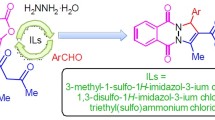
Abstract
Brønsted acidic ionic liquids, namely 2-pyrrolidonium hydrogensulfate, N-methyl-2-pyrrolidonium hydrogensulfate, N-methyl-2-pyrrolidonium dihydrogenphosphate, (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate, triphenyl(propyl-3-sulfonyl)phosphonium toluenesulfonate, l-prolinium sulfate, and l-prolinium nitrate, catalyzed efficient one-pot pseudo-four-component reaction of salicylaldehydes, malononitrile, and secondary amine for preparation of chromeno[2,3-d]pyrimidines under solvent-free conditions at room temperature. The catalysts show environmentally benign character and can be easily prepared, stored, and recovered without obvious significant loss of activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Some of the most important principles of green chemistry are elimination of hazardous solvents from chemical synthesis and avoidance of toxic solvents and waste generation [1]. Ionic liquids (ILs) were initially introduced as alternative green reaction media because of their unique chemical and physical properties. ILs show a significant role in controlling reactions as solvent or catalyst [2]. Another feature of ILs is their ability to be reused many times [3–6]. ILs are mainly classified into categories of acidic ILs, basic ILs, metal-containing ILs, chiral ILs, guanidinium ILs, and ILs containing –OH group [7].
In continuation of our research into new synthetic methods of organic synthesis using ILs as green catalysts [8–11], we developed a synthesis of chromeno[2,3-d]pyrimidine derivatives via pseudo-four-component condensation of salicylaldehydes, malononitrile, and secondary amine (Scheme 1) by using seven Brønsted acidic ionic liquids (BAILs), namely 2-pyrrolidonium hydrogensulfate ([Hnmp][HSO4]), N-methyl-2-pyrrolidonium hydrogensulfate ([NMP][HSO4]), N-methyl-2-pyrrolidonium dihydrogenphosphate ([NMP][H2PO4]), (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate, triphenyl(propyl-3-sulfonyl)phosphonium toluenesulfonate, l-prolinium sulfate [l-Pro2][SO4], and l-prolinium nitrate [l-Pro][NO3] (Fig. 1), as catalysts under mild, ambient, and solvent-free conditions.
Results and discussion
To prepare chromeno[2,3-d]pyrimidine derivatives more efficiently, the reaction of salicylaldehyde (2 mmol), malononitrile (1 mmol), and morpholine (1 mmol) was selected as a model system under solvent-free conditions at room temperature to optimize conditions. Preparation of 2-(4-morpholino-5H-chromeno[2,3-d]pyrimidin-2-yl)phenol in different amounts of acidic ILs as catalyst (5, 10, 15, and 20 mol%) (Table 1) was studied. The best result was obtained by using 5 mol% of [Hnhp][HSO4], 5 mol% of [NMP][HSO4], 5 mol% of [NMP][H2PO4], 5 mol% of (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate, 5 mol% of triphenyl(propyl-3-sulfonyl)phosphonium toluenesulfonate, 5 mol% of [l-Pro2][SO4], and 5 mol% of [l-Pro][NO3] at room temperature (Table 1).
Using these optimized reaction conditions, the scope and efficiency of the procedure were explored for synthesis of chromeno[2,3-d]pyrimidine derivatives via pseudo-four-component condensation of salicylaldehydes, malononitrile, and secondary amine at room temperature (Table 2). As shown in Table 2, salicylaldehydes with both electron-withdrawing and electron-donating substituents reacted efficiently with malononitrile and secondary amine in the presence of a catalytic amount of [Hnmp][HSO4] (5 mol%), [NMP][HSO4] (5 mol%), [NMP][H2PO4] (5 mol%), (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate (5 mol%), triphenyl(propyl-3-sulfonyl)phosphonium toluenesulfonate (5 mol%), [l-Pro2][SO4] (5 mol%) or [l-Pro][NO3] (10 mol%), forming the corresponding chromeno[2,3-d]pyrimidine derivatives without formation of any side-products in good to high yields.
The suggested mechanism is presented according to the proposed mechanism in the literature [18]. Initial Knoevenagel condensation of salicylaldehyde (1) and malononitrile (2) afforded (5), followed by Pinner reaction to form (6). Next, the cyano group of intermediate (6) can be attacked by the secondary amines (3) to produce intermediate (7). Finally, intermediate (7) reacts with another molecule of salicylic aldehyde (1) followed by proton transfer to afford the product (4) (Scheme 2).
We also compared the results of the present BAILs with other catalysts reported in the literature such as lithium perchlorate (LiClO4) [18], 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF4) [19], and piperidine [20] for preparation of chromeno[2,3-d]pyrimidine derivatives (Table 3). Table 3 clearly demonstrates that 2-pyrrolidonium hydrogensulfate, N-methyl-2-pyrrolidonium hydrogensulfate, N-methyl-2-pyrrolidonium dihydrogenphosphate, (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate, triphenyl(propyl-3-sulfonyl)phosphonium toluenesulfonate, l-prolinium sulfate, and l-prolinium nitrate are effective catalysts in terms of reaction time and yield of obtained product relative to other reported catalysts.
In green organic synthesis, catalyst recovery is more important. Thus, the reusability of the ILs as catalyst was studied in the synthesis of chromeno[2,3-d]pyrimidine derivatives. After completion of the reaction, ethanol (5 mL) and water (5 mL) were added to the reaction mixture. The IL was dissolved in ethanol and water, and filtered for separation of the crude solid product. The separated product was washed twice with ethanol (2× 5 mL) and water (2× 5 mL). To recover the ILs, the aqueous phase containing the ILs was evaporated, and the remaining viscous liquid was washed with ether (10 mL) and dried under reduced pressure. The recovered ILs were tested to study their catalytic activity in subsequent runs without adding fresh catalyst. The ILs were tested for five runs. It was seen that the ILs displayed very good catalyst reusability without any considerable loss of activity (Fig. 2).
Experimental
Chemicals and materials
All reagents were purchased from Merck and Aldrich and used without further purification. All yields refer to isolated products after purification. 2-Pyrrolidonium hydrogensulfate [Hnmp][HSO4] [12], N-methyl-2-pyrrolidonium hydrogensulfate [NMP][HSO4] [13], N-methyl-2-pyrrolidonium dihydrogenphosphate [NMP][H2PO4] [14], (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate [15], triphenyl(propyl-3-sulfonyl)phosphonium toluenesulfonate [16], l-prolinium sulfate [l-Pro2][SO4], and l-prolinium nitrate [l-Pro][NO3] [17] were prepared according to literature procedure. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance DPX 500-MHz instrument, being measured in CDCl3 relative to tetramethylsilane (TMS, 0.00 ppm). Melting points were determined in open capillaries with a BUCHI 510 melting point apparatus. Thin-layer chromatography (TLC) was performed on silica-gel Polygram SIL G/UV 254 plates.
General procedure for synthesis of chromeno[2,3-d]pyrimidine derivatives (4)
A stirred mixture of salicylaldehydes (1) (2 mmol), malononitrile (2) (1 mmol), secondary amine (3) (1 mmol), and 2-pyrrolidonium hydrogensulfate (0.009 g, 5 mol%, 0.05 mmol) or N-methyl-2-pyrrolidonium hydrogensulfate (0.0098 g, 5 mol%, 0.05 mmol) or N-methyl-2-pyrrolidonium dihydrogenphosphate (0.0098 g, 5 mol%, 0.05 mmol) or (4-sulfobutyl)tris(4-sulfophenyl)phosphonium hydrogensulfate (0.036 g, 5 mol%, 0.05 mmol) or triphenyl(propyl-3-sulfonyl)phosphonium toluenesulfonate (0.026 g, 5 mol%, 0.05 mmol) or l-prolinium sulfate (0.016 g, 5 mol%, 0.05 mmol) or l-prolinium nitrate (0.009 g, 5 mol%, 0.05 mmol) was reacted at room temperature for the appropriate times. After completion of the reaction as indicated by TLC, ethanol (5 mL) and water (5 mL) were added, and the mixture was stirred to dissolve the IL in solvents. The solid product was filtered and washed with water and ethanol to afford pure product. To recover the ILs, after isolation of insoluble products, the aqueous phase containing the ILs was evaporated, and the remaining viscous liquid was washed with ether (10 mL) and dried under reduced pressure.
All known products have been reported in the literature and were characterized by comparing their melting point and IR and NMR spectra with authentic samples [18–20]. We selected spectroscopic data (1H NMR, 13C NMR, IR) for two known compounds as below:
2-(4-(Piperidin-1-yl)-5H-chromeno[2,3-d]pyrimidin-2-yl)phenol (4c)
M.p. 168–170 °C; 1H NMR (CDCl3, 500 MHz): δ = 1.77–1.81 (6H, m), 3.45–3.47 (4H, m), 3.93 (2H, s), 6.94–6.97, 7.00–7.02, 7.11–7.14, 7.21–7.31, 7.36–7.40, 8.44–8.46 (8H, 6m, aromatic), 13.50 (1H, brs, OH) ppm; 13C NMR (CDCl3, 125 MHz): δ = 24.8, 26.1, 26.4, 49.9, 97.9, 117.5, 117.9, 119.1, 119.2, 119.9, 124.8, 128.6, 128.9, 129.6, 130.2, 133.2, 151.0, 160.8, 162.3, 165.6 ppm; IR (KBr, cm−1): 3043, 1622, 1605, 1257, 1151, 1110.
4-Bromo-2-(7-bromo-4-(morpholin-4-yl)-5H-chromeno[2,3-d]pyrimidin-2-yl)phenol (4e)
M.p. 216–218 °C; 1H NMR (CDCl3, 500 MHz): δ = 3.41–3.43, 3.81–3.83 (8H, 2t, J = 4.55 Hz), 3.90 (2H, s, CH2), 6.75–6.77, 6.97–6.99, 7.28–7.30, 7.31–7.33, 8.36–8.37 (6H, 5m, aromatic), 12.95 (1H, brs, OH) ppm; 13C NMR (CDCl3, 125 MHz): δ = 25.6, 48.8, 66.8, 97.8, 111.0, 117.2, 118.6, 118.9, 119.2, 119.9, 120.2, 121.5, 131.4, 131.5, 135.8, 149.6, 159.6, 160.9, 163.9 (aromatic) ppm; IR (KBr, cm−1): 3046, 1618, 1603, 1216, 1176, 1106, 1018.
Conclusions
We present a rapid and highly efficient method for green synthesis of chromeno[2,3-d]pyrimidine derivatives using reusable Brønsted acidic ILs as catalysts at room temperature under solvent-free conditions. We show that a number of ILs with different core, central atom (P or N), acidic functional groups, and counterions act well to perform the reaction. With such successful results, this convenient and efficient protocol should provide a superior alternative to existing methods because of its fast and clean reaction and high yield. Furthermore, the simple workup procedure makes the presented method useful and important for synthesis of chromeno[2,3-d]pyrimidine derivatives. This methodology offers significant improvements such as simplicity in operation, and green aspects by avoiding expensive or corrosive catalysts.
References
I. Yavari, E. Kowsari, Tetrahedron Lett. 48, 3753 (2007)
P. Wasserscheid, T. Welton, Ionic Liquids in Synthesis (Wiley, Stuttgart, 2002)
M.A.P. Martins, C.P. Frizzo, D.N. Moreira, N. Zanatta, H.G. Bonacorso, Chem. Rev. 108, 2015 (2008)
H. Olivier-Bourbigou, L. Magna, J. Mol. Catal. A 182–183, 419 (2002)
A. Mohammad, Green Solvents I: Properties and Applications in Chemistry, ed. by Dr. Inamuddin (Springer, Dordrecht, 2012)
A. Mohammad, Green Solvents II: Properties and Applications in Chemistry, ed. by Dr. Inamuddin (Springer, Dordrecht, 2012)
C. Yue, D. Fang, L. Liu, T.F. Yi, J. Mol. Liq. 163, 99 (2011)
H.R. Shaterian, M. Arman, F. Rigi, J. Mol. Liq. 158, 145 (2011)
H.R. Shaterian, M. Mohammadnia, F. Moradi, J. Mol. Liq. 172, 88 (2012)
H.R. Shaterian, M. Ranjbar, K. Azizi, J. Mol. Liq. 162, 95 (2011)
H.R. Shaterian, M. Ranjbar, J. Mol. Liq. 160, 40 (2011)
B. Huang, Y. Wang, K. Zhang, Y. Fang, B. Zhou, Chin. J. Catal. 28, 743 (2007)
C. Xie, H. Li, L. Li, S. Yu, F. Liu, J. Hazard. Mater. 151, 847 (2008)
H. Guo, X. Li, J.L. Wang, X.H. Jin, X.F. Lin, Tetrahedron 66, 8300 (2010)
B. Shaohua, C. Lu, J. Yongjun, Y. Jianguo, Chin. J. Chem. 28, 2119 (2010)
C. Cole, J.L. Jensen, I. Nati, K.L.T. Tran, K.J. Weaver, D. Forbes, J. Am. Chem. Soc. 124, 5962 (2002)
G.H. Tao, L. He, N. Sun, Y. Kou, Chem. Commun. 28, 3562 (2005)
R. Ghahremanzadeh, T. Amanpour, A. Bazgir, Tetrahedron Lett. 51, 4202 (2010)
A.K. Gupta, K. Kumari, N. Singh, D.S. Raghuvanshi, K.N. Singh, Tetrahedron Lett. 53, 650 (2012)
A. Zonouzi, M. Biniaz, R. Mirzazadeh, M. Talebi, S.W. Ng, Heterocycles 81, 1271 (2010)
Acknowledgments
We are grateful to the University of Sistan and Baluchestan Research Council for partial support of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaterian, H.R., Aghakhanizadeh, M. Mild preparation of chromeno[2,3-d]pyrimidines catalyzed by Brønsted acidic ionic liquids under solvent-free and ambient conditions. Res Chem Intermed 39, 3877–3885 (2013). https://doi.org/10.1007/s11164-012-0889-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0889-y