Abstract
MCM-41 silica spheres were synthesized and functionalized with 3-aminopropyltriethoxysilane (3-APTES). The Schiff base has been derived from amino groups and 5-boromo salicylaldehyde, then a tetra dentate Cu(II)–Schiff base complex was prepared. This compound was characterized by X-ray diffraction, nitrogen physisorption, UV–Vis spectrometer, transmission electron micrographys (TEM), IR-spectra, and TGA/DTA technique. The prepared grafting Cu-salen-MCM-41 over a mesoporous surface was found to be an efficient and selective catalyst for the oxidation of different sulfides into sulfoxides with urea hydrogen peroxide (UHP) giving excellent yields at room temperature. The results showed that Cu-salen-MCM-41 is an effective catalyst for the oxidation of sulfides and can be repeatedly used and regenerated with no significant decrease in its catalytic ability. They also showed that the prepared material retained a good mesoporous structure and the best catalytic properties.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Encapsulation of suitable molecular species in MCM-41 prepares new types of functional materials which have been a popular field of study [1–4]. The discovery of mesoporous materials with extremely high surface area (ca. 1,000 m2/g), large channels from 1.5 to 10 nm ordered in a hexagonal array, and large pore volume (>1.0 cm3/g), have opened up new opportunities for designing novel heterogeneous catalysts [5, 6]. Inner surfaces of MCM-41 contain Si–OH groups that can be developed basic properties by functionalization with organic components (as amino groups) [7]. Recently, metal complexes of porphyrins, Schiff-bases, phthalocyanines, etc. have been encapsulated into MCM-41 for the development of efficient oxidation catalytic phenomena [8–10].
Schiff base compounds are usually formed by the condensation of amine group with active carbonyl group, which usually linked to the aldehyde. Salicylidene Schiff base (salen) as bidentate ligand can be easily prepared by the condensation of amine derivatives and salicylaldehyde derivatives. Salen ligand is known to easily form stable complexes with transition metals [11]. Because of their high activity and selectivity of salen complexes with most transition metals (Cu(II), Co(II), Ni(II), Fe(III), and Zn(II)), they show wide applicability in biological, medicinal, industrial, and catalyst fields [12–22]. Among them, copper modifications are of considerable importance, and widely applied as oxidant catalyst in many processes because of their high stability potential and environmental importance.
In the other hand, sulfoxides are very useful compounds in organic synthesis [23] and the formation of biological active molecules [24]. They play a vital role as antihypertensive drugs [25] and cardiotonic agents [26], as well as vasodilators [27]. Various efforts have been made to develop new synthetic methods for sulfoxide synthesis [28]. For this reason, oxidation of sulfides is a simple, straight and widely accepted method for the preparation of sulfoxides, various oxidizing reagents which are now available such as PhIO, t-butylhydroperoxide (TBHP), NaOCl, H2O2, UHP, etc. [29–32]. Anhydrous urea–hydrogen peroxide complex (UHP, H2NCONH2···H2O2) [33] is a green oxidant for sulfide oxidation. Its stability at room temperature and the potential for releasing it in a controlled manner, as well as its solubility in organic solvents, make it a good and safe substitute as a “dry carrier” of the unstable and hazardous hydrogen peroxide in most oxidation reactions.
The reactions between urea–hydrogen peroxide and sulfides are slow, hence, extensive studies have been made for the discovery of new and efficient catalysts. Efficient systems for such reactions can be accomplished by supporting active phase on various materials, such as polymers [34] or inorganic solids such as zeolites [35], clays [36], alumina [37], and silica [38]. A solid catalyst capable of working under heterogeneous conditions is required to the sustainability of the process.
In this paper, we report the synthesis of eco-friendly oxidation system and characterization of Cu (Salen) complex grafted over the modified surface MCM-41 using 3-aminopropyltriethyoxysilane. The mentioned hetereogenized complex was applied as catalyst in the oxidation of sulfide using anhydrous urea hydrogen peroxide (UHP) as oxidizing agent. The results showed good catalytic activity and stability as well as the ability to be recycled for repeated use.
Experimental
General
Fourier transform infrared (FTIR) spectra of KBr disks were measured on a VRTEX 70 model Bruker FTIR spectrometer. The powder X-ray diffraction (XRD) patterns of the samples were recorded on an X Pert MPD diffractometer using Cu radiation under the conditions of 40 kV and 30 mA. The UV–Vis diffused reflectance spectra (UV–Vis DRS) were recorded on a UV–Vis Ava Spec 2048 Tec spectrometer. TGA/DTA was carried out by a Shimadzu DTG-60 instrument. N2 adsorption–desorption isotherms were measured at −196 °C with a Micromeritics ASAP 2020. The transmission electron micrographys (TEM) were recorded with a Philips CM10 microscope, working at 200 kV accelerating voltage.
The cationic surfactant cetyltrimethylammoniumbromide (CTAB, 98 %), tetraethylorthosilicate (TEOS, 98 %), sodium hydroxide, organic sulfides, solvents, and urea hydrogen peroxide) were purchased from Merck, Aldrich, and Fluka, and were used as received.
Synthesis of MCM-41 material
The MCM-41 material was prepared using the modified synthesis recipe of Chen et al. [46]. We used cetyltrimethylammoniumbromide (CTAB) as a surfactant template and tetraethylorthosilicate (TEOS) as a source of silicon for the preparation of the mesoporous material. The molar composition rate of the reactant mixture (TEOS:CTAB:NaOH:H2O) was: 60:3/0:1/0:1. TEOS was added to an aqueous solution containing CTAB, NaOH, and deionized water. After stirring for about 1 h at room temperature, the resulting homogeneous mixture was crystallized under static hydrothermal conditions at 100 °C in a teflon-lined autoclave for 96 h. After cooling at room temperature, the solid product was separated by filtration, washed with deionized water, and dried in air at 70 °C. The material was finally calcined at 550 °C for 5 h with a ramp of 2 °C/min.
Preparation of 3-APTES functionalized mesoporous silica (MCM-41-nPr-NH2)
To a suspension of calcined Si-MCM-41 (4.8 g, 80 °C) in n-hexane under nitrogen atmosphere, 4.8 g of 3-aminopropyltriethoxysilane (3-APTES) was added slowly and then refluxed for 24 h (Scheme 2). The separated white solid MCM-41-(SiCH2CH2CH2NH2) x was washed with n-hexane and dried under vacuum. Elemental analysis shows that that 3-APTES was functionalized on mesoporous silica.
Preparation of Cu-salen-MCM-41
5-Boromo salicylaldehyde (1 mmol) was added to a suspended solution of white solid MCM-41-(SiCH2CH2CH2NH2) x in ethanol and refluxed under N2 atmosphere at 80 °C for 3 h to prepare a Schiff base on the surface of MCM-41 (a bi-dentate ligand; Scheme 1).The yellow solid was filtered, Soxhlet-extracted with ethanol, and dried under vacuum. The Soxhleted Cu-salen-MCM-41 was obtained by stirring 0.5 g of the hybrid material, MCM-41–Schiff base ligand (salen-MCM-41), with Cu(NO3)2·3H2O (1 mmol) in 30 mL of ethanol at room temperature for 12 h. Then, the resulting catalyst (green powder) was filtered off, and extracted with ethanol in Soxhlet and dried in a vacuum (Soxhlet-extracted with ethanol and dried in a vacuum) to obtain the heterogenized complexes (Cu-salen-MCM-41) in almost quantitative yields (Scheme 2).
General procedure for the oxidation of sulfides to sulfoxide
To the mixture of sulfide (1 mmol) and catalyst (20 mg) in ethanol (4 mL), UHP was added (7 mmol).The reaction mixture was stirred at room temperature for the appropriate time until TLC indicated the reaction was complete. The product was extracted with ethanol (10 mL), dried over anhydrous Na2SO3, and then concentrated to get an analytically pure product.
Results and discussion
Characterization of parent supports and heterogeneous catalyst
Mesoporous materials are characterized by a variety of techniques, such as X-ray diffraction (XRD), Fourier transform infrared (FT-IR) spectroscopy, transmission electron microscopy (TEM), N2 sorption measurements, TGA/DTA analysis, and UV–Vis-spectroscopy. The main characteristics of the mesoporous silica that has been used in this survey are presented as follows.
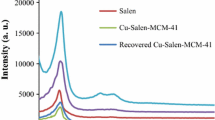
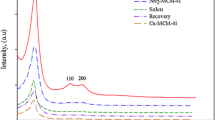
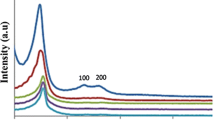
Figure 1 illustrates the XRD patterns of pure MCM-41, MCM-41-nPr-NH2, salen, Cu-salen-MCM-41 materials, and the recovered catalyst. MCM-41 is a hexagonal structured material with long-range ordered pores (Fig. 1a). The XRD pattern shows three peaks assigned to 1 0 0, 1 1 0, and 2 0 0 reflections. In fact, these peaks reflect the hexagonal unit of the structure. The ordered mesoporous structure is basically preserved after functionalizing amine (MCM-41-nPr-NH2), salen, and Cu-salen-MCM-41 that exhibited well-defined reflections of the MCM-41 meso phase. However, compared to the parent material, some broadening and decreased intensity of the 1 0 0 diffraction peak is observed, as well as the disappearance of the 1 1 0 and 1 0 0 peaks. Similar changes, which reveal some loss of order upon functionalization of MCM-41, were reported [39]. Furthermore, Fig. 1e is the recovered Cu-salen-MCM-41 and the curve clearly indicated the structural stability of catalyst after 5 reused.
The prepared heterogeneous catalysts were characterized by FT-IR. The IR spectra of MCM-41, MCM-41-nPr-NH2, salen-MCM-41, Cu-salen-MCM-41, and the recovered catalyst are exhibited in Fig. 2. The characteristic absorption bands of silica are presented as follows: bending vibration (O–Si–O) of valence angle of tetrahedral SiO4 located at 463 cm−1, symmetric and asymmetric stretching (Si–O) of tetrahedral SiO4 located at 800 and 800–1,300 cm−1 range, and stretching (O–H) of H2O of silanol groups in the surface of silica located at 3,000–3,600 cm−1 range. The transmissions around 2,900 cm−1 of the prepared catalysts were due to C–H bond stretching vibrations of the alkyl groups. The MCM-41-nPr-NH2 sample has typical (NH2) bending at 1,542 cm−1 that conformed to the MCM-41 to be functionalized. The adsorption bands around 1,655 cm−1, assigned to stretching vibrations of azomethine groups (H–C=N) bending, is the characteristic band of salen ligand that shifts this band to 1,632 cm−1 when C=N is coordinated to the metal (Cu).The peaks at 2,375 and 1,468 cm−1 can be assigned to the C=C stretching vibration of the phenyl group. The curve of the recovered complex is quite similar to that of Cu-salen-MCM-41
N2 sorption measurements have been used to determine the physical textural properties including surface area, pore size distribution, and pore volume per gram. The textural properties (surface area and pore size) of MCM-41, MCM-41-nPr-NH2, and Cu-salen-MCM-41 were determined from the N2-sorption studies carried out at liquid nitrogen temperature (Table 1). On modifying Si-MCM-41 with 3-APTES, the surface area of Si-MCM-41 decreased from 986.16 to 694.98 m2/g and the pore size reduced from 3.56 to 3.3 nm. On Cu-salen-MCM-41, a further reduction in the surface area from 694.98 to 32.84 m2/g and pore size from 3.3 to 1.19 nm was observed. The reduction in the surface area and pore size is due to the lining of the walls of the Si-MCM-41 with the organic moieties. A similar trend has also been previously observed [40].
Furthermore, adsorption–desorption isotherms of calcined MCM-41, MCM-41-nPr-NH2, and Cu-salen-MCM-41 are depicted in Fig. 3. IUPAC classification and its steep condensation behavior indicate the existence of uniformly sized mesoporous [41]. In detail, region 0.05 < P/P0 < 0.3 corresponds to the multi-layer adsorption of liquid N2 on the walls of the mesoporous compound. When the amino group was functionalized on MCM-41, the curve shows a lower value of 0.05 < P/P0 < 0.25, while after formation of the complex, the P/Po value changed to a lower value of ∼0.05 and the other adsorption takes place at 0.95 < P/P0 < 1 which we think it is due to the N2 adsorption between the grains of solid.
Thermo-gravimetric analyses (TGA) of MCM-41, MCM-41-nPr-NH2, and the Cu-salen-MCM-41 complex are shown in Fig. 4. TGA of MCM-41 indicated no alteration in weight loss by increasing the temperature after an initial weight loss of 4 % that was observed with regard to the loss of moisture inside the MCM-41 channels. The TG/DTG spectrum of MCM-41-nPr-NH2 displays two steps of weight loss, one below ∼100 °C and the other in the region of 200 to ∼300 °C. As mentioned, the former weight loss is due to desorption of physisorbed water, while the second weight loss is attributed to the loss of organo propyl amino fragments [42]. The thermal decomposition of the Cu-salen-MCM-41 complex is increased in two steps of weight loss, one at ∼100 °C and the other between 350 and 600 °C, when heated under airflow. The former weight loss (<100 °C) is usually attributed to the desorption of physisorbed water, while the latter loss is due to the decomposition/combustion of organic ligand present in the complex. A complete decomposition of the Cu-salen takes place in a temperature range of 350–600 °C which indicates that the calcination procedure used for the removal of structured directors was optimum.
The TEM micrographs and size distributions (Fig. 5) of MCM-41 demonstrated that MCM-41 possesses clear well-order channels and confirms the 2D hexagonal pore arrangement and long-range mesoporous architecture (50 and 20 nm).
Anchoring of ligands on the solid surface coordinated to transition metals was appropriately followed by diffuse reflectance of spectroscopy of the resulting catalysts [43, 44]. The UV–Vis DRS spectra of 3-APTES-modified mesoporous (MCM-41-nPr-NH2), salen-MCM-41, Cu(II)-salen mesoporous (Cu-salen-MCM-41), and the reused complex are presented in Fig. 6. No peak appears for the MCM-41-nPr-NH2. In the UV–Vis DR spectrum of salen, two bands around 255 and 312 and a band near to 420 nm are due to the π–π* and n-π* transitions, respectively. In the spectrum of Cu-salen-MCM-41, a broadening of the absorption bands at 565–785 nm when compared to the absorption bands for the salen-MCM-41 shows that this peak can be attributed to several d → d transitions. Also, the Cu-salen complex shows a red shift (π–π*) at 264 and a blue shift (n–π*) transition at 392 due to coordination of salen to copper(II). The charge transfer transition is not observed because of overlapping with π–π* and n–π* transitions. The CT and d → d transition provide evidence for complexation of Schiff base ligand with the Cu(II) ions. Moreover, Fig. 6 shows that the structure of the recovered supported catalyst is not damaged with respect to the starting complex.
Catalytic and chemoselective oxidation of sulfides into sulfoxides
In continuation of our interest in sulfur chemistry [45], we were searching for a high-yielding, catalytic, cheap, and environmentally benign reagent for sulfide oxidation and considered Cu-salen-MCM-41 with UHP as a reagent of choice. We report an exceptionally mild, general, and efficient Cu- salen-MCM-41 catalyzed for the oxidation of sulfide to sulfoxide using UHP as oxidant. In order to find the optimum reaction conditions, the influence of different factors affecting the conversion and selectivity of the oxidation reaction. such as solvent nature, catalyst concentration, and the molar ratio of UHP, were investigated. For this purpose, dibenzyle sulfide was considered as the model substrate and was subjected to different reaction conditions (Scheme 1). In the first step, the oxidation was investigated in various solvents such as ethyl acetate, ethanol, acetone, dichloromethane, acetonitrile, water, and chloroform as shown in Fig. 7.
It was found that the reaction in the eco-friendly ethanol proceeded smoothly with the high conversion and good chemo-selectivity (96 %). To study the effect of catalyst concentration on the conservation and selectivity of the dibenzyl sulfide oxidation, Cu-salen-MCM-41 catalyst loading were used in the reaction. The results are shown in Table 2. It was observed that the highest conversion and selectivity to sulfoxide were achieved in the presence of 20 mg of catalyst within 2.5 h (Table 2, entry 3). For dibenzyl sulfide, a separate experiment showed the presence of catalyst is necessary, since oxidation with UHP alone occurs rather slowly (12 h).
The various molar ratios of UHP were performed in ethanol (Table 2). As outlined in Fig. 4, the conversion of dibenzyl sulfide oxidation improved from 80 to 96 % as the molar ratio increases from 5 to 7. Thus, the reaction gave the high yield and selectivity of sulfoxide at 7 mmol of UHP. However, according to Table 2, the employment of an excess amount of UHP considerably promoted the overoxidation of sulfoxide. Based on the above observation, we come to the conclusion that 20 mg of salen catalyst and 7 mmol of UHP in ethanol as solvent is the best combination.
Based on the mentioned optimal conditions, a wide range of structurally divergent aryl alkyl sulfides was subjected to oxidation with very high selectivity and excellent yield being observed in all cases (Table 3). The high yields of oxidation products (91–96 %) obtained by this catalytic system indicate the high efficiency of Cu-salen-MCM-41 with UHP.
One of the outstanding advantages of these oxidizing systems is selectivity and chemoselectivity of the oxidation reaction, which is outlined in Scheme 3.
As is evident from Scheme 3, no side reaction such as overoxidation to sulfone or oxidation of the hydroxyl group was observed.
Due to the need to obtain catalysts for green processes, the use of recycled catalysts is required for reducing the catalytic cost. When the reaction was completed, the catalyst (insoluble) was filtered, dried, and reused. The catalyst was used and reused for five cycles with similar activity (Fig. 8)
Conclusion
The internal pore surface of the meso- structure sieve MCM-41, grafted ligands were used for the preparation of the copper(II) complex. These materials were characterized by UV–Vis, FT-IR, BET, TEM, N2 adsorption, and XRD. These materials show that the Cu complex is attached to the surface of MCM-41. Especially, UV–Vis diffuse reflectance of spectroscopy is an extremely sensitive probe for the presence of the Cu-salen complex in molecular sieves. An efficient and heterogeneous complex is active in catalyzing the oxidation of different sulfides with excellent yield, 100 % selectivity, and mild conditions using UHP as oxidant. The advantages of the use of UHP in the oxidation of sulfides are non-corrosive, high thermal stability, and easy work.
References
J. Drabowicz, P. Kielbasinski, M. Mikolaiczyk, Synthesis of sulfoxides (Wiley, New York, 1994)
H.L. Holland, Chem. Rev. 473, 88 (1988)
M.C. Carreno, Chem. Rev. 1717, 95 (1995)
S. Patai, Z. Rappoport, Synthesis of sulfones, sulfoxides and cyclic sulfides (Wiley, Chichester, 1994)
B. Kotelanski, R.J. Grozmann, J.N. Cohn, Clin. Pharmacol. Ther. 427, 14 (1973)
W.J. Parsons, V. Ramkumar, G.L. Stiles, Mol. Pharmacol. 37, 34 (1988)
S. Padmanabhan, R.C. Lavin, G.J. Durant, Tetrahedron Asymmetr. 11, 3455 (2000)
N.N. Mahamuni, P.R. Gogate, A.B. Pandit, Ultrason. Sonochem. 14, 135 (2007)
R.E. Del Rio, B. Wang, S. Achab, L. Bohe, Org. Lett. 9, 2265 (2007)
G. Maayan, R. Popovitz-Biro, R. Neumann, J. Am. Chem. Soc. 128, 4968 (2006)
C.S. Lu, E.W. Hughes, P.A. Giguere, J. Am. Chem. Soc. 63, 1507 (1941)
Ch.E. Song, E.J. Roh, B.M. Yu, D.Y. Chi, S.Ch. Kim, K.J. Lee (2000) J. Chem. Soc. Chem. Commun. 615
L. Canali, E. Cowan, H. Deleuze, C.L. Gibson, D.C. Sherrington, J. Chem. Soc. Chem. Commun. 2561 (1998)
L. Canali, E. Cowan, H. Deleuze, C.L. Gibson, D.C. Sherrington, J. Chem. Soc. Perkin Trans. I 2055 (2000)
E.F. Murphy, L. Schmid, T. Burgi, M. Maciejewski, A. Baiker, D. Gunther, M. Schneider, Chem. Mater. 13, 1296 (2001)
S.K. Kim, K.H. Kim, S.K. Ihm, Chemosphere 68, 287 (2007)
M. Kurian, S. Sugunan, Chem. Eng. J. 115, 139 (2006)
X.G. Zhou, X.Q. Yu, J.S. Huang, S.G. Li, L.S. Li, C.M. Che, J. Chem. Soc. Chem. Commun. 1789 (1999)
E. Mollmann, P. Tomlinson, W.F.J. Holderich, Mol. Cat. 206, 137 (2003)
S. Ernst, E. Fuchs, X. Yang, Microporous Mesoporous Mater. 35–36, 137 (2000)
J. Guzman, B.C. Gates, J. Chem. Soc. Dalton Trans. 3303 (2003)
R. Ganesan, B. Viswanathan, J. Phys. Chem. B 108, 1702 (2004)
S. Ray, S. Vasudevan, Inorg. Chem. 42, 1711 (2003)
J. Deutsch, H.A. Prescott, D. Muller, E. Kemnitz, J. Catal. 231, 269 (2005)
A. Galarneau, D. Desplantier-Giscard, F. Di Renzo, F. Fajula, Catal. Today 68, 191 (2001)
D. Trong On, D. Desplantier-Giscard1, C. Danumah, S. Kaliaguine. Appl. Catal. A 253, 545 (2003)
J.A. Incavo, P.K. Dutta, J. Phys. Chem. 94, 3075 (1990)
H. Chen, Y. Wang, Ceram. Int. 28, 541 (2002)
A.S. Al-Shiri, Spectrochim. Acta (A) 60, 118 (2004)
T. Chinnusamy, O. Reiser, ChemSusChem 2010, 3 (1040)
B. Yu, A.H. Liu, L.N. He, B. Li, Z.F. Diaoa, Y.N. Lia, Green Chem. 14, 957 (2012)
B. Yu, C.X. Guo, C.L. Zhong, Z.F. Diao, L.N. He, Tetrahedron Lett. 2014, 55 (1818)
H.A. El-Borae, Therm. J. Anal. Calorim. 81, 339 (2005)
Z.M. Zaki, S.S. Haggag, A.A. Sayed, Spectrosc. Lett. 31, 757 (1998)
G. Kumar, D. Kumar, C.P. Singh, A. Kumar, V.B. Rana, J. Serbian Chem. Soc. 75, 629 (2010)
V.K. Aghera, P.H. Parsania, J. Sci. Ind. Res. 2008, 67 (1083)
N. Raman, V. Muthuraj, S. Ravichandran, A. Kulandaisamy, J. Chem. Sci. 115, 161 (2003)
V.P. Lozitsky, V.E. Kuzmin, A.G. Artemenko, R.N. Lozitska, A.S. Fedtchouk, E.N. Muratov, A.K. Mescheriakov, SAR QSAR Environ. Res. 16, 219 (2005)
M.A. Villar, M.D. Failla, R. Quijada, R.S. Mauler, E.M. Valles, G.B. Galland, L.M. Quinzani, Polymer 42, 9269 (2001)
T. Joseph, M. Hartmannb, S. Ernst, S.B. Halligudi, J. Mol. Catal. A 207, 131 (2004)
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L.A. Moscou, R. Pierotti, J. Rouquerol, T. Siemieniewska, Pure Appl. Chem. 57, 603 (1985)
P. Karandikar, K.C. Dhanya, S. Deshpande, A.J. Chandwadkar, S. Sivasanker, M. Agashe, Catal. Commun. 5, 69 (2004)
S. Shylesh, S. Sharma, S.P. Mirajkar, A.P.J. Singh, Mol. Catal. A. 212, 219 (2004)
H. Yang, L. Zhang, W. Su, Q. Yang, C. Li, J. Catal. 248, 204 (2007)
A. Ghorbani-Choghamarani, M. Nikoorazm, H. Goudarziafshar, L. Shiri, Z. Chenani, Bull. Korean Chem. Soc. 30, 972 (2009)
H. Chen, Y. Wang, Ceram. Int. 28, 541 (2002)
Acknowledgment
Financial support for this work by the research affairs of Ilam University, Ilam, Iran, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jabbari, A., Mahdavi, H., Nikoorazm, M. et al. Salen copper(II) complex heterogenized on mesoporous MCM-41 as nano-reactor catalyst for the selective oxidation of sulfides using urea hydrogen peroxide (UHP). Res Chem Intermed 41, 5649–5663 (2015). https://doi.org/10.1007/s11164-014-1690-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1690-x