Abstract
Thyroid cancer has shown a parallel increase with diabetes in the last few years. This narrative review aims to explain the association between these two entities, focusing on insulin resistance as the mediator and exploring the effects of antidiabetic agents on thyroid cancer incidence and progression.
We searched Pubmed for English-written articles on insulin resistance, diabetes, antidiabetic treatments, and thyroid cancer reported from January 2019 to April 2023. Exclusion criteria were preclinical and clinical studies involving a population with thyroid dysfunction, benign nodular goiter, or those that only analyzed thyroid cancer’s association with obesity.
The results of the narrative literature review revealed 96 articles. Additionally, four studies from a manual search were retrieved. After the exclusion criteria were applied, we included 20 studies. Out of 8 studies on insulin-resistant or Metabolic Syndrome patients, all suggest a positive association with thyroid cancer. At the same time, for diabetes, four out of five publications support a link with thyroid cancer. The seven remaining studies on antidiabetics suggest that metformin might benefit thyroid cancer. In contrast, the evidence for an association between Glucagon-like peptide-1 receptor agonists (GLP-1 RA) and increased thyroid cancer findings is limited.
In conclusion, the association between thyroid cancer and diabetes may be explained by insulin resistance, as shown in observational studies. However, the causal role is yet to be defined. Although the wide use of different antidiabetic agents has been related to thyroid cancer prevalence and progression, future research with drugs such as metformin or GLP-1 RA is still needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The relationship between the thyroid and insulin resistance has been extensively reviewed, illustrating the central role of thyroid hormones in regulating carbohydrate metabolism [1, 2]. By contrast, the effect of impaired glucose metabolism on the thyroid gland continues to fuel debate.
Thyroid cancer has shown a parallel rise to diabetes [3], suggesting that metabolic syndrome (MetS), obesity, and diabetes itself might act as precipitating factors in cancer development [4, 5]. However, concerns about these observational findings have been raised due to the possibility of detection bias due to the more extensive use of diagnostic tests in susceptible populations [6].
Although the mechanistic effects of the association between thyroid cancer and metabolic abnormalities are not yet elucidated, various pathways such as hyperglycemia, hyperinsulinemia, hyperlipidemia, oxidative stress, adipokines, and inflammatory responses may be involved [7].
Insulin resistance is a leading player in this game. One of the earliest studies showing that the thyroid gland could be affected by an insulin-resistant state was performed by Rezzonico et al. [8]. In this study, where 111 women were evaluated by thyroid ultrasound, thyroid nodules were more frequently found in those women who were insulin resistant independently of their body weight. Furthermore, MetS, characterized by simple clinical criteria linked to insulin resistance [9], has also been associated with thyroid nodular disease. In a prospective study where older adults with MetS were evaluated with thyroid ultrasonograms, a positive correlation between impaired metabolic control and increased thyroid volume and nodularity was observed [9]. In agreement with these findings, Ayturk et al. found that thyroid volume was higher in MetS patients [10]. The association between MetS components and thyroid cancer was further assessed in 2018 in a meta-analysis including 42 articles and an increased risk for thyroid cancer for patients with insulin resistance, dysglycemia, high BMI and hypertension was described [11].
It is widely assumed that insulin resistance plays a key role in diabetes. Consequently, it is essential to define if thyroid cancer risk is also increased in diabetic patients. To this end, several meta-analyses [12,13,14] looking for this association have been published before our search period for this narrative review. According to the last one published in 2017 [14], including 16 cohort studies and 10,725,884 individuals, diabetic patients had a 20% increase in the incidence of thyroid cancer.
Furthermore, given the critical increase in differentiated thyroid cancer (DTC) together with the epidemic of type 2 diabetes mellitus (T2DM), it is of particular interest if the wide use of different therapies for diabetic control may affect thyroid nodule growth and cell transformation. A few studies have focused on the impact of metformin, showing that it could prevent nodular thyroid growth, but further studies are warranted to confirm this benefit [15,16,17].
This narrative review of the literature is aimed to provide clinical insights into insulin resistance as the core component of MetS and T2DM and describe its mechanistic role in cancer development. Since the topic was already covered in previous meta-analyses up to 2018, an update, including a systematic search from 2019 onwards, was performed. Those studies describing the association between thyroid cancer and insulin resistance/diabetes and those exploring anti-diabetic therapeutic drugs’ thyroid impact were included.
2 Literature search
We searched Pubmed for articles related to the section “INSULIN RESISTANCE, DIABETES, AND THYROID CANCER” of this narrative review with the details of the search terms as: ((thyroid cancer[MeSH Terms]) OR (thyroid carcinoma[MeSH Terms])) AND (insulin resistance[MeSH Terms]), and also: ((thyroid cancer[MeSH Terms]) OR (thyroid carcinoma[MeSH Terms])) AND (type 2 diabetes mellitus[MeSH Terms]).
We also searched articles for the section “INFLUENCE OF DIABETES TREATMENTS ON THYROID CANCER” with the following details: ((thyroid cancer[MeSH Terms]) OR (thyroid carcinoma[MeSH Terms])) AND (antidiabetic drugs[MeSH Terms]), and also ((thyroid cancer[MeSH Terms]) OR (thyroid carcinoma[MeSH Terms])) AND (metformin[MeSH Terms])
The search included articles published from date January 2019 to April 2023. A manual search was performed to detect studies that could be relevant but not present in the PubMed search.
One author (F.DF) screened the relevant articles and selected the studies (including reviews, retrospective studies, and prospective studies) that reported the association between thyroid cancer and diabetes or insulin resistance or treatment with antidiabetic drugs. Both Authors (G.B. and F.DF) discussed the studies that were finally selected according to inclusion and exclusion criteria.
Exclusion criteria were preclinical and clinical studies involving a population with thyroid dysfunction, benign nodular goiter, or those that only analyzed thyroid cancer’s association with obesity. Articles in another language that was not English were also excluded.
An Institutional Review Board review was not needed for a literature review.
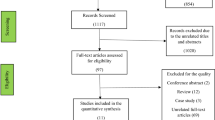
The results of the narrative literature review revealed 96 articles. Additionally, four studies from the manual search were retrieved. After the exclusion criteria were applied, we included 20 studies. (Fig. 1)
3 Definitions of insulin resistance, MetS and T2DM
It has long been known that insulin resistance results from the lack of sensitivity of cells in the body, mainly in the liver, skeletal muscle, and adipose tissue, to insulin. Therefore, glucose remains in the blood, leading to compensatory hyperinsulinemia in an attempt to process glucose. In this pathologic process, the beta cells in the pancreas wear out, leading to the development of glucose intolerance, high triglyceride serum levels, a decrease in high-density lipoprotein cholesterol concentrations, high blood pressure, smaller denser low-density lipoprotein particles, and eventually T2DM [18]. Although several plausible mechanisms could explain the pathophysiology of insulin resistance, further studies are warranted to confirm these observations, which could ultimately lead to new therapeutic strategies [19].
There are several ways to assess insulin resistance, but the gold standard is the euglycemic hyperinsulinemic clamp, which can only be performed under strict laboratory conditions [20]. An alternative is the Insulin Tolerance Test (ITT) [21], although it is still not applicable to large populations. Consequently, if insulin resistance is to be evaluated on a large scale, several indexes well correlated with the clamp, such as HOMA-IR [22] and the triglyceride-glucose (TyG) index [23], are frequently used. The TyG index is a biomarker for insulin resistance and MetS and also predicts T2DM [24]. The TyG index uses nonexpensive biomarkers such as fasting triglycerides and glucose. Different from HOMA-IR, it does not require insulin dosage. It has been reported to have good sensitivity and specificity compared to the gold standard method and good sensitivity compared to HOMA-IR [25]. Although its average values may vary according to the population studied, the proposed cut-off value to define insulin resistance with HOMA is > 2.5, while for the TyG index, the optimal cutoff point to predict incident T2DM has been described as 8.668 [24].
The specific calculation equation for HOMA-IR = Fasting Serum Insulin (µIU/ml) X Fasting Plasma Glucose (mmol/l) / 22.5 [22] and TyG index = ln[Fasting Plasma Glucose (mg/dl) × TG (mg/dl)/2] [23].
The metabolic syndrome is considered a clinical epiphenomenon of insulin resistance, and its definitions may vary according to the different Scientific Societies and the geographical areas evaluated. One of the first definitions proposed by the Adult Treatment Panel III (ATPIII) [26] requires three or more of the following five disorders: elevated waist circumference (102 cm in men and 88 cm in women), hypertriglyceridemia (1.7 mmol/l), low HDL cholesterol level (1.03 mmol/l in men and 1.3 mmol/l in women), high blood pressure (systolic blood pressure 130 mmHg and/or diastolic blood pressure 85 mmHg and/or pharmacological treatment), and elevated fasting glucose (5.6 mmol/l and/or pharmacological treatment). However, according to a joint statement of different organizations published in 2009, MetS is defined by three of the five following criteria): (i) waist circumference (WC) (for European population): 94 cm for men and 80 cm for women, (ii) elevated triglycerides (TGs): 150 mg/dL, or drug treatment for elevated TGs, (iii) reduced serum high-density lipoprotein cholesterol (HDL-C) < 50 mg/dL for men and < 40 mg/dL for women or drug treatment for reduced HDL-C, (iv) elevated blood pressure [systolic (SBP): 130 and/or diastolic (DBP): 85 mmHg or antihypertensive drug treatment in a patient with a history of hypertension, and (v) elevated fasting glucose: 100 mg/dL or drug treatment for elevated glucose as an alternate indicator [27].
Finally, the American Diabetes Association, in its article “Standards of care in Diabetes 2023”, proposes the following criteria for the diagnosis of diabetes mellitus: fasting plasmatic glucose ≥ 126 mg/dL (7.0mmol/L), defining fasting as a no caloric intake for at least 8 h, or a 2 h plasmatic glucose greater than 200 mg/dL after a glucose load (OGTT, using 75 gr of anhydrous glucose dissolved in water) or a glycated (A1c) Hemoglobin level (HbA1C) ≥ 6.5% (48mmol/mol) or patients with classic symptoms of hyperglycemia with a random value of plasma glucose ≥ 200 mg/dl (11.1mmol/L) [28].
3.1 Diabetes and cancer
Diabetes has become the focus of interest of all health systems worldwide. In the last 30 years, its numbers have increased four times, and the majority, 90%, are T2DM [29, 30].
Type 2 diabetes is frequently associated with cardiovascular risk and its fatal consequences [31]. However, the risk of cancer is another threat that has to be acknowledged. The types of cancers that have been shown at increased risk with diabetes are liver, pancreas, endometrial, colorectal, kidney, bladder, breast, and thyroid cancers [32, 33].
The association between diabetes and cancer has been studied for a long time [34], although some of its pathophysiological explanations have not yet been uncovered. Cancer and diabetes share many risk factors, such as older age, obesity, sedentary life, bad nutritional habits, smoking, and alcohol consumption [35, 36]. Although diabetes is considered an established risk factor for developing cancer [37, 38], it has been hypothesized that cancer can also increase the risk of developing diabetes [39]. A partial explanation of this paradox might be cancer cachexia, the wide use of glucocorticoids, and chemotherapy in cancer patients that can impact the dysregulation of carbohydrate metabolism.
While the interaction between cancer and diabetes is still controversial, hyperinsulinemia is the plausible mechanism that can link the development of both diseases. Furthermore, the advanced study of the Micro RNA may help to understand the common pathways involved in T2DM and cancer [40].
3.2 Mechanistic effects that may explain the association between thyroid cancer and diabetes
It has long been known that thyroid-stimulating hormone (TSH) acts directly on its receptor (TSHR), which is a cell-surface G protein-coupled receptor (GPCR) stimulating proliferation while maintaining the expression of differentiation [41]. However, insulin is required for the mitogenic action of TSH exerting a permissive action. In different species’ thyroid, insulin-like growth factor (IGF-I) and other growth factors act on its membrane receptor that possesses tyrosine kinase (TKR) activity. When insulin binds to its receptor, tyrosine phosphorylation takes place, which leads to the activation of phosphatidylinositol-3-kinase (PI-3-kinase) and glucose uptake; in addition to the activation of the mitogen-activated protein (MAP) kinase pathway responsible for cell and tissue proliferation as well as for gene expression [42]. The MAPK pathway is limited at the physiological level but plays a significant role in chronic hyperinsulinemia. At high concentrations, as in insulin-resistant states, insulin acts both on its receptor and the IGF receptor (IGFR) [43].
The TSHR and IGF1R can interact by forming a multi-protein complex called signalosome. As proposed by Krieger et al., the two receptors are in close proximity to crosstalk, and this complex is stabilized by β-arrestin 1 in the absence of ligand binding [44].
Research in recent years has demonstrated that dysregulation of the insulin/IGF system is a critical factor in tumor progression and metastasis. This system includes three ligands—insulin, IGF1, and IGF2 and cognate receptors, including the insulin receptor (IRc), with its two isoforms —isoform A (IRc-A) and isoform B (IRc-B)—the IGF1R and the IGF2R. IRc-A is the primary mediator of the mitogenic effects of insulin, whereas IRc-B is the primary mediator of the metabolic effects of insulin [45]. Both IRc and IGF1R are highly homologous and can form heterodimeric hybrid receptors [46].
Many cancer cells exhibit an aberrant expression of growth factors and their receptors and are, therefore, more responsive to the mitogenic effects of insulin. In thyroid cancer, the density of IGF1R and the two isoforms of IRc is higher, supporting their biological significance in cancer development [47].
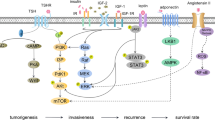
In summary, the mechanistic effects that may link the hyperinsulinemia characteristic of T2DM patients with thyroid cancer include the direct overstimulation of MAPK pathways, the stimulation of IGF1R leading indirectly to overstimulation of the same mitogenic pathway, the permissive effects on the TSH mitogenic action, the inhibition of the production of IGFBP-1 and 2 which results in increased levels of IGF1 and 2 and the stimulation of hybrid receptors (IRc/IGFR) [48, 49]. (Fig. 2)
The worldwide parallel increased incidence of diabetes and differentiated thyroid cancer (DTC) can be explained in large part by insulin resistance. Insulin exerts its metabolic actions through its own tyrosine kinase receptor (TKR) and when insulin binds to its receptor, tyrosine phosphorylation takes place, which leads to the activation of phosphatidylinositol-3-kinase (PI-3-kinase) to increase glucose uptake and to the activation of the Rat Sarcoma Virus - mitogen-activated protein (Ras-MAP) kinase pathway responsible for cell and tissue proliferation as well as for gene expression. The MAPK pathway is limited at the physiological level but plays a significant role in chronic hyperinsulinemia. At high concentrations, as in insulin-resistant states, insulin acts both on its receptor and the IGF receptor (IGFR). Besides, the stimulation of IGF1R leads indirectly to overstimulation of the same mitogenic pathway, thereby inhibiting the Tuberous Sclerosis Complex 1/2 (TSC 1/2) protein complex and activating Mammalian Target Of Rapamycin (mTOR) signaling. Moreover, the nuclear stimulation of Adenosine Mono-Phosphate-Activated Protein Kinase (AMPK) increases the activation of TSC2 and decreases the activation of mTOR. In the thyroid gland in particular, the thyroid-stimulating hormone (TSH) also contributes to proliferation. TSH acts directly on its receptor (TSHR), which is a cell-surface G protein-coupled receptor (GPCR) stimulating proliferation through the PI3K/AKT pathway, sharing this mechanism with insulin and growth factors.
3.3 Insulin resistance, diabetes, and thyroid cancer
In the last four years, several observational studies have evaluated a potential association between insulin resistance, MetS, diabetes, and thyroid cancer.
As regards insulin resistance, in a systematic review by Harikrishna et al. [50] looking for a correlation between thyroid cancer, obesity, and insulin resistance, fifteen articles were included. Still, only four of them were about insulin resistance. The first of these four articles [51] described a case-control retrospective study of 48 DTC cases compared to 70 patients with primary hyperparathyroidism and 102 internal medicine in-patients as the control groups. The diabetes-adjusted odds ratio was 3.178 (95% CI 1.202–8.404, p = 0.0198) when comparing cases versus primary hyperparathyroidism controls and 2.237 (95% CI 1.033–4.844, p = 0.0410) for pooled controls. The second one [52] also reported on a case-control retrospective study. In this study, 20 female patients with DTC were compared to 20 well-matched controls, showing that HOMA-IR > 2.5, considered as insulin resistance, was statistically increased in DTC patients (50%) compared with control patients (10%). The third article [53] described a study with a cross-sectional design and looked for the association between insulin resistance and the occurrence of DTC. The two groups compared were 735 female patients with papillary thyroid cancer (PTC) vs. 537 female non-PTC subjects. Higher fasting serum insulin levels, glucose levels, and HOMA-IR (all p < 0.001) were found in the PTC group. It was shown that the prevalence of PTC was statistically associated with increased insulin levels (OR = 2.88), glucose levels (OR = 9.32), and HOMA-IR (OR = 4.07). The fourth article [54] included a retrospective study of 38 PTC patients followed for a median of 5.5 years. It was observed that those patients with intermediate ATA risk of recurrence (ATA RR) and without insulin resistance (HOMA-IR < 2.5) had a higher rate of initial excellent response to treatment (36 vs. 11%, p = 0.01) compared to those with HOMA-IR > 2.5. Furthermore, at the final follow-up, the frequency of structural persistent disease in both low and intermediate ATA RR patients was higher in insulin-resistant patients (10 vs. 0%, p = 0.02 and 45 vs.7%, p = 0.01, respectively).
In 2021, Zhao et al. [55] published a meta-analysis of case-control studies evaluating the prevalence of thyroid cancer in patients with insulin resistance. With a different strategic literature search than Harikrishna et al. [50], after excluding 1264 studies, fourteen studies were included, with 2024 patients with thyroid cancer as cases and 1460 patients as controls. The control group included both healthy individuals and patients with benign thyroid disease. It was found that hyperinsulinemia and higher HOMA-IR were more prevalent in DTC patients. To assess insulin resistance in particular, the Authors meta-analyzed four studies with 326 DTC and 199 controls. According to HOMA-IR, the OR for insulin resistance was 3.16 (95% CI 2.09–4.77, p < 0.01).
Xu et al. [56] published a cross-sectional study evaluating the association between insulin resistance and thyroid cancer in 233 patients without insulin resistance-related risk factors such as diabetes mellitus, cardiovascular disease, and hypertension history. The final analysis included 61 patients with DTC and 262 with benign nodules as a control group. The HOMA-IR in DTC was 5.4 ± 6.9 vs. 2.8 ± 1.3 in the control group (p < 0.001). HOMA-IR > 2.7 was considered insulin resistance, and the adjusted OR for DTC in insulin-resistant patients resulted in 1.572 (95% CI: 1.277–1.935). As this study was performed only with Chinese participants, it may be not easy to extrapolate its findings to other geographic areas.
Alkurt et al. [57] took over a retrospective cross-sectional study of 254 patients with DTC. They compared it to 128 patients who underwent thyroid surgery for benign thyroid disease to evaluate the role of the TyG index in thyroid cancer risk. The TyG in non-malignant patients was statistically lower (8,303.46 ± 5,896.20) than in patients with DTC (10,603.06 ± 9,795.03). Although the levels of triglycerides in both groups were similar, glucose levels were markedly increased in the PTC group, explaining their higher TyG index. Possible limitations of this study include differences between the compared groups regarding TSH values and lack of information on body mass index (BMI) values and the nature of the non-malignant thyroid disease group.
In another study by Zhao et al. [58], the aim was to evaluate the impact of abnormal glucose metabolism parameters on the aggressiveness of DTC. The study design was cross-sectional, and 377 patients with DTC who underwent surgery and were scheduled to receive iodine 131 treatment were included. An oral glucose tolerance test including plasma glucose, insulin, and C-peptide levels (fasting, 1, 2, and 3 h) was obtained, and several indexes of insulin resistance were calculated under levothyroxine deprivation with an overt hypothyroid status. These calculations revealed insulin resistance in most of the studied population, in line with what had been previously reported in patients with DTC and acute overt hypothyroidism [59]. It is worth noting that when the aggressiveness of DTC was considered, T staging was associated with a higher area under the curve of C-peptide and abnormal insulin/C-peptide sensitivity. This trend was also observed in patients without a history of diabetes. A delayed peak of insulin secretion was positively related to capsule invasion. Furthermore, 3-hour glucose, insulin, and C-peptide levels were statistically associated with the number of lymph node metastases. Given that all the measurements were performed under the confounding effect of hypothyroidism, it would be interesting to find out if similar results can be reproduced under euthyroid status.
Regarding MetS, Park et al. [60] published a large-scale cohort study based on the Korean population database provided by the National Insurance Service, followed up until the date of development of thyroid cancer, which was identified according to the hospitalization records, death, or the date of the end of recruitment. The mean follow-up period for the 9,890,917 subjects was 7.2 years, and 77,133 thyroid cancer cases were newly identified in the study period. At baseline, 26.7% of the population had MetS, and it was found that they were more predisposed to develop thyroid cancer (HR 1.15; 95% CI 1.13–1.17). It is also worth noting that a higher number of MetS components correlated with the risk of developing thyroid cancer, in particular within the obese MetS group (1 component: HR: 1.18, 95% CI: 1.15–1.20; 2 components: HR: 1.31, 95% CI 11.28–1.34; 3 components: HR: 1.33, 95% CI: 1.29–1.36; 4 components: HR: 1.37, 95% CI: 1.33–1.41; 5 components: HR: 1.39, 95% CI: 1.33–1.44). It should be stated that the combined effect of obesity and MetS was only observed in males.
Song et al. [61] performed a cross-sectional study about the association between the components of MetS and DTC. This study included 745 patients undergoing thyroid surgery and central lymph node dissection, of which 145 had MetS. Metabolic syndrome was defined according to the Chinese Diabetes Society criteria. The proportion of multifocal disease, AJCC stage, and radioactive iodine therapy was similar between patients with and without MetS. Nevertheless, MetS was a risk factor for more extensive tumors (OR = 2.29, 95% CI: 1.31–4.03), more number of lymph node metastasis (OR = 1.97, 95% CI: 1.11–3.51), and higher clinical stage (OR = 7.92, 95% CI: 1.59–39.34) after correction for age, sex, TSH level, and BMI. Taking account of the five components of MetS, only high blood pressure and low HDLc were positively correlated with larger tumors and lymph node metastases. Among the limitations reckoned by the Authors are the small sample size, the lack of information about the follow-up of patients beyond one year of surgery, and confounders related to MetS, such as duration, treatment, and concurrent smoking habit.
All these findings have been recently included, together with previous studies, by Li et al. [62] in a review, where the authors concluded that MetS and its components could be associated with an increased risk and aggressiveness of thyroid cancer.
In summary, the observed association between DTC and insulin resistance, or MetS as a surrogate of an insulin-resistant state, is present in several populations evaluated. To which extent insulin resistance plays a role in these patients’ tumor progression is still quite intriguing. (Table 1)
As regards the association of diabetes and thyroid cancer, several studies with controversial results have been published in the search period between January 2019 and April 2023.
In a case-control study by Zhang et al. [63], the levels of HbA1C and lipids were compared between 23 patients with T2DM and DTC vs. 120 with T2DM without DTC. The mean serum HbA1C (10.47 vs. 9.07%), total cholesterol (226 vs. 208 mg/dL), and triglycerides (269 vs. 231 mg/dL) levels were significantly higher, and the levels of HDL-C lower (28 vs. 45) mg/dL) in DTC patients vs. non-DTC patients, respectively. One of the main limitations of this study is its small sample size.
Wang et al. [64] also analyzed the associations between diabetes and the risk of thyroid cancer in a cross-sectional study where 2,937 thyroid cancer cases and 2,937 healthy controls were included. No association was found either in the diagnosis of diabetes or its duration. Furthermore, it was described that patients older than 40 years old, female sex, overweight/obese, and those with a positive family history of diabetes had a lower risk of thyroid cancer than the control group. One possible explanation for these controversial findings is the unaccounted use of metformin, an antidiabetic drug with potential antiproliferative action in thyroid cancer tissue [65]. Since the diagnosis of diabetes was self-reported and only 293 subjects had diabetes, this study may also be limited by its small sample size.
On the other hand, a positive association between thyroid cancer and diabetes has been recently reported in a meta-analysis of 20 cohort studies published between 1991 and 2019 [66]. These studies were performed in nine countries from three different continents, with a sample size of more than 300,000 patients, developing a total of 11,091 cases of thyroid cancer. Sex-specific risk analysis revealed that the risk of thyroid cancer in female patients with diabetes (36%) was slightly higher than in male patients (26%) with diabetes. In the subgroup analysis, those patients with T2DM had a RR of 1.34 for developing DTC (95% CI, 1.12–1.37), being slightly higher for females than for men (RR: 1.37, 95% CI 1.17–1.53 vs. RR 1.32, 95% CI 1.12–1.54 respectively). Four studies reported a 30% higher risk of thyroid cancer in gestational diabetes. Only one study reported the risk of thyroid cancer in T1DM, with a higher risk in female patients (RR 1.51) without any association in male individuals. One of the limitations of this study is the lack of assessment of the severity of diabetes since the difference between controlled and uncontrolled diabetes was not evaluated.
As regards the clinical aggressiveness of DTC and the presence of prediabetes and diabetes, Elbasan et al. [67] retrospectively evaluated 526 DTC patients with total thyroidectomy and/or radioactive iodine ablation followed for over four years in a single center. Patients were divided into three groups: diabetes, prediabetes, and normoglycemia. Histological aggressiveness of DTC at the time of diagnosis and clinical response at the time of the last clinical visit, according to the criteria suggested by the ATA 2015 guideline, were similar in the three groups. These negative findings might be limited by the retrospective design of the single-center study and the lack of data about the duration of diabetes and its treatment.
Nonetheless, in another retrospective case-control study by Li et al. [68], an association between diabetes and more advanced thyroid cancers was observed. In this study conducted on 942 patients with DTC, 471 patients were diagnosed with T2DM for over a year, and 471 non-diabetic patients matched by gender and age served as controls. Tumor migration was higher in the diabetic patients than in the control group, mainly reflected in the rates of extra-thyroidal extension (34.82%) and lymph node metastasis (42.68%). However, there was no significant difference in primary tumor size, multifocality, or T stage (T3/T4).
As mentioned previously, although the association between thyroid cancer and diabetes is well described, it is still not clear if the development of thyroid cancer can be fully explained by diabetes. Furthermore, it might be hypothesized that thyroid cancer could also play a causal role. Regarding the inverse association between thyroid cancer and the risk of developing T2DM, Roh et al. [69] designed a case-control study with 36,377 patients with thyroid cancer matched to 36,337 non-cancer healthy subjects. They found that post-thyroidectomy, patients with thyroid cancer had a 43% higher risk of T2DM (95% CI 1.39–1.47), and this risk was higher when the surgical approach was total thyroidectomy compared to hemithyroidectomy. Higher adjusted HRs of T2DM were observed among the subgroups of the female sex, low BMI, absence of current smoking, heavy alcohol consumption, hypertension, and presence of dyslipidemia. Besides, patients with thyroid cancer were categorized by quartiles of the mean levothyroxine dosage (< 101, 101–127, 128–149, and 150 µg/day), and it was found that those patients in the lowest and highest quartiles had the highest risk for T2DM.
In summary, the association between thyroid cancer, insulin resistance, MetS, and diabetes has been evidenced in several observational studies, leading to the hypothesis that impaired glucose alterations might be involved in the increased incidence of thyroid cancer. Given the relevance of this association, further investigation is warranted to expand these findings. (Table 2)
3.4 Influence of diabetes treatments on thyroid cancer
3.4.1 Role of metformin in oncology
Metformin is a biguanide containing two coupled molecules of guanidine with additional substitutions. Metformin is derived from galegine, a natural product from the plant Galega officinalis, used in herbal medicine in medieval Europe. As it was not designed to target a particular pathway or disease, its molecular mechanisms of action remain much debated [70]. Animal and human studies have shown that metformin acts in the liver, inhibiting gluconeogenesis by blocking a mitochondrial redox shuttle. However, a complete understanding of metformin’s mechanism of action remains elusive, and the drug’s effects are likely pleiotropic [71].
Several studies have shown that metformin is emerging as a drug with numerous beneficial effects outside diabetes, such as body weight control, reduction of cardiovascular risk, and treatment of non-alcoholic fatty liver disease, MetS, and cancer [72, 73]. In this regard, epidemiological data documented the association between metformin therapy and a lower risk of developing breast, colon, pancreatic, and liver cancers in diabetic patients.
A meta-analysis by Yin et al. included 13,008 cancer patients with T2DM. It revealed that the administration of metformin to these patients was associated with a significantly reduced risk for death compared with those without the drug (HR = 0.66; 95% CI: 0.55–0.79). In a fixed model analysis stratified by cancer type, it was found that, except for lung cancer, metformin was associated with a significantly reduced risk for death in those patients with breast (HR: 0.94, 95% CI 0.90–0.99), prostate (HR: 0.68, 95% CI 0.51–0.90), pancreatic (HR: 0.65, 95% CI 0.49–0.86), and colorectal cancer (HR: 0.65, 95% CI 0.56–0.77) [74]. Another meta-analysis by Gandini et al. [75] included 65,540 cancer patients with diabetes from 47 studies and demonstrated an association of metformin with a reduction in overall cancer incidence by 31% [Summary relative risk (SRR: 0.69; 95% CI: 0.52–0.90], although between-study heterogeneity was considerable and no reduction was found when the analysis was restricted to prospective studies or randomized clinical trials. (SRR: 0.71; 95% CI: 0.47–1.07; SRR: 0.95; 95% CI: 0.69–1.30, respectively). It is worth mentioning that when the analysis was organ-specific based, the risk of reduction with metformin use in unadjusted analyses reached statistical significance only for liver (SRR: 0.47; 95% CI: 0.28–0.78) and lung cancer (SRR: 0.82; 95% CI: 0.67–0.99. Analysis of prospective studies confirmed this association for liver but not lung cancer.
Thakur et al. [76] proposed potential mechanisms of action of metformin in endocrine tumors: metformin is a negatively charged molecule that enters the cell through organic cation transporters (OCTs). After entering the cells, it can inhibit tumorigenesis through different pathways. The first is through its inhibitory effect on complex one or mGPDH, either alone or together, to inhibit oxidative phosphorylation (OXPHOS), which lowers intracellular ATP production and causes cellular stress in cancer cells. Besides, the lower levels of ATP activate the AMP-activated protein kinase (AMPK) that leads to the activation of tuberous sclerosis complex 1/2 (TSC 1/2), which inhibits the mechanistic target of rapamycin (mTOR) pathway. In the second place, metformin can inhibit insulin or insulin-like growth factor-1 (IGF-1)-mediated activation of the RAS-MEK-ERK and PI3K-AKT pathways, inhibiting the mTOR pathway. Thirdly, metformin-mediated activation of AMPK can inhibit the phosphorylation of insulin receptor substrate-1 (IRS-1). IRS-1 is downstream of the IGF-1 pathway and upstream of the PI3K-AKT pathway. Inhibition of IRS-1 activity hampers AKT phosphorylation, which causes activation of the tuberous sclerosis complex (TSC) complex and inhibits the mTOR pathway. Lastly, metformin has other targets in endocrine cells, including STAT3, GSK3, and ATF3. These targets are components of biologically essential cellular metabolism, proliferation, survival, and apoptosis pathways. Besides these, numerous other targets of metformin have been identified in cancer cells, such as cyclins (CCND1, CCNE2), miRNAs (miR-26a, miR-34a), and mitochondrial genes (mGPDH).
3.5 The role of metformin in thyroid cancer
A narrative review by García-Sáenz et al. [77] describes the molecular pathways of metformin involved in thyroid cancer. Metformin inhibits cell proliferation in thyroid cancer cell lines and promotes apoptosis as previously described for all endocrine tumors (AMPK, TSC2/mTOR, and IRS-1/IGF-1R/PI3K/AKT pathways) [76]. However, an AMPK-independent pathway is also mentioned for metformin, acting through the inhibition of the unfolded protein response (UPR) that causes apoptosis, prevents angiogenesis, and induces toxicity on cancer stem cells. Furthermore, an increased expression of binding of immunoglobulin protein (BIP), CCAAT/enhancer-binding protein homologous protein (CHOP), and caspase-12 markers of endoplasmic reticulum (ER) stress are postulated as another apoptotic mechanism. This same review mentions that metformin may increase thyroid cancer cells’ sensitivity to radiation due to cell redifferentiation, with a subsequent increase in RAI uptake and describes the potential benefit of metformin treatment in the follow-up of DTC patients.
To assess the clinical impact of metformin in thyroid cancer, Wang et al. [78] carried out a systematic review and meta-analysis of literature articles published from January 1970 to December 2021, including seven studies (three prospective cohort studies, three retrospective cohort studies, and one case-control study). Four studies examined the incidence of thyroid cancer within 1,705,123 participants (930,486 metformin users and 774,637 non-users), yielding a total of 3,238 thyroid cancer events. The SRR of thyroid cancer showed that metformin was non-significantly associated with decreased thyroid cancer (SRR: 0.743; 95% CI: 0.453–1.220). However, the heterogeneity among the studies was high; one of the prospective studies included patients with self-reported thyroid cancer, and the incidence of cancer in the whole population was small, both in the exposed and not exposed patients. Moreover, both in the retrospective and big sample size prospective studies, a significant decrease in the incidence of thyroid cancer among patients receiving metformin was noticed, while in the single case-control designed study, a non-significant risk between both groups was found. Three studies analyzed the association between metformin and prognostic risk of thyroid cancer patients, with a total of 27,513 patients included (25,059 on metformin) and a total of 4972 diagnosed thyroid cancer cases. The SRR showed a trend towards a better prognosis in metformin users’ cancer outcomes than other antidiabetic drugs and nonusers (SRR = 0.504; 95% CI: 0.178–1.430). Nevertheless, the heterogeneity among studies was high: the only study with a positive outcome in favor of metformin users was a retrospective American cohort study with a small sample size (34 metformin users versus 21 nonusers) and a low incidence of thyroid cancer (6 and 9 for each group respectively).
In summary, the benefit of metformin on thyroid cancer remains inconclusive. More prospective studies are needed to establish a strong correlation between the use of metformin and the incidence and prognosis of thyroid cancer. (Table 3)
3.6 The implications of glucagon-like peptide-1 (GLP-1) in thyroid cancer
Glucagon-like peptide-1 (GLP-1) is an incretin hormone secreted by enteroendocrine cells known as L-cells. It controls plasma glucose levels by several mechanisms, including stimulation of insulin secretion upon nutrient intake, reduction of postprandial glucose absorption through delayed gastric emptying, and simultaneous inhibition of glucagon secretion [84].
The successful use of synthetic GLP-1 receptor (GLP-1 R) agonists (GLP-1 RA) as anti-obesity and anti-diabetes drugs has been well demonstrated [85]. Several GLP-1RAs, such as exenatide, liraglutide, lixisenatide, albiglutide, dulaglutide, and semaglutide, are available worldwide [86].
The expression of GLP-1 R is distributed along the whole body, and its presence in mice brains with the putative effect of GLP-1 RA on controlling food intake [87, 88] is an excellent example of the pleiotropic effects of this hormone. The thyroid is no exception. The calcitonin-producing C-cells harbor GLP-1 R that can be stimulated by GLP-1 RA, as shown in rodent models [89]. However, GLP-1 R thyroid expression in humans is minimal [90]. In this connection, the relationship between GLP-1 RA and thyroid cancer development has recently raised attention.
Recent findings reported about GLP-1 RA and thyroid cancer have been summarized in Table 3.
In a meta-analysis by Hu et al. [79], the use of different GLP-1 RAs and the occurrence of thyroid disorders, including thyroid cancer, were compared with placebo or other interventions. Out of 45 randomized control trials, 18 trials, with 24,787 patients, used liraglutide as the experimental agent, and together, the LEADER trial, where liraglutide was investigated, and the REWIND trial with dulaglutide as the tested drug, contributed to more than 75% of the weight of the meta-analysis. Although, in these trials, thyroid events were regarded as safety results and not as the primary outcome, and the type of thyroid cancer was not specified, it was shown that GLP-1 RA use was not associated with thyroid cancer (RR 1.30, 95% CI 0.86–1.97).
In a different setting than the RCTs arena, a recent prospective study also confirmed the safety of using incretins regarding thyroid cancer [80]. In this study, US health plan patients were followed for 17 months on liraglutide or exenatide and other non-incretin antidiabetic drugs as comparator medications. Relative risks from intention-to-treat analyses (ITT) ranged from 1.00 (95% CI 0.56–1.79) versus metformin to 1.70 (95% CI 1.03–2.81) versus all comparators excluding exenatide. It was observed that patients on liraglutide had a higher prevalence of microcarcinomas and shorter time-to-diagnosis, suggesting increased surveillance bias. All possible significant differences observed among liraglutide initiators disappeared after adjusting the primary analyses (ITT) for latency. One potential limitation of this study may be the short follow-up, considering that thyroid cancer may develop in further years. Another one is that obesity, one of the main indications of liraglutide, is a risk factor for thyroid cancer and can act as a confounder.
By contrast, a recent real-world nationwide population-based study has reported an increased incidence of thyroid cancer attributed to GLP-1 RA, raising some controversy [81]. Out of 2562 thyroid cancer patients matched with 45,184 controls subjects; it was reported that the use of GLP-1 RA for 1–3 years was associated with increased risk of all thyroid cancer (adjusted hazard ratio [HR] 1.58, 95% CI 1.27–1.95) and medullary thyroid cancer (adjusted HR 1.78, 95% CI 1.04–3.05). With its case-control design, this study is accompanied by a commentary from Thomson et al. [91], highlighting some limitations. Increased monitoring for thyroid cancer in GLP-1 RA recipients due to their primary obesity or diabetic condition may be considered detection or surveillance bias. As in other studies, this one is also limited by a short latency period to detect thyroid cancer from initiating the drugs, adding another potential surveillance bias. Furthermore, as medullary thyroid cancer accounted for > 15% of all thyroid cancers detected, this three-fold higher frequency than previously described [92] might suggest potential measurement bias.
In a retrospective study where 11,243 cases of thyroid cancer were included, 236 were recorded using GLP-1 RA [82]. These patients were identified from the spontaneous reporting system of the European Pharmacovigilance Database (EudraVigilance). The association was strongest for liraglutide, followed by exenatide and dulaglutide, with a proportional reporting ratio (PRR) of 27.5 (95% CI 22.7–33.3), 22.5 (95% CI 17.9–28.3) and 13.1 (95% CI 9.4–18.3), respectively.
The study may be limited by the lack of precise estimation of the actual number of thyroid cancers due to the way information on these thyroid cancer cases was collected. In the future, additional pharmacovigilance monitoring the incidence of medullary thyroid cancer, in particular, is warranted. Meanwhile, GLP-1 RA use is not recommended in patients with a personal or family history of this type of cancer or multiple endocrine neoplasia (MEN) type 2 [83].
4 Conclusion
The parallel increased incidence of thyroid cancer and metabolic diseases, such as MetS or diabetes, has led to the design of several observational studies reporting a link between both conditions. Multiple molecular mechanistic pathways support this hypothesis showing how insulin resistance may be central to this association.
Metformin, among the most widely used antidiabetic drugs, has evidenced potential antiproliferative effects in thyroid cancer, which require further confirmation. The newer GLP-1 RAs have been associated with sporadic cases of thyroid cancer. However, these findings may be related to surveillance bias and do not appear to increase thyroid cancer incidence.
Given the global epidemy of metabolic diseases such as diabetes and MetS, further investigation is warranted to define their role in thyroid cancer and its potential therapeutical approach.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Brenta G. Why can insulin resistance be a natural consequence of thyroid dysfunction? J Thyroid Res. 2011;2011:152850.
Teixeira P, Dos Santos PB, Pazos-Moura CC. The role of thyroid hormone in metabolism and metabolic syndrome. Ther Adv Endocrinol Metab. 2020;11:2042018820917869.
Aschebrook-Kilfoy B, Schechter RB, Shih YC, Kaplan EL, Chiu BC, Angelos P, et al. The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiol Biomarkers Prev. 2013;22(7):1252–9.
Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, et al. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011;20(3):464–72.
Zhang W, Bai X, Ge H, Cui H, Wei Z, Han G. Meta-analysis in the association between obesity and risk of thyroid cancer. Int J Clin Exp Med. 2014;7(12):5268–74.
Kitahara CM, Obesity. Physical activity, and thyroid Cancer risk: Disentangling True associations from Detection Bias. Thyroid. 2023;33(3):276–7.
Kim WG, Cheng SY. Mechanisms linking obesity and thyroid Cancer Development and Progression in Mouse models. Horm Cancer. 2018;9(2):108–16.
Rezzonico J, Rezzonico M, Pusiol E, Pitoia F, Niepomniszcze H. Introducing the thyroid gland as another victim of the insulin resistance syndrome. Thyroid. 2008;18(4):461–4.
Blanc E, Ponce C, Brodschi D, Nepote A, Barreto A, Schnitman M, et al. Association between worse metabolic control and increased thyroid volume and nodular Disease in elderly adults with metabolic syndrome. Metab Syndr Relat Disord. 2015;13(5):221–6.
Ayturk S, Gursoy A, Kut A, Anil C, Nar A, Tutuncu NB. Metabolic syndrome and its components are associated with increased thyroid volume and nodule prevalence in a mild-to-moderate iodine-deficient area. Eur J Endocrinol. 2009;161(4):599–605.
Yin DT, He H, Yu K, Xie J, Lei M, Ma R, et al. The association between thyroid cancer and insulin resistance, metabolic syndrome and its components: a systematic review and meta-analysis. Int J Surg. 2018;57:66–75.
Noto H, Tsujimoto T, Sasazuki T, Noda M. Significantly increased risk of cancer in patients with Diabetes Mellitus: a systematic review and meta-analysis. Endocr Pract. 2011;17(4):616–28.
Yeo Y, Ma SH, Hwang Y, Horn-Ross PL, Hsing A, Lee KE, et al. Diabetes Mellitus and risk of thyroid cancer: a meta-analysis. PLoS ONE. 2014;9(6):e98135.
Li H, Qian J. Association of Diabetes Mellitus with thyroid cancer risk: a meta-analysis of cohort studies. Med (Baltim). 2017;96(47):e8230.
Rezzonico J, Rezzonico M, Pusiol E, Pitoia F, Niepomniszcze H. Metformin treatment for small benign thyroid nodules in patients with insulin resistance. Metab Syndr Relat Disord. 2011;9(1):69–75.
Sui M, Yu Y, Zhang H, Di H, Liu C, Fan Y. Efficacy of Metformin for Benign thyroid nodules in subjects with insulin resistance: a systematic review and Meta-analysis. Front Endocrinol (Lausanne). 2018;9:494.
Klubo-Gwiezdzinska J, Costello J Jr., Patel A, Bauer A, Jensen K, Mete M, et al. Treatment with metformin is associated with higher remission rate in diabetic patients with thyroid cancer. J Clin Endocrinol Metab. 2013;98(8):3269–79.
Reaven GM. Pathophysiology of insulin resistance in human Disease. Physiol Rev. 1995;75(3):473–86.
Lee SH, Park SY, Choi CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. 2022;46(1):15–37.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–23.
Bonora E, Moghetti P, Zancanaro C, Cigolini M, Querena M, Cacciatori V, et al. Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J Clin Endocrinol Metab. 1989;68(2):374–8.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Park HM, Lee HS, Lee YJ, Lee JH. The triglyceride-glucose index is a more powerful surrogate marker for predicting the prevalence and incidence of type 2 Diabetes Mellitus than the homeostatic model assessment of insulin resistance. Diabetes Res Clin Pract. 2021;180:109042.
da Silva A, Caldas APS, Rocha D, Bressan J. Triglyceride-glucose index predicts independently type 2 Diabetes Mellitus risk: a systematic review and meta-analysis of cohort studies. Prim Care Diabetes. 2020;14(6):584–93.
Expert Panel on Detection E. Treatment of high blood cholesterol in A. Executive Summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–97.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640-5.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of Diabetes: standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):19–S40.
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of Diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional Diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843.
Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and Cardiovascular Disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–46.
Bjornsdottir HH, Rawshani A, Rawshani A, Franzen S, Svensson AM, Sattar N, et al. A national observation study of cancer incidence and mortality risks in type 2 Diabetes compared to the background population over time. Sci Rep. 2020;10(1):17376.
Ling S, Brown K, Miksza JK, Howells L, Morrison A, Issa E, et al. Association of type 2 Diabetes with Cancer: a Meta-analysis with Bias Analysis for Unmeasured Confounding in 151 cohorts comprising 32 million people. Diabetes Care. 2020;43(9):2313–22.
Joslin EP, Lombard HL, Burrows RE, Manning MD. Diabetes and cancer. N Engl J Med. 1959;260(10):486–8.
Shi Y, Hu FB. The global implications of Diabetes and cancer. Lancet. 2014;383(9933):1947–8.
Cignarelli A, Genchi VA, Caruso I, Natalicchio A, Perrini S, Laviola L, et al. Diabetes and cancer: pathophysiological fundamentals of a ‘dangerous affair’. Diabetes Res Clin Pract. 2018;143:378–88.
Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–85.
Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide burden of cancer attributable to Diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. 2018;6(6):e6–e15.
Hwangbo Y, Kang D, Kang M, Kim S, Lee EK, Kim YA, et al. Incidence of Diabetes after Cancer Development: a Korean National Cohort Study. JAMA Oncol. 2018;4(8):1099–105.
Natalicchio A, Montagnani M, Gallo M, Marrano N, Faggiano A, Zatelli MC, et al. MiRNA dysregulation underlying common pathways in type 2 Diabetes and cancer development: an Italian Association of Medical Oncology (AIOM)/Italian Association of Medical Diabetologists (AMD)/Italian Society of Diabetology (SID)/Italian Society of Endocrinology (SIE)/Italian Society of Pharmacology (SIF) multidisciplinary critical view. ESMO Open. 2023;8(3):101573.
Maenhaut C, Brabant G, Vassart G, Dumont JE. In vitro and in vivo regulation of thyrotropin receptor mRNA levels in dog and human thyroid cells. J Biol Chem. 1992;267(5):3000–7.
Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE, Roger PP. Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev. 2001;22(5):631–56.
Clement S, Refetoff S, Robaye B, Dumont JE, Schurmans S. Low TSH requirement and goiter in transgenic mice overexpressing IGF-I and IGF-Ir receptor in the thyroid gland. Endocrinology. 2001;142(12):5131–9.
Krieger CC, Neumann S, Gershengorn MC. TSH/IGF1 receptor crosstalk: mechanism and clinical implications. Pharmacol Ther. 2020;209:107502.
Belfiore A, Malaguarnera R, Vella V, Lawrence MC, Sciacca L, Frasca F, et al. Insulin receptor isoforms in Physiology and Disease: an updated view. Endocr Rev. 2017;38(5):379–431.
Belfiore A, Rapicavoli RV, Le Moli R, Lappano R, Morrione A, De Francesco EM et al. IGF2: a role in Metastasis and Tumor Evasion from Immune Surveillance? Biomedicines. 2023;11(1).
Vella V, Pandini G, Sciacca L, Mineo R, Vigneri R, Pezzino V, et al. A novel autocrine loop involving IGF-II and the insulin receptor isoform-A stimulates growth of thyroid cancer. J Clin Endocrinol Metab. 2002;87(1):245–54.
Kushchayeva YS, Kushchayev SV, Startzell M, Cochran E, Auh S, Dai Y, et al. Thyroid abnormalities in patients with Extreme insulin resistance syndromes. J Clin Endocrinol Metab. 2019;104(6):2216–28.
Kushchayeva Y, Kushchayev S, Jensen K, Brown RJ. Impaired glucose Metabolism, anti-diabetes medications, and risk of thyroid Cancer. Cancers (Basel). 2022;14(3).
Harikrishna A, Ishak A, Ellinides A, Saad R, Christodoulou H, Spartalis E, et al. The impact of obesity and insulin resistance on thyroid cancer: a systematic review. Maturitas. 2019;125:45–9.
Oberman B, Khaku A, Camacho F, Goldenberg D. Relationship between obesity, Diabetes and the risk of thyroid cancer. Am J Otolaryngol. 2015;36(4):535–41.
Rezzonico JN, Rezzonico M, Pusiol E, Pitoia F, Niepomniszcze H. Increased prevalence of insulin resistance in patients with differentiated thyroid carcinoma. Metab Syndr Relat Disord. 2009;7(4):375–80.
Bae MJ, Kim SS, Kim WJ, Yi YS, Jeon YK, Kim BH, et al. High prevalence of papillary thyroid cancer in Korean women with insulin resistance. Head Neck. 2016;38(1):66–71.
Pitoia F, Abelleira E, Bueno F, Urciuoli C, Schmidt A, Niepomniszcze H. Insulin resistance is another factor that increases the risk of recurrence in patients with thyroid cancer. Endocrine. 2015;48(3):894–901.
Zhao J, Zhang Q, Yang Y, Yao J, Liao L, Dong J. High prevalence of thyroid carcinoma in patients with insulin resistance: a meta-analysis of case-control studies. Aging. 2021;13(18):22232–41.
Xu N, Liu H, Wang Y, Xue Y. Relationship between insulin resistance and thyroid cancer in Chinese euthyroid subjects without conditions affecting insulin resistance. BMC Endocr Disord. 2022;22(1):58.
Alkurt EG, Sahin F, Tutan B, Canal K, Turhan VB. The relationship between papillary thyroid cancer and triglyceride/glucose index, which is an indicator of insulin resistance. Eur Rev Med Pharmacol Sci. 2022;26(17):6114–20.
Zhao J, Tian Y, Jia Z, Yao J, Liao L, Dong J. Abnormal glucose metabolism parameters and the aggressiveness of differentiated thyroid carcinoma: a hospital-based cross-section study in China. Front Endocrinol (Lausanne). 2022;13:806349.
Brenta G, Celi FS, Pisarev M, Schnitman M, Sinay I, Arias P. Acute thyroid hormone withdrawal in athyreotic patients results in a state of insulin resistance. Thyroid. 2009;19(6):665–9.
Park JH, Choi M, Kim JH, Kim J, Han K, Kim B, et al. Metabolic syndrome and the risk of thyroid Cancer: a Nationwide Population-based Cohort Study. Thyroid. 2020;30(10):1496–504.
Song JL, Li LR, Yu XZ, Zhan L, Xu ZL, Li JJ, et al. Association between metabolic syndrome and clinicopathological features of papillary thyroid cancer. Endocrine. 2022;75(3):865–71.
Li LR, Song JL, Liu HQ, Chen C. Metabolic syndrome and thyroid Cancer: risk, prognosis, and mechanism. Discov Oncol. 2023;14(1):23.
Zhang B, Song Z, Ren L, Wang J, Gu Y, Feng C, et al. Relationship between changes in blood glucose and blood lipid levels and the risk of thyroid Cancer in patients with type 2 Diabetes Mellitus. Dis Markers. 2023;2023:4814328.
Wang M, Gong WW, Lu F, Hu RY, He QF, Yu M. The association between Diabetes and thyroid cancer risk: a hospital-based case-control study in China. BMC Endocr Disord. 2021;21(1):21.
Cho YY, Kang MJ, Kim SK, Jung JH, Hahm JR, Kim TH, et al. Protective effect of metformin against thyroid Cancer Development: a Population-based study in Korea. Thyroid. 2018;28(7):864–70.
Dong WW, Zhang DL, Wang ZH, Lv CZ, Zhang P, Zhang H. Different types of Diabetes Mellitus and risk of thyroid cancer: a meta-analysis of cohort studies. Front Endocrinol (Lausanne). 2022;13:971213.
Elbasan O, Yavuz DG. Effects of concomitant obesity and Diabetes on the aggressiveness and outcomes of differentiated thyroid cancer patients. Arch Endocrinol Metab. 2021;65(4):455–61.
Li C, Kuang J, Zhao Y, Sun H, Guan H. Effect of type 2 Diabetes and antihyperglycemic drug therapy on signs of Tumor invasion in papillary thyroid cancer. Endocrine. 2020;69(1):92–9.
Roh E, Noh E, Hwang SY, Kim JA, Song E, Park M, et al. Increased risk of type 2 Diabetes in patients with thyroid Cancer after thyroidectomy: a Nationwide Cohort Study. J Clin Endocrinol Metab. 2022;107(3):e1047–e56.
Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–85.
Flory J, Lipska K. Metformin in 2019. JAMA. 2019;321(19):1926–7.
Zhou J, Massey S, Story D, Li L. Metformin: An Old Drug with New Applications. Int J Mol Sci. 2018;19(10).
Amin S, Lux A, O’Callaghan F. The journey of metformin from glycaemic control to mTOR inhibition and the suppression of tumour growth. Br J Clin Pharmacol. 2019;85(1):37–46.
Yin M, Zhou J, Gorak EJ, Quddus F. Metformin is associated with survival benefit in cancer patients with concurrent type 2 Diabetes: a systematic review and meta-analysis. Oncologist. 2013;18(12):1248–55.
Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila). 2014;7(9):867–85.
Thakur S, Daley B, Klubo-Gwiezdzinska J. The role of an anti-diabetic drug metformin in the treatment of endocrine tumors. J Mol Endocrinol. 2019;63(2):R17–R35.
Garcia-Saenz M, Lobaton-Ginsberg M, Ferreira-Hermosillo A. Metformin in differentiated thyroid Cancer: Molecular pathways and its clinical implications. Biomolecules. 2022;12(4).
Wang Z, Luo J, Zhang Y, Xun P, Chen Z. Metformin and thyroid carcinoma incidence and prognosis: a systematic review and meta-analysis. PLoS ONE. 2022;17(7):e0271038.
Hu W, Song R, Cheng R, Liu C, Guo R, Tang W, et al. Use of GLP-1 receptor agonists and occurrence of thyroid disorders: a Meta-Analysis of Randomized controlled trials. Front Endocrinol (Lausanne). 2022;13:927859.
Funch D, Mortimer K, Ziyadeh NJ, Zhou JDS, Ng L. Risk of thyroid Cancer Associated with Use of Liraglutide and other antidiabetic Drugs in a US commercially insured Population. Diabetes Metab Syndr Obes. 2021;14:2619–29.
Bezin J, Gouverneur A, Penichon M, Mathieu C, Garrel R, Hillaire-Buys D, et al. GLP-1 receptor agonists and the risk of thyroid Cancer. Diabetes Care. 2023;46(2):384–90.
Mali G, Ahuja V, Dubey K. Glucagon-like peptide-1 analogues and thyroid cancer: an analysis of cases reported in the European pharmacovigilance database. J Clin Pharm Ther. 2021;46(1):99–105.
Smits MM, Van Raalte DH. Safety of Semaglutide. Front Endocrinol (Lausanne). 2021;12:645563.
Kuhre RE, Deacon CF, Holst JJ, Petersen N. What is an L-Cell and how do we study the secretory mechanisms of the L-Cell? Front Endocrinol (Lausanne). 2021;12:694284.
Knudsen LB, Lau J. The Discovery and Development of Liraglutide and Semaglutide. Front Endocrinol (Lausanne). 2019;10:155.
Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 Diabetes - state-of-the-art. Mol Metab. 2021;46:101102.
Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest. 2014;124(6):2456–63.
Blundell J, Finlayson G, Axelsen M, Flint A, Gibbons C, Kvist T, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19(9):1242–51.
Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151(4):1473–86.
Waser B, Blank A, Karamitopoulou E, Perren A, Reubi JC. Glucagon-like-peptide-1 receptor expression in normal and diseased human thyroid and pancreas. Mod Pathol. 2015;28(3):391–402.
Thompson CA, Sturmer T, Putting. GLP-1 RAs and thyroid Cancer in Context: additional evidence and remaining doubts. Diabetes Care. 2023;46(2):249–51.
Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022;10(4):264–72.
Funding
No financial or non-financial interests directly or indirectly related to the work submitted for publication are present.
Author information
Authors and Affiliations
Contributions
G.B. had the idea for the article, F.DF. performed the literature search and data analysis, and G.B. and F.DF. drafted and critically revised the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors did not receive support from any organization for the submitted work.
Consent to participate
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brenta, G., Di Fermo, F. Thyroid cancer and insulin resistance. Rev Endocr Metab Disord 25, 19–34 (2024). https://doi.org/10.1007/s11154-023-09849-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-023-09849-7