Electrodynamic properties of composite (BeO + TiO2)-ceramics in the frequency range 8 – 12 GHz are studied. An increase in ceramic sintering temperature from 1823 to 1933 K after introducing TiO2 micro- and nanopowders into BeO mixtures makes it possible to influence the efficiency of (BeO + TiO2)-ceramics as an absorbing component of microwave radiation by increasing the modulus of imaginary and real parts of the dielectric constant. Additional reduction annealing in a hydrogen atmosphere at 1073 K further changes the ceramic electrical conductivity and enhances the effect of its ability to absorb UHF-radiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Recently the problem has become acute of electromagnetic security in view of the development of powerful UHF-radiation devices, by means of which it is possible to disarm electronic engineering components, rocket, aircraft and drone guidance systems, etc. In this case a requirement arises for developing powerful UHF-emission absorbents based upon radio-adsorbent materials (RAM). An important task in this is selection of functional materials exhibiting simultaneously good mechanical properties and a capacity for UHF-radiation absorption, having considerable thermal conductivity and density. Within RAM due to dielectric and magnetic losses there are reflection dissipation, transmission, and absorption of electromagnetic energy and its transformation into other forms, in particular thermal. In order to achieve the required weakening parameters for electromagnetic radiation (EMR) over a wide frequency range with minimum density, and consequently weight, it is necessary to optimize the composition, increase efficiency and to create the required RAM structural components.
Currently there is extensive use for creating radio-absorbent coatings of composite materials based upon thermoplastic polymers, modified with certain admixtures [1,2,3]. For achievement of these UHF-absorbents it is possible to refer to their high chemical stability, satisfactory mechanical properties, and ease of creating a certain concentration of modifying additions. A disadvantage concerns the comparatively weak thermal conductivity. This limits their extensive application in creating powerful UHF EMR absorbers.
Existence of unique physicochemical properties for ceramic materials finds application in electronic engineering [4,5,6,7,8,9,10,11,12,13]. Ceramics based upon oxides of aluminum (KT-30) and beryllium (BT-30) with addition of an optimum amount of titanium dioxide (30 wt.%) find extensive application. There is special interest in composite (BeO + TiO2) ceramic capable of absorbing electromagnetic UHF energy [3, 5, 6, 14,15,16] exhibiting comparatively good thermal conductivity.
It is well known that thermal conductivity of pure (without additive) BeO-ceramic at 300 K may be 320 W/(m·K) [1,2,3, 5,6,7,8,9,10]. The low thermal conductivity of TiO2 (about 5 W/(m·K)) significantly reduces the overall composite ceramic BT thermal conductivity. As measurement has shown, the overall thermal conductivity of BeO-ceramic with addition of 30 wt.% TiO2 at 300 K is 130 – 140 W/(m·K) [5, 6, 9, 10]. Presence of developed interfaces and intergranular reactions, and also a number of other factors connected with volumetric and surface properties of BeO and TiO2, may have a marked effect on physicochemical and operating properties of composite BeO-ceramic.
During a study of ceramic properties by impedance spectroscopy methods in the frequency range 100 Hz – 100 MHz [1] it has been established that addition of TiO2 micropowder to BeO-ceramic is accompanied by a change in electrical conductivity and capacity to absorb electromagnetic radiation [3, 5,6,7,8, 10, 14]. In this case the sintering temperature affecting ceramic microstructure and its electrical properties is limited to 1803 K, so that with an increase in this value there is a reduction in strength of the ceramic obtained due to coarsening of a specimen crystal structure [1]. For the possibility of sintering at higher temperatures to the composition of this ceramic there was addition of nanosize TiO2 power from 0.1 to 20 wt.%, which significantly slows down ceramic specimen microcrystal recrystallization.
This work is devoted to evaluation of the effect of adding nanosize TiO2 powder on the electrodynamic properties of composite (BeO + TiO2) ceramic and also the possibility of increasing specimen sintering temperature with addition of nanosize TiO2 with the aim of achieving higher EMR absorption indices. Frequency dependence as studied in this work for electrodynamic parameters of composite (BeO + TiO2) ceramic in the range 8 – 12 GHz, which is used actively in radio location where EMR of considerable power is often encountered.
RESEARCH METHODS AND SPECIMENS
The amount of additive and degree of TiO2 reduction affects electrical conductivity of (BeO + TiO2) ceramic [3, 6, 14] and consequently its absorption properties. The effect of a different amount of TiO2 powder additive, its size and shape (micron or nanopowder) on thermal conductivity of composite (BeO + TiO2) ceramic has been studied in [9]. Separately electrodynamic properties of the original TiO2 micro- and nanopowders have been studied in [17].
In order to measure spectral characteristics of complex dielectric permittivity a transition line method was used with modernization of the measurement unit providing fixation of a powder specimens directly in the wave insert used [18].
In order study to the electrodynamic properties of beryllium ceramic three series of specimens were synthesized in the form of plates with dimensions of 2 × 23 × 10 mm with addition micro- and nano-particles of TiO2 [1] of the compositions:
1 — BeO + 29.9 wt.% TiO2 (μm) + 0.1 wt.% TiO2 (nm);
2 — BeO + 29.5 wt.% TiO2 (μm) + 0.5 wt.% TiO2 (nm);
3 — BeO + 29.0 wt.% TiO2 (μm) + 1.0 wt.% TiO2 (nm);
4 — BeO + 28.5 wt.% TiO2 (μm) + 1.5 wt.% TiO2 (nm);
5 — BeO + 28.0 wt.% TiO2 (μm) + 2.0 wt.% TiO2 (nm).
The first series of specimens was sintered at a maximum temperature of 1823 K, and the second at 1933 K. After sintering at 1933 K some of the ceramic specimens were additionally heat treated in a hydrogen atmosphere at 1073 K. In [1] a study has been made of the electrodynamic properties of specimens obtained in the frequency range from 100 Hz to 1oo MHz.
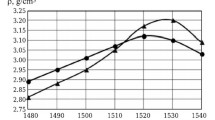
Ceramic density was determined by a hydrostatic method using three fragments obtained by cleaving a single ceramic specimen. Results of measuring the density of composition 2 sintered at different temperatures are provided in Table 1.
Ceramic specimen microstructure sintered at 1823 and 1933 K was studied (Fig. 1). Microphotographs of shears of the test ceramic specimens of composition 2 sintered at different temperatures in a reflection regime (BSE) and secondary electrons (SE) were obtained.
The TiO2 grain size in ceramic specimens sintered at 1823 K did not exceed 10 μm, whereas the size of grains of specimens prepared at 1933 K was on average not more than 40 μm.
In order to study specimen electrodynamic properties in the UHF range a contactless measurement method was used whose application implies location of a specimens directly using a transfer line and measurement of the impedance frequency dependence of this filled line.
Electrodynamic properties of materials are first determined for complex dielectric permittivity, which also be given the main attention in this work.
In order to study frequency properties of complex electrical permittivity of ceramic specimens a transmission line method was used making it possible to study electrodynamic properties of a specimen with an arbitrary step with respect to frequency, which makes it possible to observe not just certain frequency points as would be the case in using a resonance measurement technique. Use of a transmission line method assumes direct measurement of the scattering matrix coefficient for a test specimen, located using a transmission line and filling it entirely, with subsequent transformation of measurement parameters into values of complex dielectric permittivity by means of an appropriate mathematic model.
In order to measure matrix scattering coefficients (S-parameters) there was use of a vector analyzer circuit (VAC). An important advantage of using a VAC is the possibility of taking account of frequency properties of the whole equipment due to conducting a calibration procedure corresponding to the type used for a transmission lime. This makes it possible to reduce significantly the S-parameter measurement error, and consequently calculated values of the relative complex dielectric permittivity. We consider propagation of an electromagnetic wave through a test specimen (Fig. 2)
Propagation of an electromagnetic wave through test material: a1) electromagnetic wave descending in material; b1) electromagnetic wave reflected from a specimen surface; b2) electromagnetic wave passing through material; Γ1 and Γ2) reflection coefficients from specimen surface; z1, z2) electromagnetic wave damping coefficients within the volume of test material in direct and reverse directions.
Measurement of the dissipation matrix coefficient, reflection coefficient S11, and transmission coefficient S21 may be written in terms of Γ and Z as:
By expressing parameters z and Γ in terms of measured values of S11 and S21 it is possible to calculate the value of the complex dielectric permittivity using an NRW mathematical device:
where λ0 is wavelength in the transmission line used; λc is the critical wavelength in the transmission line used.
CERAMIC SPECIMEN ELECTRODYNAMIC PROPERTIES
Complex dielectric permittivity of a composite ceramic RAM depends upon the amount of relative content of absorbing components, particle shape, their sizes, etc. Complex dielectric permittivity was studied within ceramic specimen 1.9 mm thick, represented by a plate, corresponding with respect to shape of the cross section used in transmission line. In order to find the frequency characteristics of relatively complex dielectric permittivity a transmission line method was used with use of special software [19] making it possible to conduct conversion of matrix dissipation parameters in complex values of dielectric permittivity. Conversion was performed by means of an NRW mathematical model [20, 21]. In order to perform measurements there was use of a right angle wave guide standard WR90 with a frequency range of 8 – 12 GHz and cross section of 23 mm by 10 mm. measurement of matrix dissipation coefficients was conducted using an R&S ZVA50 vector circuit analyzer. Taking account of frequency properties of the measuring equipment there is calibration of the measuring system using TRL technology [22]. The error in determining the absolute value of dielectric permittivity for the measuring equipment used was a value of the order of 5% [23].
In order to verify the correctness of the calibration performed there was measurement of a standard specimen with known properties. For this a specimen of material was used based upon reinforced fluoroplastic entirely isotropic in all directions with measured values of dieletric permittivity of 3.01 and dielectric loss tangent angle 0.003275 at a frequency of 10.02347 GHz. The error in values of the active part of dielectric permittivity did not exceed 3%. Measured values of complex relative dielectric permittivity at a frequency of 10 GHz comprise 3.008–i0.0008 (Fig. 3).
Frequency dependences for the complex relative dielectric permittivity of pure BeO ceramic (without additive) are shown in Fig. 4. Frequency dependences of both active ε′, and also imaginary ε′′ parts of the relative dielectric permittivity depend weakly upon frequency and are constants within the frequency range in question, which corresponds to data I [24, 25].
Values of complex relative dielectric permittivity of BeO ceramic at a frequency of 10 GHz are 6.98 – 10.11. In order to study the effect of on electrodynamic properties of annealing temperature and addition to the composition of TiO2 nanopowder based upon the BeO ceramic, as a basis ceramic was taken based upon BeO with addition of 30 wt.% TiO2 micropowder
(VT-30). Results of measuring the complex relative dielectric permittivity of ceramic VT-30 with a sintering temperature of 1803 K, represented by average data for a series of five specimens, are shown in Fig. 5. Deviation between individual specimens within the series did not exceed 5%.
Then a series of specimens was studied with a different TiO2 nanopowder concentration (Fig. 6). Measurements were performed in the range from 8 to 12 GHz, but for convenience of comparing the data obtained results are only provided for frequencies 8, 10, and 12 GHz.
An increase in TiO2 nanopowder concentration to 1.5% within the volume of mixture of micro and nanopowders, and correspondingly a reduction in the concentration of micropowder, leads to a weak change in electrodynamic properties ε′ and ε′′, and increase in TiO2 nanopowder concentration above 1.5% leads to a sharp reduction.
Electrical conductivity of the dielectric and the imaginary part of dielectric permittivity is connected by an expression:
where \({\upvarepsilon }_{r}^{{\prime}{\prime}}\) is the imaginary part of dielectric permittivity; σ is dielectric electrical conductivity ε0 is vacuum dielectric permittivity; f is electromagnetic oscillation frequency.
A significant reduction in \({\upvarepsilon }_{r}^{{\prime}{\prime}}\) point to a commensurate reduction in the electrical conductivity of these specimens. With a reduction in the amount of free charge there is also a reduction in the amount of dipoles, which make a contribution to the value of the actual part of dielectric permittivity, which appears as a reduction in this property.
A sharp reduction in \({\upvarepsilon }_{r}^{{\prime}{\prime}}\) values with addition of TiO2 nanopowder may point to the fact that this additive prevents formation of conducting parts within the ceramic volume, thereby reducing the value of test specimen conductivity. This is directly confirmed by microphotographs of ceramic shears of composition 2, obtained in reflected electrons (see Fig. 1).
In order to evaluate the effect of sintering temperature for BeO ceramic specimens with addition of TiO2 micro- and nanopowders electrodynamic properties were measured for ceramic specimens with nanopowder concentrations of 0.2 – 2.0% sintered at 1933 and 1823 K.
An increase in sintering temperature led to a marked increase in both ε′ and ε′′ (Fig. 7) and consequently to an increase in electrical conductivity and a reduction in dipole size within the structure of the ceramic obtained.
Microphotographs of (BeO + TiO2)-ceramic composition 2 shears sintered at 1933°C obtained in reflected electrons (see Fig. 1) indicate that ceramic grains are coarsened considerably, and this as assumed led to an increase in specimen conductivity. Scatter of ε′ values for test specimens in relation to TiO2 nanopowder concentration (see Fig. 7a ) is very considerable and comprises about 20%.
A series of specimens after sintering at 1933 K was additionally reduced in a hydrogen atmosphere at 1073 K. In order to evaluate the effect of this reduction there was comparison of the electrodynamic properties of specimens sintered at 1933 K before and after reduction (Fig. 8).
Data obtained by experiment indicate that reduction in a hydrogen medium for test specimens made it possible to increase values of ε′ and ε′′. The dependence of ε′ on TiO2 nanopowder concentration for reduced specimens sintered at 1933 K (see Fig. 9a) became similar to the same property for a specimen sintered at 1823 K (see Fig. 7a ). In contrast to ceramic specimens sintered at 1823 K, the maximum of the ε′′ modulus for ceramic reduction is found at a concentration of 0.5 wt.% of TiO2 nanopowder. With an increase in concentration there is a significant reduction in dielectric losses. An increase in the value of ε′′ after firing in a hydrogen atmosphere agrees with results of studying data for specimens in the frequency range from 100 Hz to 100 MHz [1], where a marked reduction is observed in the resistance of ceramic specimens at room temperature. As has been shown [1], annealing in a hydrogen atmosphere may lead to hydroxide formation, lower titanium oxide (predominantly Ti3O5) and hydride TiH2, and also a significant change in dependences of ε′ and ε′′ for BeO-ceramic on the concentration of TiO2 micro- and nanopowders.
CONCLUSIONS
Addition of a mixture of TiO2 micro- and nanopowders to the composition of BeO ceramic makes it possible to change ε′ and ε′′ significantly over a wide range of values, which provides the possibility of obtaining material with prescribed electrodynamic properties within a certain property range. An increase in ceramic sintering temperature from 1823 to 1933 K, which became possible after introducing TiO2 nanopowder into the composition, makes it possible to increase significantly TiO2 ceramic efficiency as an absorbing component due to a marked increase in the modulus of ε′′ by more than a factor of four (from 8 to 32). The value of ε′ in this case also increases, but to a significantly less extent (from 20 to 35). It has been demonstrated that reduction of ceramic sintered at 1923 K in a hydrogen atmosphere leads to a change in the dependence of actual and imaginary parts of ceramic dielectric permittivity, which may be connected with a change in phase composition and stoichiometry of a TiO2 powder surface as a result of action of reduction annealing. This may lead to modification of a TiO2 surface by formation of additional impurity phases such as hydroxide lowering titanium oxide (TinO2n-1, predominantly Ti3O5), TiH2 hydride and titanium to a metallic condition. In order to establish the UHF radiation absorption mechanism for this ceramic with a change in properties it is necessary to study ceramic sintering and reduction regimes under carefully controlled thermodynamic conditions.
Research was carried out with financial support of the Russian scientific Fund, grant No. 21-79-10394, https://rscf.ru/project/21-79-10394/
References
N. A. Drokin, V. S. Kiiko, A. I. Malkin, et al., “Electrophysical properties of BeO + 30 wt.% TiO2 ceramics sintered at elevated temperatures,” Refract. Ind. Ceram., 63(3), 315 – 320 (2022). DOI: https://doi.org/10.1007/s11148-022-00728-3.
N. A. Drokin, V. S. Kiiko, A. I. Malkin, et al., “BT-30 ceramic electrophysical properties,” Refract. Ind. Ceram., 61(3), 341 – 348, 2020. DOI: https://doi.org/10.1007/s11148-020-00484-2.
V. S. Kiiko, S. N. Shabunin, and Yu. N. Makurin, “Preparation, physicochemical properties and passage of UHF-radiation for ceramic based upon BeO,” Ogneupor. Tekhn. Keram., No. 10, 8 – 17 (2004).
R, A, Belyaev, Beryllium Oxide [in Russian], Atomizdat, Moscow (1980).
V. S. Kiiko and A. V. Pavlov, “Composite (BeO + TiO2)-ceramic for electronic engineering and other fields of technology (Review),” Refract. Ind. Ceram., 58(6), 687 – 692 (2018). DOI: https://doi.org/10.1007/s11148-018-0168-6.
V. S, Kiiko, Yu. N. Makurin, and A. L. Ivanovskii, Ceramic Based upon Beryllium Oxide: Preparation, Physicochemical Properties and Use [in Russian], UrO RAN, Ekaterinburg, (2006).
V. Ya. Vaispapir and V. S. Kiiko, “Beryllium ceramic for temporary field of engineering,” Vestnik Vozdush.-Kos. Oborony., Art. 17, No. 1 (2018).
V. S, Kiiko, “Effect of titanium oxide additions on ceramic physicochemical and luminescence properties,” Neorgan Materialy, 30(5), 688 – 693 (1994).
V. S. Kiiko, A. V. Pavlov, and V. A. Bykov, “Production and thermophysical properties of BeO ceramics with the addition of nanocrystalline titanium dioxide,” Refract. Ind. Ceram., 59(6), 616 – 622 (2019). DOI: https://doi.org/10.1007/s11148-019-00284-3.
V. S. Kiiko, and V. Ya. Vaispapir, “Thermal conductivity and application prospects for BeO-ceramic in electronic engineering,” Steklo i Keramika, No. 11, 12 – 16 (2014).
V. S. Kiiko, “Transparent beryllia ceramics for laser technology and ionizing radiation dosimetry,” Refract. Ind. Ceram., 45(3), 266 – 272 (2004). DOI: https://doi.org/10.1023/B:REFR.0000046509.70557.d6.
A. L. Ivanovskii, I. R. Shein, Yu. N. Makurin, et al., “Beryllium oxide electron structure and properties,” Neorgan. Materialy, 45(3), 263 – 275 (2009).
V. Kortov, I. Milman, A. Slesarev, et al., “New BeO ceramics for TL ESR dosimetry,” Radiation Protection Dosimetry, 47(1 – 4), 267 – 270 (1993).
V. S. Kiiko, M. A. Gorbunova, Yu. N. Makurin, et al., “Microstructure and electric conductivity of composite (BeO + TiO2) ceramics,” Refract. Ind. Ceram., 48(6), 429 – 434 (2007). DOI: https://doi.org/10.1007/s11148-008-9012-8.
V. N. Batygin, N. D. Efimova, A. V. Inozemtseva, et al., “Volumetric absorption for powerful LBV,” Élektronika SVCh, No. 11, 95 – 102 (1970).
S. G. Mikhailov, “Some properties of titanium-manganese and titanium-beryllium oxide UHF oscillation absorbents and electron bombardment on their composition,” Ukrain. Phys. Zh., 12(9), 1415 – 1416 (1967).
A. Malkin, A. Korotkov, N. Knyazev, et al., “Approbation of the measurement method to determining the permittivity of microand nanopowders of titanium dioxide,” 2019 International Multi-Conference on Engineering, Computer and Information Sciences (SIBIRCON) (2019)
A. Malkin, A. Korotkov, and S. Knyazev, “Measurement of electrodynamic parameters of powder materials,” 2019 Ural Symposium on Biomedical Engineering, Radioelectronics and Information Technology (USBEREIT) (2019).
A. I. Malkin and N. S. Knyazev, “Dielectric permittivity and permeability measurement system,” CEUR Workshop Proceedings. CEUR Workshop Proceedings, 1814, 45 – 51 (2017).
A. M. Nicolson and G. F. Ross, “Measurement of the intrinsic properties of materials by time-domain techniques,” IEEE Transactions on Instrumentation and Measurement, 19(4), 377 – 382 (1970).
W. B. Weir, “Automatic measurement of complex dielectric constant and permeability at microwave frequencies,” Proceedings of the IEEE, 62(1), 33 – 36 (1974).
R. B. Marks, “A multiline method of network analyzer calibration,” IEEE Transactions on Microwave Theory and Techniques, 39(7), 1205 – 1215 (1991).
A. Malkin, V. Chechetkin, A. Korotkov, et al., “Estimation of uncertainty of permittivity measurement with transmission line method in the wide frequency range,” 29th Telecommunications Forum (TELFOR). Belgrade, Serbia: IEEE, 1 – 3 (2021).
V. S. Kiiko and V. Y. Vaispapir, “Production features of preparation and properties of ceramic objects made from a mixture of lightly- and highly-fired BeO powder,” Refract. Ind. Ceram., 57(4), 423 – 426. (2016). DOI: https://doi.org/10.1007/s11148-016-9997-3.
G. A. Komandin, O. E. Porodinkov, I. E. Spektor, et al., “Electrodynamic properties of beryllium oxide in the submillimetre infrared range,” Fiz. Tverdogo Tela, 57(12), Art. 2319 (2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 10, pp. 45 – 52, September 21, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malkin, A.I., Drokin, N.A., Kiiko, V.S. et al. Electrodynamic Properties of (BeO + TiO2)-Ceramics in the Centimeter Wavelength Range. Refract Ind Ceram 64, 555–561 (2024). https://doi.org/10.1007/s11148-024-00889-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-024-00889-3