The change in LTEC is analyzed on heating and cooling ceramic materials of the Al2O3–ZrO2 system in the range 200 – 1500°C. The effect of structural and phase components and measurement regime (heating/cooling) on form of the relationship Δl/l 0 = f(t), and also the dependence of LTEC on composite chemical composition are established. Results obtained are discussed from the point of view of material phase composition and published data are analyzed. Data are provided for average and true LTEC values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that composite ceramic of the ZrO2(Y)–Al2O3 system exhibits such extremely important properties as increased strength and crack resistance, and it is used extensively as a refractory, heat insulation, and structural material [1,2,3]. Within material of the ZrO2–Al2O3 system a special strengthening mechanism is realized providing improvement of mechanical properties of composite ceramic with mono-zirconia and mono-corundum refractories [4,5,6]. Since Al2O3 has low solubility in ZrO2 at high temperature, then during sintering of this composite conditions are created for suppression of grain growth of both components, as a result of which there is formation of a fine grain structure within the system. Presence for one of the components of polymorphic transformation with volumetric inversion in the sintering temperature range leads to a nonlinear dependence of a change in sintered specimen density on composition [7]. It may might be expected that other important properties of the composite will have their own dependence on composition. For effective use of composite in combination with other materials LTEC was studied for a composite in relation to the ratio of ZrO2 and Al2O3. Determination of LTEC is important since similar values are required in order to provide good contact between functional materials and to prevent cracking or separation during heating, cooling, and thermal cycling. For engineering calculations both average and true values of LTEC are used during heating and cooling. For example, in order to calculate internal stresses arising within material with a change in temperature, it is necessary to use true LTEC values, but in order to calculate the change in object dimensions as a result thermal expansion it is convenient to use the average LTEC value calculated for the temperature range t 1 – t 2, since LTEC depends on phase and chemical composition and the nature of heat treatment.
For pure Al2O3 with an α-corundum structure LTEC during heating and cooling changes uniformly. With presence in a composite of ZrO2, which may have structural changes (phase transformations), the uniform change in LTEC on heating and cooling is disturbed [3]. The nature of change in LTEC for ZrO2(Y)–Al2O3 composite should depend on its composition.
The aim of research is a comprehensive study of the change in LTEC on heating and cooling for ceramic of the ZrO2–Al2O3 system in the range 200 – 1500°C, and evaluation of the effect of structural components and ratio of oxides on the level of LTEC.
Experimental Section
Composite ceramic of the composition (x)Al2O3 + (100 – x)ZrO2(Y), where x = 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100, was studied. The starting materials used were zirconium dioxide powder, stabilized with 3.5 mol.% Y2O3; powders synthesized by combined deposition, washing the residue, drying and calcining at 1100°C for 3 h. Aluminum oxide was prepared by calcining hydroxide at 1350°C for 4 h. The phase composition of zirconium dioxide powder YSZ-3.5 is mainly represented by a tetragonal structure, and the content of monoclinic phase is within the limits of 2 – 10%. Aluminum oxide had the structure of corundum. Specimens of the prescribed composition were molded in the form of cylinders 10 mm in diameter and 30 mm long by technology described in publication [7]. Then samples were dried and fired at a temperature providing preparation of specimens with density of not less than 0.99 of the relatively theoretical density, in order to exclude the effect of ceramic porosity on thermal expansion.
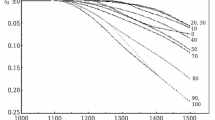
Dilatometric measurements of ceramic materials were performed by means of a DIL 402 C dilatometer from Netzsch Gerätebau, in a heating regime up to 1500°C and subsequent cooling at a constant rate of 5°C/min. Experiments were carried out in an air atmosphere with constant flushing with air at a rate of 100 ml/h. The accuracy of temperature maintenance in the dilatometer region of a specimen did not exceed 0.1°C. The accuracy of measuring length l, determined by nonlinearity of the dilatometer movement transducer transmission function, was 0.133%. Values of (l – l 0)/l 0 are shown in Fig. 1 for the relative change in specimen length of composite ceramic of compositions xAl2O3 + (100 – x)ZrO2(Y) in the concentration range 0 ≤ x ≤ 100.
Results and Discussion
Dilatometric data show that the nature of thermal expansion of composite ceramic depends on the Al2O3/ZrO2(Y) ratio. Three typical dilatometric curves are shown in Fig. 1. A uniform change in length on heating and cooling (curve 1) is observed for ceramic with predominant corundum (0.8 ≤ x ≤ 1.0). The second type of dependence is observed for ceramic with corundum concentration in the range 0.2 ≤ x ≤ 0.6. At 600°C ceramic of these compositions experiences significant expansion, replaced by compression. With a subsequent increase in temperature the path of dilatometric curves is uniform, the same as for ceramic with predominant corundum (0.8 ≤ x ≤ 1.0). On cooling below 300°C an expansion effect is observed. The temperatures of these extremes are given in Table 1.
The third type of dependence is observed for ceramic with a low corundum concentration in the range 0 ≤ x ≤ 0.1. In contrast to the second type there is absence of an effect of expansion on specimen cooling. The data obtained provide a basis for assuming that with an increase in corundum concentration (0.8 ≤ x ≤ 1.0) the ZrO2 in ceramic is single phase, since the compressive stresses in the matrix decrease the probability of tetragonal-monoclinic polymorphic transformation of ZrO2 grains and facilitate retention within a composite of tetragonal ZrO2 throughout the whole temperature range for heating and cooling. Therefore LTEC retains a uniform change on heating and cooling. With transition in the region of reduced corundum concentration inflexions appear on curves, probably caused by transformation conversion of ZrO2 proceeding within the material. This is partly confirmed by data in [8] about the fact that presence of Al2O3 stabilizing additions in ZrO2 entirely or partly lose their stabilizing properties. A similar phenomenon is observed with excess Al2O3, but the corundum matrix is capable of holding an insignificant amount of ZrO2 grains in tetragonal phase. Presence and location of peaks is explained by properties of the monoclinic ZrO2 phase itself and the fact that tetragonal-monoclinic transformation in ZrO2 shifts energetically in this temperature range, since it depends on many factors (particle size, compressive action of matrix, and stabilizer content).
The temperature of inflections on curves (see Fig. 1) does not depend on the ratio Al2O3/ZrO2: t 1 = (490 ± 100)°C (expansion peak), t 2 = (600 ± 40)°C (end of expansion on heating), t 3 = (290 ± 25)°C (start of expansion on cooling), t 4 = (180 ± 30)°C (expansion peak). On the other hand, the amplitude of D depends on composition. The maximum expansion amplitude in the heating branch corresponds to the composition x = 40 wt.%. For ceramic made of corundum (x = 0) a significant amount of expansion is also typical.
Average values of LTEC were calculated for cooling sections in the range 1300 – 400°C from the expression LTEC = Δl/l·1/Δt. Calculated values of LTEC are given in Table 2 from which it follows that LTEC decreases with an increase in corundum concentration (Fig. 2). Experimental data of the concentration dependence of LTEC is described satisfactorily by a nonlinear regression equation LTEC = 1.15 × 10–5 – A·x, where A is proportionality coefficient; x is Al2O3 weight fraction in composite ceramic. With x = 40 – 50%, when the volume fraction of corundum and zirconium oxide phases is similar, deviation of observed from the general dependence for the change in value of LTEC.
Values of LTEC for single-phase ceramic (x = 0, x = 100) agree satisfactorily with reference data [9]: for tetragonal ZrO2 (2 – 4 mol.% YSZ) LTEC = 11.4 × 10–6 1/K (200 – 1000°C), for Al2O3 LTEC = 9.5 × 10–6 1/K (200 – 1000 °C). The uniform change in average LTEC with a change in ZrO2/Al2O3 ratio points to an equal contribution of both phases in thermal expansion over the whole concentration range and contradicts the concept of existence of a considered two-phase system in the form of a continuous matrix of main phase within which grains of second phase are disseminated. This assumes that the main thermal expansion should be determined by matrix material. Only in the region of equal volumes of phase fractions should thermal expansion be the average of two phases.
A dependence of LTEC on temperature is shown in Fig. 3, interpolated by a linear function. Here there also is a marked difference in the behavior of ceramic with predominance of corundum (0.8 ≤ x ≤ 1.0) due to the rest of components (0.1 ≤ x ≤ 0.6) and pure ZrO2 (x = 0). Angles of inclination of the temperature dependences for LTEC decrease uniformly with an increase in proportion of ZrO2. This is due to the different nature of change in the value of LTEC for single-phase ceramic (x = 0, x = 100) due to temperature (see Fig. 3). For pure ZrO2 the effect of temperature on change in LTEC value is less clearly expressed than for Al2O3.
Conclusion
-
1.
According to features of thermal expansion the test composite ceramic xAl2O3 + (1 – x)ZrO2(Y) is divided into three groups: 0.8 ≤ x ≤ 1.0, 0.1 ≤ x ≤ 0.6, and x = 0. From the group 0.1 ≤ x ≤ 0.6 the composition x = 0.4 is separated both for the value of average LTEC, and for the temperature dependence of true LTEC.
-
2.
It has been demonstrated that all composite ceramics xAl2O3 + (1 – x)ZrO2(Y) with x ≤ 0.8 experience monoclinictetragonal transformation in a temperature range independent of x. Amplitude of the effect is at a maximum for the composition x = 0.4.
-
3.
The average value of LTEC decreases uniformly from 11.9 × 10–6 to 9.6 × 10–6 1/K with an increase in corundum concentration from 0 to 100 wt.%.
-
4.
Temperature dependences for true values of LTEC differ for different x. The angle of inclination of straight lines of the dependence for the change in LTEC on x increases with an increase in corundum concentration.
References
V. Naglieri, P. Palmero, L. Montanaro, “Preparation and characterization of alumina-doped powders for the design of multi-phase nanocomposities,” Therm. Anal. Calorim., 97(1), 231 – 237 (2009).
R. H. J. Hannik, P. M. Kelly, and B. C. Muddle, “Transformation toughening in zirconia-containing ceramics,” J. Amer. Ceram. Soc., 83(3), 461 – 487 (2000).
M. C. Moraes, C. N. Elias, J. D. Filho, and L. G. Oliviera, “Mechanical properties of alumina-zirconia composites for ceramic abutments,” Mater. Res., No. 7(4), 643 – 649 (2004).
C. Santos, L. H. P. Teixiera, J. K. M. F. Daguano, et al., “Mechanical properties and cytotoxicity of 3Y-TZP bioceramics reinforced with Al2O3 particles,” Ceram. Internat., 35, 709 (2009).
F. A. T. Guimares, K. L. Silva, and V. Trombini, “Correlation between microstructure and mechanical properties of Al2O3 / ZrO2 nanocomposites,” Ceram. Internat., 35, 741 – 745 (2009).
W. H. Tuan, R. Z. Chen, and T. C. Wang, “Mechanical properties of Al2O3/ZrO2 composites,” J. Europ. Ceram. Soc., 22, 2827 – 2833 (2002).
Yu. I. Komolikov, I. D. Kashcheev, and V. D. Khrustov, “Sintering of composite ceramic based on zirconium and aluminum oxide powders,” Refract. Indust. Ceram., 56(4), 418 – 420 (2015).
V. I. Strakhov, E. A. Pavlova, and S. I. Gershkovich, “Phase transformations in in stabilized ZrO2-Al2O3 composites and zirconia refractory properties,” Ogneupory, No. 12, 5 – 8 (1995).
I. K. Kikoin (editor), Physical Quantity Tables: Handbook [in Russian], Atomizdat, Moscow (1976).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 9, pp. 59 – 62, September, 2016.
Rights and permissions
About this article
Cite this article
Komolikov, Y.I., Kashcheev, I.D. & Khrustov, V.R. Thermal Expansion of Composite Ceramic of the Zirconium Dioxide – Aluminum Oxide System. Refract Ind Ceram 57, 516–519 (2017). https://doi.org/10.1007/s11148-017-0015-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-017-0015-1