Pulverized bakelite properties are provided. The effect of novolac molecular weight and particle size, amount of hardener, and oxygen in the atmosphere during carbonization, on the amount of pulverized bakelite coke residue is considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pulverized bakelite is a thermoreactive polymer that exhibits high strength on hardening and provides good stability of a refractory obtained. In addition, it is distinguished from other binders for refractory materials by increased adhesion, high coke residue on carbonization, and insignificant liberation of harmful substances.
The main advantage of pulverized bakelite as a binder for carbon-containing refractories includes a high coke residue (Table 1).
A mechanism proposed for carbonization of phenolformaldehyde resins is presented in Fig. 1. Due to the high cross-linking density and carbon content of about 80% pulverized bakelite is capable of easy carbonization. The high density of the three-dimensional polymer network creates favorable conditions for forming carbon during pyrolysis (see Fig. 1). Theoretical calculations show that based on an 80% carbon content in an ideal cement of pulverized bakelite it is possible to expect a coke residue with pyrolysis at a level of 55 – 70%.
Pulverized bakelite carbonization mechanism [1].
The amount of pulverized bakelite coke residue depends on the novolac molecular weight, amount of hardener, oxygen in the atmosphere during carbonization, and also on novolac particle size.
Novolac Molecular Weight
Special tests demonstrate that the coke residue of pure novolac depends strongly on molecular weight. Coke residue is about 20% with a molecular weight of about 400 g/mole and it increases to 50% with a molecular weight of 1000 g/mole (Fig. 2).
Dependence of coke residue on novolac molecular weight [2].
Amount of Hardener in Pulverized Bakelite
Pulverized bakelite consists of a mixture of phenolformaldehyde resin of the novolac type and a hardener. The hardener used is hexamethylenetetramine, more well known within Russia under the name urotropin or dry fuel. The amount of hexamethylenetetramine affects the amount of coke residue. A relationship is shown in Fig. 3 between the amount of hexamethylenetetramine, added to novolac, and coke residue of novolacs with molecular weights of 600 and 750 g/mole. The amount of coke residue achieves its own maximum when the amount of hexamethylenetetramine added is in the range 7 – 10%. The reason suggested is as follows: part of the novolac with low molecular weight, which normally evaporates on heating, enters into reaction with hexamethylenetetramine and forms a non-volatile polymer with high molecular weight. This means a reduction in part of the amount of volatile components, and this leads to an increase in pulverized bakelite efficiency. However, with a further increase in the amount of hexamethylenetetramine the coke residue decreases, since coke residue of hexamethylenetetramine itself is insignificantly small. Consequently, it is necessary of correct the amount of hexamethylenetetramine added to novolac to an appropriate value taking account of the coke residue required and carrying out a hardening reaction.
Relationship between amount of hexamethylenetetramine added to novolac and a novolac coke residue with molecular weight 600 (♦) and 750 (■) g/mole [1].
Amount of Oxygen in the Atmosphere during Carbonization
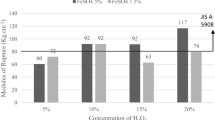
It has been established that even an insignificant oxygen content in the atmosphere during carbonization reduces the amount of coke residue of novolac hardened with urotropin by 10 – 30% (Fig. 4). The effect of a reduction in amount of coke residue nonetheless is less, the lower the novolac molecular weight. This indicates that optimum cross linking of novolac increases oxidation resistance. Oxidation may also be slowed down with addition an antioxidizing additive (see Fig. 4).
Coke residue of hardened pulverized bakelite in different atmospheres [2].
Novolac Particle Size
Particle size also has an effect on coke residue. Coke residue increases in an inert atmosphere with large particle sizes. The effect is due to the low degree of mechanical destruction of novolac and low diffusion rate of volatile organic components from a mass of pulverized bakelite with a large particle size. This effect is partly or entirely lost in the presence of oxygen,
Thus, pulverized bakelite is suitable as a binder in the production of carbon-containing refractories. It is important to select the most appropriate grade of pulverized bakelite for corresponding use, and to select the optimum hardening and pyrolysis conditions.
References
Phenolic Resins: A Century of Progress, Springer-Verlag, Berlin – Heidelberg (2010).
Phenolic Resins, Springer-Verlag, Berlin – Heidelberg (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
1Proceedings of International Conference of Refractory Workers and Metallurgists (3 – 4 April, 2014, Moscow).
Translated from Novye Ogneupory, No. 8, pp. 8 – 9, August 2014.
Rights and permissions
About this article
Cite this article
Yunusov, R.I. Factors Affecting Coke Residue Quality in Binder Based on Pulverized Bakelite1 . Refract Ind Ceram 55, 288–290 (2014). https://doi.org/10.1007/s11148-014-9710-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-014-9710-3