateful to Damghan University Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiyani, H., Darbandi, H., Mosallanezhad, A. et al. 2-Hydroxy-5-sulfobenzoic acid: an efficient organocatalyst for the three-component synthesis of 1-amidoalkyl-2-naphthols and 3,4-disubstituted isoxazol-5(4H)-ones. Res Chem Intermed 41, 7561–7579 (2015). https://doi.org/10.1007/s11164-014-1844-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1844-x