Results are provided for a laboratory study of the applicability of refractories in the Al2O3–Cr2O3 system in bubble-agitated double-chamber laboratory furnaces during processing of a broad spectrum of iron-containing materials, including technogenic waste. Laboratory tests are conducted for refractory life in a medium of molten iron. Conditions are modeled for exploitation of a refractory in melting and reducing zones of a bubble-agitated type furnace. Optical microscopy is used to study zones and the overall depth of crucible reaction with melt at the melt – refractory crucible interface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
An important problem for humanity is accumulation of an enormous amount of technogenic wastes. The exiting level of development of processing technology does not make it possible to process them ecologically safely and economically effectively. A thermal method is most promising for waste treatment. The main chemical elements contained in technogenic waste are iron, silicon, calcium, carbon, and a considerable amount of other elements: aluminum, chromium, magnesium, zinc, copper, etc. The technology developed should be waste-free, making it possible to extract all useful components from waste into finished products, and also be energy and economically effective. The most promising technology answering these specifications is processing waste in a bubble-agitated type furnace operating in a liquid slag bath treatment regime with an oxygen-containing blast. Operating units of this type are known: Russian Romelt, foreign HiSmelt and Ausmelt, operating in ferrous and nonferrous metallurgy. The advantage of the process for waste treatment in furnaces of the bubble-agitated type is the possibility of total combined treatment of raw materials. In our opinion all of the potentially useful components may be transferred into finished products: metal alloy, slag suitable for preparing building objects, and concentrates of a number of nonferrous metals captured in gas cleaning system. In this case there is no secondary waste formation.
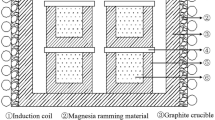
In September 2017 in the industrial base MK Stal’kron (Mtsensk) an MP (MISiS process) a bubble-agitated type pilot plant unit was put into operation for the technology developed of processing a broad range of iron-containing ores, concentrates, and technogenic waste. The general appearance of the unit is shown in Fig. 1. The aggregate had two working zones: melting, within which charge materials are melted, and reducing, within which there is final reduction of iron in melt. In parallel there are processes for recovering nonferrous metals. The discharge of melting products is accomplished from a four-hearth receiver. Five tuyeres are installed in the pilot plant furnace for flushing with oxygen, air, a mixture of them, and natural gas. Development of technology was conducted in NITU MISiS with participation of specialists of OOO New Metallurgical Technologies. The first test campaign confirmed the operating capacity of this structure. In the course of a campaign the whole equipment complex was tested in an operating regime and the main production processes were developed.
An important element of the MP aggregate structure is the lining, primarily the hearth of the unit. Considering the intended industrial furnace within which technologies will be developed and proven, such as coal gasification, ferroalloy production, utilization of solid domestic waste, processing of metallurgical slags and slurries, etc., it is necessary to have highly resistant refractory material. The main specifications for refractory material for the hearth of the PM semi-industrial furnace: good corrosion resistance towards the action of high-iron lag (Fetot up to 60%) at a working temperature of 1400 – 1600°C, and increased heat resistance. Most often for severe operating conditions in furnaces of various types for high temperature material processing such as ferroalloy furnaces, nonferrous metallurgy units, glass melting furnaces, etc., corundum-chromium oxide and chrome-oxide corundum refractories are recommended. Foreign technical publications talk about alumina-chromeoxide or chromecorundum refractories [1,2,3].
A feature of refractories in the Al2O3–Cr2O3 system is the fact that both oxides exhibit a similar crystal lattice, and consequently have any ratio (Fig. 2). This characteristic makes it possible to select refractory under specific operating conditions by varying the oxide ratio. In addition, during object sintering, molded in hydraulic presses, there is a practically identical structure varying in direction parallel and perpendicular to the compaction force. These refractories due to structural features and phase composition exhibit good corrosion resistance, especially in a furnace oxidizing atmosphere. An effect develops of suppression of slag corrosion, into which chromium oxide is transferred; slag viscosity increases and the rate of refractory dissolution within it slows down.
The reason for which Cr2O3-containing refractories exhibit good corrosion resistance in a furnace oxidizing atmosphere is connected with the effect of suppression of corrosion under action of Cr2O3, which is dissolved in slag. Slag viscosity increases due to dissolved Cr2O3, which leads to a slowdown in dissolution rate. If metallurgical unit operation is accomplished discontinuously over a prolonged period of time, furnace operating conditions become more severe.
In order to check the action of molten iron slag laboratory studies were performed using a crucible method combined with petrographic and x-ray structural analyses for the refractory – slag reaction zone. Crucibles were sawn from the refractory (Fig. 3). Crucible height 130 mm, side of the base 100 mm. In the center of a crucible a hole about 100 mm deep and 50 mm in diameter was drilled. The high hardness of the refractory material did not make it possible to manufacture crucibles with identical crucible cavity geometry. Therefore, before each experiment the height of the hole was measured for identical (with respect to weight) filling of the cavity with molten slag during an experiment, and subsequent comparative analysis of the depth of melt penetration into crucible material.
Melting was carried out in an electrical resistance furnace (Fig. 4). Specimens of iron-containing materials were installed in the cavity of a refractory crucible, which was placed in the furnace space and then heated to 1500°C at a rate of 7°C/min. After melting iron-containing material through an upper hole in the laboratory furnace an additional portion of this material was charged in order to increase melt volume within the crucible. A sample of material for melting of one type of combination (material + scale, material + scale + coke, material + DSP dust) in crucibles was identical for each. Briquettes were used as iron-containing material in laboratory tests prepared by stiff extrusion from technogenic waste, intended for further processing in the PM industrial furnace.
The aim of this research in a laboratory furnace was modeling refractory operating conditions in both zones of a pilot plant unit in order to confirm the validity of the choice of refractory material. In order to model melting zones after heating and melting of iron-containing materials the melt was held in the crucible (experiments Nos. 4, 6, 8, and Nos. 10, 12, 13) for 2 and 12 h respectively.
During modeling of reduction zones after melting at the surface of a melt additionally each 10 min coke fines were added, used as a reducing agent for the pilot plant unit. The volume of coke fines charges was calculated from the stoichiometric ratio of iron and reducing agent for reaction of direct reduction; in this case the content of bi- and trivalent iron in an original charge was considered. After charging the required volume of coke fines and holding for 30 min the crucible together with melt cooled in the furnace to room temperature (experiments Nos, 5, 7, 9). Then the supply was switched off and the crucible together with melt was cooled in the furnace to room temperature.
The chemical composition of the original iron-containing materials is given in Table 1, coke fines composition, wt.%: moisture 9.4, ash 17.8, S 0.60, volatiles 2.5, Cfix 79.1. Coke fines ash chemical composition, wt.% Fe2O3 9.35, SiO2 49.44, Al2O3 16.48, CaO 12.12, MgO 7.54, TiO2 0.46, MnO2 0.16, Na2O 1.18, K2O 1.20, BaO 0.20, NiO 0.03, CoO 0.21, Cr2O3 0.05, ZnO 0.70, Sr) 0.15, P2O5 0.67, balance 0.06. Samples before exposure were collected after complete melting by melt freezing on a metal rod, and also after melt crystallization in a crucible. Results of chemical analysis of the collected samples are given in Table 2. Chemical analysis was performed in the NOTs IMT NITU Miss and ZAO MK Stal’kron laboratories. Spectral, atomic emission with an inductively-coupled plasma, and chemical analysis methods were used.
After exposure of specimens in a laboratory furnace crucibles were cut over the vertical axis. During visual examination of a refractory crucible saw cut no changes were observed in the wall and bottom geometry (erosion). Visually it was impossible to reveal the presence and size of an impregnation zone (Fig. 5). Results of chemical analysis showed that impregnation of iron melt by aluminum oxide proceeds as a result of chemical reaction of melt with crucibles of refractory material and is accompanied by melt penetration into refractory.
Changes in corundum-chrome refractory were studied in the laboratory center of collective usage of the Skolkovo “Systems for microscopy and analysis” fund. The number of characteristic zones at the melt – refractory crucible interface after conducting a test for slag resistance, the thickness of each zone, and overall depth of crucible reaction with melt at the melt – refractory crucible interface, were determined by optical microscopy. Polished specimens were on the instrument table of a Leica DM LM 2 optical microscope and areas of interest were photographed at different magnifications. Zonality and thickness of zones were determined in sections of a cut crucible specimen (Fig. 6), and the microstructure of zones was studied using an optical microscope (Fig. 7). Refractory specimens after a test for slag resistance have four visibly different zones: slag zone (slag skin), zone of slag component penetration, impregnation and reaction zone, and least changed zone.
1. The slag skin consists of rhombic crystals, primarily spinelid, between which dendrite-like skeletal inclusions are observed, predominantly wustite, rarely baddeleyite, and a silicate matrix within which all crystals are immersed: in specimens with participation of a reducing agent iron metal is observed. Pores in slag are a bubble type and round in shape. At the contact with refractory a dense layer is observed, consisting of complex spinelid crystals.
2. The zone of slag component penetration is expressed in presence in refractory at contact with slag of local areas containing complex spinelid, similar to the contact spinelid in slag.
3. The zone of impregnation and reaction of refractory phases with slag components has different penetration thickness. It is expressed in grain decomposition and partial transfer of baddeleyite beyond the boundary of grains in slag and in compaction due to sintering, phase formation, reaction of mullite with corundum, and impurities of finely milled component. Pores of two types: individual coarse, or directional shape and fine, and closed. Transfer between the impregnation zone and the least changed area is unclear, blurred, and visually poorly separated.
4. The least changed zone is similar to a specimen before a test.
Analysis of the chemical element distribution at the surface of a microsection taking account of structural components was conducted in a perpendicular section of specimens in the region of reaction of melt and refractory crucible. The number of typical zones and their thickness were determined by x-ray fluorescence spectroscopy (ORBIS PC x-ray fluorescence spectrometer from EDAX) on the basis of comparing data for the elemental composition at each point and the level of signal in the x-ray detector. An example of a silicon distribution map for an area analyzed of a specimens in experimental No. 4 is shown in Fig. 8. The distribution of the concentration of the following elements was studied: Mg, Al, Si, P, Fe, Zr, S, K, Ca, Ti, Cr, and Mn.
Analysis of the microstructure of zones, their amount, and thickness, chemical element distribution in zones through the section of a specimen in the region of melt and refractory crucible interaction was performed by means of a scanning electron microscope (SEM) with an energy-dispersion microanalyzer in a MAPS regime, and numerical analysis of SEM data. The SEM method preferably examines a specimen at low magnification (×85 – ×150) in order to evaluate the uniformity of object distribution, which is necessary to measure and reveal presence of a zonal structure. A CBS detector for a photograph makes it possible to obtain an image of the polished section surface in a contrast regime, depending on element atomic number in the D. I. Mendeleev periodic system of elements. As a result nonmetallic inclusions, pores, and cracks are reflected by a dark color, and inclusions containing chemical elements with high atomic number (Z > 36) are reflected as white. In a MAPS regime in double-beam scanning-ion microscope FEI Versa 2D mapping was carried out for the microstructure for the test zone of a polished section. The amp obtained was used for subsequent specification and revelation of typical structural components (zones). An example of this map is shown in Figs, 9 and 10.
Then tests were conducted on each separated zone of a specimen by the SEM method using an energy-dispersion spectrometer (SEM/EDS) in order to determine the mineral and chemical composition and typical features of each zone, and also in order to construct a distribution map for chemical elements over the area of a region of interest at a polished section surface. The size of micro-inhomogeneities of the structure (thickness, depth of zone extent) perpendicular to the melt-crucible interface in specimens is given in Table 3. It is seen from Table 3 that with exposure for the first 2 h the maximum impregnation rate is 2.75 mm/h, and with exposure for 12 h it is 0.67 mm/h. The fastest impregnation rate for material is for that with an increased zinc content. The rate of refractory impregnation in a reduction regime (reduction zone) corresponds approximately to the impregnation rate under conditions the melting zone in a PM pilot plant furnace.
Microstructure of specimen section in experiment No. 4 after slag resistance test. SEM., MAPS technology. Boundary: □) slag and refractory;  ) zone of slag component penetration and impregnation zone;
) zone of slag component penetration and impregnation zone;  ) upper part of impregnation zone containing baddeleyite, with lower part;
) upper part of impregnation zone containing baddeleyite, with lower part;  ) impregnation zone with least changed zone.
) impregnation zone with least changed zone.
Conclusion
-
1.
Reaction of corundum-chromium oxide refractories with molten iron slag commences with impregnation through a milled component by means of solid-phase diffusion of silicon, calcium, andiron ions with formation of complex silicates between crystals, i.e., finely milled component particles.
-
2.
Chromium and aluminum oxides from refractory are carried into slag with formation of complex spinelids.
-
3.
In All specimens independent of composition of iron-containing material and experiment atmosphere there are identical processes of formation of zones and phase formation. With an experiment duration impregnation is deeper.
-
4.
A section of melt interaction of iron-containing slag with corundum-chromium oxide refractory includes four zones: slag skin zone of slag component penetration, an impregnation and reaction zone, which is similar in microstructure to the zone of slag component penetration, but does not contain complex spinelid from slag, and a least changed zone, similar in structure and phase composition to refractory before testing.
-
5.
Presence of zinc in slag increases impregnation rate. As a rule zinc enters into the composition of complex spinelids, fixed in slag at the boundary with refractory and in a surface zone of slag penetration into the refractory structure.
-
6.
It has been revealed that an increase in impregnation duration from 2 to 12 h leads to a reduction in impregnation rate by more than a factor of four. The impregnation process is limited with respect to a time character. This signifies that this form of refractory may be recommended for further use in the MP pilot plant unit.
-
7.
Within the scope of further development of technology for processing different forms of materials in a bubble-agitated furnace, including technogenic waste, there will be continuation of testing of refractory materials with prospects for evaluating their use in industrial units, including taking account of atmosphere quality in a heating unit.
References
M. Dietrich and S. Postrach, “Chrome corundum: an alternative to isostatically pressed products,” RHI Bulletin, No. 2, 27 – 30 (2013).
Yasutaka Yoshimi, “Wear of Al2O3–Cr2O3 bricks in waste smelting rotary kiln,” J. Technical Association of Refractories, 36(3), 16 – 18 (2016).
Shixian Zhao, Binli Cai, Pengtao Li, et al., “Thermodynamic evaluation of Cr2O3–Al2O3 refractory corrosion by smelting reduction ironmaking slag,” China’s Refractories, 24(2), 6 – 10 (2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 5, pp. 3 – 9, May, 2018.
Rights and permissions
About this article
Cite this article
Podgorodetskii, G.S., Aksel’rod, L.M., Agapov, E.A. et al. Resistance of Refractories of the Al2O3–Cr2O3 System Under Liquid-Phase Reduction Conditions of Iron-Containing Technogenic Waste. Refract Ind Ceram 59, 231–236 (2018). https://doi.org/10.1007/s11148-018-0212-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-018-0212-6