Abstract
The objective of this work was to prepare novel magnetic Fe3O4/polyurethane foam (Fe3O4/PUF) composites applied to the carriers of immobilized microorganisms for toluene-containing wastewater treatment. The morphology and structure of Fe3O4/PUF composite were characterized by X-ray diffraction, Fourier transform IR spectroscopy, thermogravimetric analysis, differential scanning calorimetry, scanning electron microscopy, and magnetic property measurement system. These morphological investigations revealed that Fe3O4 nano-particles were well dispersed into the matrix of PUF with nano-scale diameter particles. TG experiments indicated that the initial thermal weight loss temperatures of composite with the content of 2.5 wt% and 7.5% Fe3O4 were increased by 7 and 16 °C, compared with pure PUF. The degradation efficiency of toluene with magnetic PUF composite was much higher than that of pure PUF carrier, and the reason why the immobilization of microbial biomass of microorganisms on the magnetic PUF composite was much higher than that of the pure PUF. The prepared magnetic Fe3O4/PUF composite offered excellent thermal stability and medium paramagnetic properties. And this composite could not only increase the immobilized biomass of the microorganisms, but also enhance the COD removal efficiency of wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past few decades, immobilized microorganism technology has attracted more and more interest in the field of wastewater treatment [1]. Many researchers have focused on the selection of immobilization carriers including ceramics [2, 3], ion-exchange resins [4], polyurethane (PU) [5, 6], chitosan [7], PVA [8], cellulose [9, 10], agar [11], alginate [12], and carrageenan [13]. Among these investigated matrices available for immobilization, polyurethane form (PUF) offers several advantages, such as high mechanical strength, resistant to attack from organic solvents and microbes, easy handling, good regeneration ability, and especially very low cost. Oh et al. [14] prepared one hybrid PUF by adding chitin and inorganic nutrient into a polyether polyol mixture to increase the absorption and degradation abilities to oil from surface water. Lupton and Zupancic [15] manufactured a PUF composite contained granulated activated carbon for the microorganism immobilization and the treatment of phenol-containing wastewater. The experimental results found that this PUF composite could maintain the pollutant concentration at a mediate constant level, which would not inhibit the microorganism growth and thus enhance the degradation efficiency of phenol. From the above-mentioned summary, we can thus conclude that the PUF or newly synthesized hybrid PUFs are good carriers for the immobilized microorganisms applied to wastewater treatment.
Recently, Sakai et al. [16] found that, in the process of activated sludge treatment, the balance of the growth and death of microorganisms could be controlled by adding magnetic powder into activated sludge, and that the sludge bulking was also prevented from increasing the processing efficiency of wastewater treatment. Yavuz et al. [17] also found that the removal efficiency of glucose was 44% higher than that of without applied magnetic field when they investigated the effect of magnetic fields on the removal efficiency of synthetic wastewater treated by activated sludge. Rao et al. [18] also found that the magnetic field with a certain strength could promote the growth of microorganisms and accelerate the degradation rate of phenol. All of this research demonstrated that weak magnetic fields could efficiently stimulate the growth of bacteria, shorten the growth cycle, and increase the activity of microorganisms, which are beneficial to proliferation and metabolism of microorganisms. However, to our knowledge, no hybrid magnetic PUF composite has yet been developed for the immobilized carrier of microorganisms applied to wastewater treatment.
Therefore, the designing and synthesizing of a novel magnetic PUF carrier which could adjust the propagation and metabolism of microbial cells should be an interesting topic in the area of immobilized microorganism. Thus, in this paper, a novel magnetic nano-particles Fe3O4/PUF composite was synthesized by using in situ blend methods from the polymerization of toluene-2,4-diisocyanate (TDI) and polyether polyol, blending with Fe3O4 nano-particles. And the composition and morphology of this prepared composite was characterized by means of various modern analysis technologies, such as X-ray diffraction (XRD), Fourier transform IR spectroscopy (FT-IR), thermogravimetric (TG) analysis, differential scanning calorimetry (DSC), scanning electron microscopy (SEM), and magnetic property measurement system (MPMS), respectively. Furthermore, the resultant magnetic PUF composite was applied to the immobilized carrier of microorganisms for toluene-containing wastewater treatment.
Experimental section
Materials
Toluene-2,4-diisocyanate (TDI) and polyether polyol were all A.R. grade purchased from Baiyin Yinguang Chemical (Baiyin, China). Toluene was purchased as analytic regent from Guangzhou chemical factory. The pure PUF purchased from Baiyin Yinguang Chemical, was washed three times with deioned water before use for experiments, and other solvents and reagents used in this work were all A.R. grade.
Preparation of magnetic Fe3O4 nanoparticles and Fe3O4/PUF composite
The typical synthesis procedure of Fe3O4 nanoparticles is similar to the method described in [19]. Twenty grams NaOH was dissolved in 250 mL deionized water which was deoxygenated by bubbling N2 gas for 30 min at 1,000g stirring for 10 min. In another beaker, 0.85 mL of 12.1 N HCl and 25 mL deionized water was mixed, and then 8.1 g FeCl3 and 3.1 g FeCl2 were successively dissolved in the solution under stirring. Then, the resultant solution was added drop-wise into the above-mentioned NaOH solution under vigorous stirring, and black precipitate was obtained and harvested by centrifugation, and then washed with deionized water and ethanol several times, respectively, and finally dried in the air at 80 °C for 2 h. Then, the harvested Fe3O4 nanoparticle composite was used for the next step in the preparation of the magnetic PUF carrier.
The composition and the recipe for preparation of the magnetic PUF composite are listed in Table 1. The main ingredient of solution A is Polyol-330 (mixed with blowing agent), while solution B is the mixture of toluene-2,4-diisocyanate (TDI), Polyol-330 and curing agents. Different mass fraction of prepared Fe3O4 powder was completely soaked into solution B and dispersed by the ultrasonic for further 15 min, and then mixed with solution A by using a mechanical stirrer at 500g for about 10 s. Finally, the mixture was kept for curing for 2 days at room temperature.
Preparation of immobilization microorganism and biodegradation experiment
The pure and magnetic PUF carrier was rinsed with distilled water, then dried and autoclaved at 120 °C for 20 min before the bacteria was immobilized. Bacillus cereus S1 used in this experiment was originally obtained from a wastewater treatment facility of Guangzhou Petrochemical Corporation [20]. The medium was composed of 0.080 g toluene (COD = 250 mg/L), 21.75 g K2HPO4·H2O, 33.40 g Na2HPO4·12H2O, 8.50 g KH2PO4, 40 g NH4Cl, 22.50 g MgSO4, 36.4 g CaCl2, 0.25 g FeCl3, 0.04 g MnSO4·H2O, 0.04 g ZnSO4·H2O, 0.04 g (NH4)6Mo7O24·4H2O and 1 L deionized water. Bacteria was grown on the enrichment-medium (1.0% peptone, 0.5% malt extract and NaCl) at 30 °C with a shaking speed of 120g for 24 h, then were harvested by centrifuge at 4 °C with a speed of 10,000g for 10 min, and washed three times with 0.05 mol/L sterile potassium phosphate buffer (pH 7.5). Then, the washed cells were resuspended in the sterile phosphate buffer, resulting in a 10% cell suspension [21]. Initial immobilization of Bacillus cereus S1 was carried out in a 250-mL serum bottle with 50 mL suspended cell and 0.5 g pure PUF and magnetic PUF carrier. Then the mixture was inoculated and incubated for further 48 h to get the immobilized microorganism onto pure PUF and magnetic PUF carrier. The bacteria mass on the carrier and in the suspension can be measured in terms of dry weight (105 °C for 24 h). The net immobilized biomass can be calculated by subtracting the weight of carrier from the total weight of immobilized microorganism.
When the biodegradation experiment of toluene was performed, a certain amount of toluene and 0.5 g immobilized microorganism loading onto pure PUF or magnetic PUF were added into 100 mL medium and sealed into a 250-mL serum bottle, and the degradation of toluene (the initial COD concentration of 250 mg/L) was carried out at 30 °C in a rotary incubator at 120g. Samples were collected at regular time intervals for the analysis of chemical oxygen demand (COD) to determine the removal efficiency of toluene-contained wastewater.
Apparatus and analytical methods
The phase identification and the crystalline size calculations of Fe3O4 were taken by using a Siemens D5000 X-ray diffractometer with Cu K α radiation (λ = 0.15405 nm) in the interval 2θ of 20–80° with steps of 0.5°. FT-IR spectra were recorded on a model 750 Nicolet Magna-IR (USA) spectrometer after the samples (PUF, Fe3O4/PUF) were first dried by liquid nitrogen, and then mulled with KBr pellets to get the chips for FT-IR measurement. The differential scanning calorimeter (DSC) analysis was measured under a N2 atmosphere with a model DSC 10 TA Instruments (USA), at a heated range between −70 and 110 °C with the heating rate of 10 °C/min, after the samples were treated in a vacuum oven at 100 °C for 24 h and then quickly put into liquid nitrogen until the temperature reached −70 °C to obtain the amorphous samples. Magnetic hysteresis loops of Fe3O4 nano-particles and magnetic foam were measured by a XL-7 model magnetic property measurement system (MPMS; Quantum Design, USA.). The COD concentration was determined by the standard method based on the oxidation of potassium chromate (K2Cr2O7) and sulfuric acid digestion.
Results and discussion
XRD pattern and magnetic analysis of Fe3O4 particles
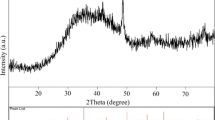
The wide-angle XRD patterns of prepared Fe3O4 particles are shown in Fig. 1. From the figure, the XRD peaks at 2θ = 30.18, 35.54, 43.16, 53.10, 57.10, and 62.88 degrees corresponding to plane reflection of Fe3O4 (220), (311), (400), (422), (511) and (440), respectively, were easily found in the profile. These peaks confirmed the existence of face-centered cubic Fe3O4 phase (JCPDS card. No. 19-629), which is in good agreement with the reported literature [22]. The average calculated value of crystal lattice parameter extracted from the XRD data, α, was about 0.084 nm, and the mean crystal size of the resultant nanometer Fe3O4 were calculated as 12 nm from the broadening of the XRD peaks of Fe3O4 according to the Scherrer equation.
The magnetic property of prepared nanometer Fe3O4 was also confirmed by MPMS at room temperature. The hysteresis loop reveals that a ferromagnetic behavior with a saturation magnetization value of 34 emu/g was obtained for the prepared nanometer Fe3O4, which further confirmed the existence of Fe3O4 particles [23].
FT-IR analysis
Figure 2 shows the FT-IR results of prepared Fe3O4/PUF composite blending with different amounts of Fe3O4 nanoparticles. It can be seen from the IR spectra that all samples have similar absorption peaks at 3,300 cm−1 (hydrogen-bonded N–H stretching), 1,550 cm−1 (N–H deformation), and 1,720–1,695 cm−1 (C=O stretching) as assigned to the urethane structure. The bands at 2,960 cm−1 are corresponding to the C–H asymmetrical stretching of the methylene hydrogen of polyether [24]. Also, Fig. 2 illustrates a broadening and weakening of the –OH absorption peak at 3,300 cm−1 with the increase of the content of nanoparticles, which should result from the coordination of Fe3O4 nanoparticles with nitrogen-containing functional groups presented in PUF [25]. This is because this kind of coordination effect would, obviously, be suitable to prepare stable PUF composite.
Thermogravimetric analysis and differential scanning calorimetry
Thermogravimetric (TG) analysis was carried out to investigate the thermal properties of the Fe3O4/PUF composite, and the relative results are shown in Fig. 3. As can be seen from the figure, the addition of nano-particles would improve the thermal stability of the PUF composite, and the contents of nano-particles had influence on the thermal stability of the PUF composite. That is, when the content of nano-particles was increased, the initial thermal weight loss temperature would be increased as well. The initial thermal weight loss temperature of magnetic PUFs with the content of 2.5 and 7.5% Fe3O4 as well as pure PUF were obtained as 281, 290, and 274 °C, respectively. The thermal decomposition temperature increased 16 °C, suggesting that thermal stability of hybrid PUF composite was improved considerably. The possible reason for this behavior can be interpreted that the introduction of Fe3O4 into PUF can reduce the mobility of PUF chains, inhibit the PUF chain reaction, and slow the degradation process [26, 27].
To prove the free volume change, differential scanning calorimeter (DSC) was also employed to observe the glass transition temperature (T g) of the prepared Fe3O4/PUF composites, and the results are shown in Fig. 4. The T g values for Fe3O4/PUF composite with contents of 0, 2.5 and 7.5 wt% were gained as −60, −59, and −58 °C, respectively, according to the loss of endothermic peaks. It can be observed that, for the hybrid PUFs composite blending with Fe3O4 nano-particles, the T gs are slightly higher than that of pure PUF. When the content of Fe3O4 increased, the T g was also slightly raised to a higher temperature. Similar to the afore-mentioned TG data, these results also verified that the introduction of Fe3O4 nano-particles would improve the stability of composite PUFs. These phenomena were also previously reported by Kuan et al. [28], who found that the T gs of polysilicic acid (PSA)/waterborne PU composite increased with the increase of PSA contents. This indicates that Fe3O4 nano-particles maybe act as a cross-linking agent to inhibit the movement of PU soft segment and therefore increase the T g value of PUF composite.
Surface morphology analysis of Fe3O4/PUF composite
SEM images of magnetic Fe3O4/PUF composites with different contents of Fe3O4 nanoparticles are displayed in Fig. 5. It can be seen that the pore size of pure PUF was ~300 μm, and the opening ratio was over 90%. With the increase of the content of nano-particle Fe3O4, the pore size became much smaller and the opening ratio slightly decreased, which had little influence on the pore structure of the composite. But when the ratio of nano-particle Fe3O4 exceeded 5.0%, the pore structure of the PUF composite became blurred. And the uniform porous structure and the high opening ratio will be lossed completely when the percentage of nanoparticle Fe3O4 is higher than the value of 5.0%. Thus, these may suggest that the better structured magnetic Fe3O4/PUF composite would be guaranteed when the nanoparticles Fe3O4 did not exceed 5.0%. And the resultant PUF composite should have a high opening ratio and large amounts of pores which maybe enhance the convenient mass transfer in the wastewater treatment [29].
To further confirm the existence of nano-particles Fe3O4 in the composites, the surface energy-dispersive spectra (EDS) was also applied to investigate the dispersion of nano-particles Fe3O4 into the matrix of the PUF composite. The results of EDS for magnetic PUF composite with the content of 2.5% Fe3O4 are shown in Fig. 6. As seen from the figure, the hybrid magnetic PUF composite was composed of C (0.250 keV), O (0.523 keV), Cl (2.621 keV), and Fe (6.398 keV), as well as minor amounts of Al (1.486 keV). However, the weight of Fe element introduced as an magnetic additive into the composites was obtained with the mass fraction of 0.84% by using surface energy dispersive spectrum. Thus, the amount was much lower than that of the theoretical value of Fe element of 1.81% calculated according to the chemical composition analysis. This may indicate that Fe3O4 nano-particles were evenly dispersed in the PU matrix as well as the surface, and that these results were also consistent to the data of TG analysis and DSC.
Magnetic properties analysis
The hysteresis loops of the Fe3O4/PUF composites prepared with different content of Fe3O4 were also measured at room temperature, and the data are shown in Fig. 7. From the figure, the specific saturation magnetization of various magnetic PUF composites increased quickly with the increase of the contents of the Fe3O4 nanoparticle, but no hysteresis, remanence, and coercivity were observed. The saturation magnetization of magnetic the PUF composite was much smaller than that of the Fe3O4 nanoparticles with 12 nm reported in the early phases. When the content of nano-particle Fe3O4 were varied from 0.5 to 10%, the specific saturation magnetizations of Fe3O4/PUF composites varied from 0.25 to 3.96 emu/g, which means that the prepared Fe3O4/PUF composites were all super paramagnetic, and could be magnetized and modulated via an external magnetic field [30–32]. Thus, these properties of prepared Fe3O4/PUF composites would make them an excellent carrier of separation and transportation as well as the immobilized carrier for microorganisms.
The application of magnetic Fe3O4/PUF for immobilized carrier for microorganism
In order to observe the effect of magnetic properties of the carrier on the removal efficiencies of organic pollutants from wastewater, a commercial pure PUF and the prepared magnetic Fe3O4/PUF composite with 5.0% content of Fe3O4 were both used as carriers for the immobilized microorganisms. The compared results are shown in Fig. 8. From the figure, we readily found that toluene concentrations decrease quickly by using both commercial PUF and the prepared composite as carriers of immobilized microorganisms. However, the removal efficiency of COD concentration with immobilized microorganisms onto Fe3O4/PUF composite was always higher than that with immobilized microorganisms onto pure PUF. The COD removal efficiency reached 77.7% for Fe3O4/PUF composite, while only 70.1% was obtained for pure PUF carrier, after 8 h treatment with immobilized microorganisms. By measuring the biomass loaded onto PUF and Fe3O4/PUF, we can also readily find that the biomass of microorganisms on the magnetic PUF composite was much higher (25.9 mg dry wt. cells/g carrier) than that of the pure PUF (18.8 mg dry wt. cells/g carrier). Thus, this is the reason why the magnetic PUF composite carriers would increase proliferation and metabolism of immobilized microorganisms [33] and then finally accelerate the toluene degradation.
Conclusions
A novel magnetic Fe3O4/PUF composite was prepared to apply to the carriers of immobilized microorganisms for toluene-containing wastewater treatment. Confirmed by experiments of both thermogravimetric analysis and differential scanning calorimetry, it is found that the thermal stability of prepared magnetic Fe3O4/PUF was increased slightly by the dispersion of nano-particles Fe3O4 in the PUF. The magnetic properties analysis indicated that the prepared Fe3O4/PUF composites were all super paramagnetic, and would be magnetized and modulated via an external magnetic field. By measuring the biomass loaded onto PUF and Fe3O4/PUF, it is easily found that the biomass of microorganisms on the magnetic PUF composite was much higher than that of the pure PUF. And the removal efficiencies of COD concentration with microorganisms immobilized onto the Fe3O4/PUF composite were always higher than that with microorganisms immobilized onto pure PUF.
References
L.C. Zhou, G.Y. Li, T.C. An, J.M. Fu, G.Y. Sheng, Recent Pat. Eng. 2, 28 (2008)
X.C. Quan, H.C. Shi, Y.M. Zhang, H.L. Wang, Y. Qian, Sep. Purif. Technol. 34, 97 (2004)
H.R. Kariminiaae-Hamedaani, K. Kanda, F. Kato, J. Biosci. Bioeng. 95, 128 (2003)
J. Savic, V. Vasic, B. Dnadevic, Hemijska Industrija 61, 13 (2007)
K. Na, Y. Lee, W. Lee, Y. Huh, J. Lee, M. Kubo, S. Chung, J. Biosci. Bioeng. 90, 368 (2000)
E. Quek, Y.P. Ting, H.M. Tan, Bioresour. Technol. 97, 32 (2006)
W.T. Qi, J. Ma, W.T. Yu, Y.B. Xie, W. Wang, X.J. Ma, Enzym. Microb. Technol. 38, 697 (2006)
R.F. Martins, F.M. Plieva, A. Santos, R. Hatti-Kaul, Biotechnol. Lett. 25, 1537 (2003)
P.Y. Yang, T. Ma, T.S. See, N. Nitisoravut, Water Sci. Technol. 29, 487 (1994)
M. Matsumura, T. Yamamoto, P.C. Wang, K. Shinabe, K. Yasuda, Water Res. 31, 1027 (1997)
A.Y. Fedorov, E.V. Volchenko, I.N. Singirtsev, V.I. Korzhenevich, G.M. Shub, Appl. Biochem. Microbiol. 36, 50 (2000)
X.L. Guo, G. Deng, J. Xu, M.X. Wang, Enzym. Microb. Technol. 39, 1 (2006)
J.L. Wang, Y. Qian, Chemosphere 38, 3109 (1999)
Y.S. Oh, J. Maeng, S.J. Kim, Appl. Microb. Biotechnol. 54, 418 (2000)
F.S. Lupton, D.M. Zupancic, US Patent no. 4983299 (1991)
Y.A. Sakai, K.A. Tani, F.U. Takahashi, J. Ferment. Bioeng. 74, 413 (1992)
H. Yavuz, S.S. Celebi, Enzym. Microb. Technol. 26, 22 (2000)
T.B. Rao, R.L. Sonolikar, S.P. Saheb, Chem. Eng. Sci. 52, 4155 (1997)
S. Cui, X.D. Shen, B.L. Lin, Rare Met. 25, 426 (2006)
G.Y. Li, Z. He, T.C. An, X.Y. Zeng, G.Y. Sheng, J.M. Fu, J. Chem. Technol. Biotechnol. 83, 1019 (2008)
F. Zeng, K.Y. Cui, J.M. Fu, G.Y. Sheng, H.F. Yang, Water Air Soil Pollut. 140, 297 (2002)
L.Q. Xu, J. Du, P. Li, Y.T. Qian, J. Phys. Chem. B 110, 3871 (2006)
Y.H. Zheng, Y. Cheng, F. Bao, Y.S. Wang, Mater. Res. Bull. 41, 525 (2006)
J. Mehrdad, Y. Hamid, N.H. Mehdi, Polym. Int. 57, 1385 (2008)
L. Chen, W.J. Yang, C.Z. Yang, J. Mater. Sci. 32, 3571 (1997)
J. Chen, Y.M. Zhou, Q.L. Nan, Y.Q. Sun, X.Y. Ye, Z.Q. Wang, Appl. Surf. Sci. 253, 9154 (2007)
M.N. Gururaj, H. Erika, J. Margaret, B. Valery, Polym. Int. 57, 1083 (2008)
H.C. Kuan, H.Y. Su, C.M. Ma, J. Mater. Sci. 40, 6063 (2005)
L.C. Zhou, X. Bai, Y.F. Li, P.C. Ma, Environ. Eng. Sci. 9, 1235 (2008)
J.L. Rivera-Armenta, T. Heinze, A.M. Mendoza-Martinez, Eur. Polym. J. 40, 2803 (2004)
G.L. Qiu, Y.L. Li, K. Zhao, Enzym. Microb. Technol. 39, 770 (2006)
H. Yavuz, S.S. Celebi, J. Bioact. Compat. Polym. 16, 221 (2001)
S.Y. Shaw, Y.J. Chen, J.J. Ou, L. Ho, Enzym. Microb. Technol. 39, 1089 (2006)
Acknowledgments
This is contribution No. IS-1170 from GIGCAS. The authors gratefully acknowledge the financial support from the Science and Technology Project of Guangdong Province, China (2007A032301002, 2006A36701002 and 2009B030400001) and the Chinese Postdoctoral Science Foundation (20080430848).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, L., Li, G., An, T. et al. Synthesis and characterization of novel magnetic Fe3O4/polyurethane foam composite applied to the carrier of immobilized microorganisms for wastewater treatment. Res Chem Intermed 36, 277–288 (2010). https://doi.org/10.1007/s11164-010-0134-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-010-0134-5