Abstract
Background
Thyroglobulin is a well-established disease marker during follow-up in paediatric differentiated thyroid cancer. However, no conclusive data on the role of endogenously stimulated thyroglobulin after thyroidectomy (ptTg) in predicting disease-specific outcomes are available. This review aims to establish the prognostic value of ptTg in children with DTC.
Methods
Online medical databases were searched for studies evaluating the association between ptTg and disease-specific outcomes in DTC-affected children. Documents not in English, preclinical studies, other review articles, case reports, and small case series were excluded. The risk of bias was assessed with the QUADAS-2 tool.
Results
Twelve studies, analysing 1043 children in total, were included in the review. They all had a retrospective design and were published between 2016 and 2022. Of all patients, 1008 (97%) and 849 (81%) had undergone thyroidectomy and RAI, respectively. Eight studies (756 children) evaluated the correlation between ptTg and disease persistence/relapse: six reported a significant association between these parameters; a specific ptTg cut-off (10–14 ng/ml) was identified at the multivariate analysis in three studies. The remaining four studies assessed the link between ptTg levels and disease extension, with three reporting a correlation between ptTg and lung/nodal metastases.
Discussion
ptTg is a readily available and inexpensive parameter, bearing a strong prognostic power in identifying disease persistence, relapse, and the presence of metastases in children affected by DTC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Paediatric differentiated thyroid cancer (DTC) is the most frequent endocrine tumour during childhood and adolescence, and its incidence has increased in the last decades [1]. This tumour’s pathological and molecular features are favourable, and its prognosis is excellent [2, 3]. However, at first diagnosis, patients with paediatric DTC often present a more advanced disease stage than their adult counterpart and multicentric tumour with extensive lymph node and lung metastases is observed in up to 20% of patients [2]. Considering these peculiar clinical characteristics, proper prognostic factors are highly required to correctly identify the risk of disease persistence/progression after initial treatment, which includes thyroidectomy and radioactive iodine therapy (RAI) [2, 3].
Serum Thyroglobulin (Tg) is a well-established marker of disease during the follow-up of paediatric DTC after thyroidectomy and RAI [2, 3]. Moreover, its role as an independent prognostic factor, when evaluated immediately after surgery and before thyroid remnant ablation, has been recognised in adults by the American Thyroid Association (ATA) DTC guidelines, which suggest using this biomarker to estimate the risk of disease persistence or recurrence [4]. Although the higher the value of this stimulated Tg (ptTg), the higher the probability of relapse [4,5,6,7], no definitive cut-off Tg values have been identified. However, several studies and one meta-analysis conducted on a large number of patients showed that DTC patients with ptTg levels < 10 ng/ml are at lower risk of disease relapse [5, 8, 9]; conversely, patients with ptTg levels > 10 ng/ml have a higher likelihood to show lymph-node and distant metastases on post-therapeutic 131I whole-body [10] and high risk of recurrence after RAI [6, 7]. More recently, some authors proposed the use of ptTg to identify which patients may benefit from RAI ablation and reported that DTC patients with low or undetectable ptTg levels should not undergo RAI, especially if previously classified at low or intermediate risk of disease persistence [11, 12]. However, in this setting, no definite and reliable ptTg cutoff values have been determined [13].
The 2015 paediatric ATA guidelines identified a ptTg value of 10ng/ml as the threshold above which there is a strong indication for RAI [3]. Conversely, this approach has never been backed up by evidence-based data; the most recent ETA guidelines do not include ptTg as a pivotal biomarker to select which patients deserve RAI [2].
This review aims to clarify the predictive role of ptTg in paediatric DTC patients treated with thyroidectomy and RAI. Indeed, we systematically searched the literature to identify all the original studies reporting the predictive value of ptTg on locoregional/distant metastases and its association with disease-specific outcomes.
2 Materials and methods
The systematic review was conducted according to a predefined protocol and written according to the PRISMA statement [14].
Search Strategy.
Two authors (A.P. and F.F.) searched the available literature, performing the article selection independently. The search and selection process consisted of four separate steps. In the first step, so-called “sentinel” studies were identified in PubMed/Medline by entering different combinations of the following keywords: thyroid cancer, paediatric, thyroglobulin, preablative, postoperative, RAI, and prognosis. In the second step, the results were used to identify specific MeSH terms in PubMed/Medline. In the third step, PubMed/Medline, CENTRAL, Scopus, Web of Science and the web were searched using the selected MeSH terms. In the final step, we only included the studies that performed an adequate univariate or multivariate analysis considering the main DTC prognostic factors (e.g., age, sex, tumour dimension, lymph node and distant metastases), and ptTg (homogenously stimulated across the population and with clearly defined values). Original articles without these peculiar characteristics were excluded; studies with possible overlapping cohorts were also excluded. Review articles, case reports, and small case series (< 5 subjects) were excluded.
The references of the included studies were searched to identify other potential matches. The search process was concluded on July 31th 2023. Considering the heterogeneity of the studies, a meta-analysis was not planned or performed.
2.1 Data extraction
Two authors (A.P. and F.F.) independently performed the data extraction, including:
-
1.
General characteristics of the studies (authors, year of publication, country, study design, population).
-
2.
Treatment and Technical parameters (type of surgery, prophylactic lymph node dissection, type of TSH stimulation, AbTg levels, other considered prognostic parameters, RAI activity, follow-up time).
-
3.
Prognostic parameters considered and those associated with DTC outcome.
-
4.
Standard of reference (SOR).
In the evaluation phase, full-text articles and their supplementary materials were included. The extracted data were cross-checked, and any discrepancy was discussed through a consensus meeting.
2.2 Quality assessment
The risk of bias in the studies was assessed by two authors (F.F and P.T.) by using the QUADAS-2 method [15]. For each study, an evaluation of the seven QUADAS-2 items was performed, and each point was scored as having a high, low or unclear risk of bias. High and unclear risk of bias were assigned 1 and 0.5 points, respectively; studies totalling a QUADAS-2 score of four or higher were excluded by the systematic review.
3 Results
3.1 Literature search outcome
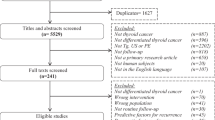
99 records were initially identified after duplicate removal, and their titles and abstracts were assessed; 12 articles had to be excluded since they reported single cases or small cases series (< 5 subjects) or did not include human beings. Of the remaining 87 records, 75 were excluded because they did not meet the set inclusion criteria. Therefore, 12 articles were finally selected (Fig. 1).
3.2 Qualitative analysis
The twelve articles in the systematic review were published between 2016 and 2022 [16,17,18,19,20,21,22,23,24,25,26,27]. All studies have a retrospective design. Three studies were conducted in China and two others in Italy, while Israel, USA, Brazil, Republic of Korea, Croatia, Greece and Canada contributed with one study each. The characteristics of the studies and their patients’ populations are summarised in Table 1. Treatments and technical aspects are described in Table 2, the predictive and prognostic role of ptTg is elucidated in Table 3, and the quality assessment of included studies is reported in Table 4.
3.3 Technical aspects
Thyroidectomy was performed in 100% of patients in all studies but one (in this study, only 65% of patients underwent a complete removal of the gland [20]). Prophylactic dissection of the central compartment was never proposed to younger DTC patients. However, therapeutic central and lateral lymph-node dissections were performed across the 12 studies in a proportion of patients ranging from 18 to 72% and from 14.5 to 82% respectively. After total thyroidectomy, most patients (ranging from 83 to 100%) underwent a post-surgical RAI by means an adequate TSH stimulation which was endogenous in all cases. Indeed, a great variability of RAI activity was used as reported in Table 2. However, in all but one study [27] the empiric approach rather than the dosimetric one was used to calculate the RAI activity.
In 9 out of 12 studies the anti-thyroglobulin antibodies (AbTg) were negative, or in the case of positive AbTg the patients were not included [16,17,18,19, 22, 23, 25,26,27]. This aspect was not specified in the other 3 studies [20, 21, 24].
Eight out of 12 studies investigated the association between ptTg levels and disease persistence/relapse after initial treatment [16, 18, 20,21,22,23, 26, 27]. Among these 8 studies, a multivariate analysis was conducted in 6 [16, 20, 22, 23, 26, 27]. In four out of these six studies, the paediatric ATA risk classification system was included among the risk factors assessed in the statistical analysis.
On the other hand, 4 studies evaluated the ability of ptTg levels to predict the presence of distant metastases [17, 19, 24] or peculiar DTC features [25], and a multivariate analysis was conducted in two [17, 25]. Only one study included the paediatric ATA risk classification in the multivariate analysis.
Predictive role of ptTg.
The twelve articles selected for the systematic review included populations consisting of 32 to 276 paediatric patients affected by DTC (Table 1). The 8 studies [16, 18, 20,21,22,23, 26, 27], which analysed the association between ptTg and disease persistence, counted 756 paediatric patients. On the other hand, the 4 studies [17, 19, 24, 25] analysing the association between ptTg and disease localisation included 287 patients. Table 3 details the predictive role of ptTg in these 12 studies.
ptTg was reported to be significantly associated with disease persistence/relapse after initial treatment in most studies facing this important issue [18, 20, 22, 23, 26, 27].
At the univariate level, a ptTg cut-off value of 31.5 ng/ml could predict outcomes with a sensitivity and specificity of 100% [18]. In addition, the association between disease persistence and ptTg was confirmed at univariate and multivariate levels after introducing a ptTg threshold ranging from 10 to 14 ng/ml [22, 23, 26]. Interestingly, in these 3 studies, ptTg was an independent risk factor of disease persistence independently on the ATA risk classification system [22, 23, 26] (Table 3).
In our systematic review, we also found that high levels of ptTg may predict the presence of lung metastases. Indeed, levels of ptTg above 150ng/ml were significantly associated with distant metastases [17, 19] with very high sensitivity ranging from 87.7 to 100%. In addition, ptTg above 10 ng/ml is significantly associated with lymph node metastases [25].
No studies investigated the association between ptTg levels and overall survival.
3.4 Quality Assessment of the Studies
The quality assessment of the studies according to the QUADAS-2 method is reported in Table 4. The risk of bias for the evaluated studies ranged from none to 3.5; therefore, no study had to be excluded due to an excess risk. Overall, the risk of bias scores were low, with a median value of 1 and a mean of 1.5. The most common source of bias was identified by the “applicability” signalling questions in the patient selection domain. Conversely, none of the studies showed problems related to the timing.
4 Discussion
Our systematic review evaluated the predictive and prognostic role of ptTg measured after thyroidectomy in a not negligible number of paediatric DTC patients immediately before RAI under endogenous TSH stimulation. We found that this parameter can identify patients at higher risk of developing disease persistence/relapse after initial treatment (i.e., thyroidectomy and RAI). Indeed, after using proper statistical analysis, a significant association between Tg levels and outcome was proved in most studies included in our review [18, 20, 22, 23, 26, 27]. By using these analyses, it was found that, even at multivariate levels, ptTg remains statistically associated with outcome, although other powerful prognostic factors, such as the ATA risk classification system [22, 23, 26], were considered in the statistical model. This was particularly evident when the authors considered ptTg a dichotomous variable after identifying an adequate cut-off value [22, 23, 26]. In these studies, patients with higher ptTg levels (i.e., > 10 ng/mL) were 2.5 to 28 times more likely to experience structural recurrence after initial treatment than those with lower Tg levels. These findings seem to support what was proposed “a priori” by the ATA Guidelines, which identified 10 ng/ml as a warning cut-off value above which patients should be considered at risk of disease persistence and RAI should always be considered [3]. However, this does not automatically imply that all patients with ptTg < 10 ng/ml should be spared ablative or adjuvant RAI treatment. This review includes patients mostly treated with RAI and, therefore, does not allow evaluation of what the outcome of patients with low ptTg could have been if they had not undergone the treatment.
Only 2 out of the 8 studies considering the patients’ outcome found no association between the ptTg levels and disease persistence [16, 21]. Indeed, one [16], including 54 patents, performed a multivariate analysis and found that the ATA and the Schneider Children’s Medical Center of Israel (SCMCI) scores were the only parameters associated with disease persistence. However, histological DTC features and ptTg levels are required to calculate the SCMCI score. Patients presenting levels of ptTg > 10ng/ml were systematically classified at intermediate/high risk of relapse [16]. From this point of view, SCMCI score and ptTg may be considered collinear variables, so they could not independently predict the outcome.
Another interesting point of this review is the high accuracy of ptTg in predicting DTC locoregional or distant metastases. Considering the low sensitivity of computed tomography (CT) to identify lung micrometastases [3], high levels of ptTg (e.g., above 60 ng/ml) [17, 19, 24] can help the physician to suspect a lung metastatic involvement and correctly schedule patients for high 131I activity. In the same direction, ptTg levels above 10 ng/ml were found to be significantly associated with neck lymph node metastases [25]. This observation is again of great clinical value, considering that enlarged lymph nodes are often present in this young population and can be non-univocally interpreted on the neck US [2]. On the one hand, high levels of ptTg could prompt the physician to focus better on doubtful or suspicious lymph node findings before proceeding with RAI treatment. Conversely, lower levels of ptTg could reinforce the physician to correctly define negative, uncertain and nonspecific nodes.
Regarding the comparison of our systematic review on the value of ptTg in paediatric patients with that published in 2012 for adult patients [9], it is important to underline that most papers of that meta-analysis focused their attention on the high negative predictive (NPV) of low levels of ptTg. Indeed, the meta-analysis reported that a patient with ptTg < 10 ng/ml had a 94% likelihood of being disease-free at follow-up [9]. On the contrary, the studies included in our analyses principally investigated paediatric patients’ risk of showing disease persistence; this information allows, in turn, to identify which patients are amenable to more aggressive treatments and closer monitoring. This difference probably lies in the fact that in the paediatric population, which often presents a more aggressive onset with a more frequent nodal and pulmonary involvement [2], the prevalence of disease persistence after initial treatment is higher than in adults [3, 28, 29].
Although our systematic review reported interesting and useful results for physicians to understand the clinical utility of ptTg in paediatrics, some limitations should also be considered. First, we investigated a limited number of studies, all showing a retrospective design. However, the selection criteria limited us to only investigate those studies with an adequate statistical approach in a multivariate analysis in most cases. Second, the parameters other than the ptTg considered in the multivariate analyses were not always the same across the studies. However, the predictive value of ptTg was tested by introducing in the statistical model strong clinical parameters as the ATA risk classification systems or the one-year response assessment [16, 22, 23, 26, 27]. Third, the clinical follow-up was considered a gold standard to confirm whether disease persistence was different among studies or not well-specified. However, the observation time in the studies that have analysed the predictive value of ptTg on disease recurrence has never been less than 5 years. Finally, we did not perform a meta-analysis due to the heterogeneity among studies included in our systematic review (i.e. differences in patients characteristics, RAI activity, statistics, outcome measures, etc.).
5 Conclusion
ptTg provides important information for clinicians facing paediatric DTC patients. Indeed, this readily available and inexpensive parameter is a powerful independent risk factor that can predict the presence of locoregional/distant metastases and disease persistence after initial treatment. Thus, being aware of the meaning of ptTg is of great help to customise treatment and follow-up of young DTC patients.
Abbreviations
- AbTg:
-
Thyroglobulin Antibodies
- ATA:
-
American thyroid association
- BMI:
-
Body mass index
- CT:
-
X-Ray computed tomography
- DTC:
-
Differentiated thyroid cancer
- IQR:
-
Interquartile range
- H:
-
High
- L:
-
Low
- M:
-
Multivariate
- N.A.:
-
Not applicable
- NPV:
-
Negative predictive value
- N.R.:
-
Not reported
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PT:
-
Post-Therapeutic
- ptTg:
-
Post-thyroidectomy stimulated thyroglobulin
- QUADAS-2:
-
Quality Assessment of Diagnostic Accuracy Studies-2
- R:
-
Retrospective
- RAI:
-
Radioactive Iodine
- SCMCI:
-
Schneider Children’s Medical Center of Israel
- SOR:
-
Standard of reference
- SPECT/CT:
-
Single-photon emission computed tomography/X-ray computed tomography
- Tg:
-
Thyroglobulin
- TSH:
-
Thyroid-stimulating hormone
- U (Table 3):
-
Univariate
- U (Table 4):
-
Unknown
- US:
-
Ultrasound
- WBS:
-
Whole-body scintigraphy
References
Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64.
Lebbink CA, Links TP, Czarniecka A, Dias RP, Elisei R, Izatt L et al. 2022 european thyroid Association Guidelines for the management of pediatric thyroid nodules and differentiated thyroid carcinoma. Eur Thyroid J. 2022;11.
Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM et al. Management guidelines for children with thyroid nodules and differentiated thyroid Cancer. Thyroid. 2015;25.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE et al. 2015 american thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid Cancer: the american thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid Cancer. Thyroid 2016;26.
Tae YK, Won BK, Eun SK, Jin SR, Jeong SY, Seong CK et al. Serum thyroglobulin levels at the time of 131I remnant ablation just after thyroidectomy are useful for early prediction of clinical recurrence in low-risk patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2005;90.
Piccardo A, Arecco F, Puntoni M, Foppiani L, Cabria M, Corvisieri S et al. Focus on high-risk DTC patients: high postoperative serum thyroglobulin level is a strong predictor of disease persistence and is associated to progression-free survival and overall survival. Clin Nucl Med. 2013;38.
Piccardo A, Siri G, Ugolini M, Fiz F, Puntoni M, Bottoni G et al. A three-domain scoring system to customize the risk of relapse of differentiated thyroid carcinoma. Cancers (Basel). 2021;13.
Piccardo A, Siri G, Raffa S, Castellana M, Foppiani L, Bottoni G et al. How to better stratify the risk of differentiated thyroid carcinomas: the key role of radioactive iodine therapy, age, and gender. Eur J Nucl Med Mol Imaging. 2020.
Webb RC, Howard RS, Stojadinovic A, Gaitonde DY, Wallace MK, Ahmed J, Burch HB. The utility of serum thyroglobulin measurement at the time of remnant ablation for predicting disease-free status in patients with differentiated thyroid cancer: a meta-analysis involving 3947 patients. J Clin Endocrinol Metab. 2012;9:2754–63.
Souza Do Rosário PW, Guimarães VC, Ribeiro Maia FF, Fagundes TA, Purisch S, Padrao EL et al. Thyroglobulin before ablation and correlation with posttreatment scanning. Laryngoscope. 2005;115.
Ibrahimpasic T, Nixon IJ, Palmer FL, Whitcher MM, Tuttle RM, Shaha A et al. Undetectable thyroglobulin after total thyroidectomy in patients with low- and intermediate-risk papillary thyroid cancer - is there a need for radioactive iodine therapy? Surg (United States). 2012;152.
Orlov S, Salari F, Kashat L, Freeman JL, Vescan A, Witterick IJ et al. Post-operative stimulated thyroglobulin and neck ultrasound as personalized criteria for risk stratification and radioactive iodine selection in low- and intermediate-risk papillary thyroid cancer. Endocrine. 2015;50.
Li S, Ren C, Gong Y, Ye F, Tang Y, Xu J et al. The role of Thyroglobulin in Preoperative and postoperative evaluation of patients with differentiated thyroid Cancer. Front Endocrinol (Lausanne). 2022.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB et al. Quadas-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011.
Lazar L, Lebenthal Y, Segal K, Steinmetz A, Strenov Y, Cohen M, Yaniv I, Yackobovitch-Gavan M, Phillip M. Pediatric thyroid Cancer: postoperative classifications and response to initial therapy as prognostic factors. J Clin Endocrinol Metab. 2016;101:1970–9.
Livhits MJ, Pasternak JD, Xiong M, Li N, Gosnell JE, Yeh MW, Chiu HK, PRE-ABLATION THYROGLOBULIN, AND THYROGLOBULIN TO THYROID-STIMULATING HORMONE RATIO MAY BE ASSOCIATED WITH PULMONARY METASTASES IN CHILDREN WITH DIFFERENTIATED THYROID CANCER. Endocr Pract. 2016;22:1259–66.
Zanella A, Scheffel RS, Pasa MW, Dora JM, Maia AL. Role of postoperative stimulated thyroglobulin as prognostic factor for differentiated thyroid Cancer in children and adolescents. Thyroid. 2017;27:787–92.
Liu L, Huang F, Liu B, Huang R. Detection of distant metastasis at the time of ablation in children with differentiated thyroid cancer: the value of pre-ablation stimulated thyroglobulin. J Pediatr Endocrinol Metab. 2018;26:31:751–6.
Byeon HK, Kim SB, Oh HS, Kim HK, Choi IH, Kim H, Cho JG, Oh KH, Baek SK, Woo JS, Kwon SY, Kim HY, Jung KY. Clinical analysis of Pediatric thyroid Cancer: a single Medical Institution experience of 18 years. Ann Otol Rhinol Laryngol. 2019;128(12):1152–7.
Prpić M, Franceschi M, Jukić T, Kust D, Dabelić N, Varjačić T, Lucijanić M, Bolanča A, Kusić Z. DIFFERENTIATED THYROID CANCER IN PEDIATRIC POPULATION (≤ 18 YEARS): POSTOPERATIVE TREATMENT WITH RADIOACTIVE IODINE (I-131). Acta Clin Croat. 2019;58(1):119–27.
Liu L, Zhang X, Tian T, Huang R, Liu B. Prognostic value of Pre-Ablation Stimulated Thyroglobulin in Children and Adolescents with differentiated thyroid Cancer. Thyroid. 2020;30(7):1017–24.
Karapanou O, Tzanela M, Rondogianni P, Dacou-Voutetakis C, Chiotis D, Vlassopoulou B, Vassiliadi D, Kanaka-Gantenbein C, Tsagarakis S. Long-term outcome of differentiated thyroid cancer in children and young adults: risk stratification by ATA criteria and assessment of pre-ablation stimulated thyroglobulin as predictors of disease persistence. Endocrine. 2020;70(3):566–74.
Chesover AD, Vali R, Hemmati SH, Wasserman JD. Lung metastasis in children with differentiated thyroid Cancer: factors Associated with diagnosis and outcomes of Therapy. Thyroid. 2021;31(1):50–60.
Tian T, Jiang L, Zhang X, Huang R, Liu B. Association between clinical and tumor features with postoperative thyroglobulin in pediatric papillary thyroid cancer. Surgery. 2020;168:1095–100.
Klain M, Zampella E, Manganelli M, Gaudieri V, Nappi C, D’Antonio A, Piscopo L, Volpe F, Pace L, Schlumberger M, Cuocolo A. Risk of structural persistent disease in pediatric patients with low or intermediate risk differentiated thyroid cancer. Endocrine. 2021;71(2):378–84.
Cistaro A, Quartuccio N, Garganese MC, Villani MF, Altini C, Pizzoferro M, Piccardo A, Cabria M, Massollo M, Maghnie M, Campennì A, Siracusa M, Baldari S, Panareo S, Urso L, Bartolomei M, De Palma D, Mazzoletti A, Dondi A, Bertagna F, Giubbini F, Albano R. D.
Enomoto Y, Enomoto K, Uchino S, Shibuya H, Watanabe S, Noguchi S. Clinical features, treatment, and long-term outcome of papillary thyroid cancer in children and adolescents without radiation exposure. World J Surg. 2012;36:1241–6.
Palmer BA, Zarroug AE, Poley RN, Kollars JP, Moir CR. Papillary thyroid carcinoma in children: risk factors and complications of disease recurrence. J Pediatr Surg. 2005;40:1284–8.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
None of the authors has any conflict of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Piccardo, A., Fiz, F., Bottoni, G. et al. Does it work in childhood and adolescence? The predictive role of postoperative/preablative stimulated thyroglobulin levels in paediatric thyroid cancer. A systematic review of the literature. Rev Endocr Metab Disord 25, 53–63 (2024). https://doi.org/10.1007/s11154-023-09835-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-023-09835-z