Abstract
Selective hydrogenation of acetylene was studied using palladium catalysts supported on different transition alumina spheres. The alumina supports were prepared by calcining pseudo boehmite alumina sphere at different calcination temperatures; the ρ-Al2O3 used was flash calcined alumina. The alumina supports were characterized using different techniques like XRD, surface area, pore volume and acidity. The Pd/Al2O3 catalyst were prepared, palladium penetration depth was measured using microscopic imaging and the catalyst reducibility were studied by Temperature Programmed Reduction (TPR). The microscopic imaging using TEM were done to study the palladium particle size and nature of particle clusters on the support surface. The catalyst performance evaluation was done in a fixed bed reactor with 1% acetylene in nitrogen feed and studied the impact of support properties on the catalytic activity. The catalyst prepared on γ-Al2O3 support showed the highest conversion and lowest selectivity, whereas the catalyst on α-Al2O3 support has the lower conversion and highest selectivity. Thermogravimetric analysis coupled with mass spectroscopy analysis were done for the spent catalyst to understand the possibilities of heavy component deposition of the catalyst. The catalyst prepared on α-Al2O3 support had the lowest hydrocarbon deposit on the surface whereas the γ and δ alumina showed the highest hydrocarbon deposits on the catalyst surface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ethylene is one of the major chemicals used in the petrochemical industry. The majority of the ethylene is produced from steam cracking of naphtha like feedstocks. Ethylene from the steam cracker typically contains 0.1−1% acetylene. Acetylene impurities are typically removed by selective hydrogenation and by using adsorption technique. Selective hydrogenation is the most commonly used method [1]. Acetylene removal is important as it act as poison for the polyethylene catalyst. Selective hydrogenation for acetylene can be done in two-configurations: front-end hydrogenation and tail end hydrogenation. The tail end hydrogenation is the more common configuration due to its better process controllability. Pd/ Al2O3 first generation commercial catalysts were introduced in early 1960s after twenty years. The first bi metallic catalyst, palladium and silver on alumina was introduced after almost twenty years, which is still used successfully in the industry. The Pd–Ag alloy catalyst had good selectivity and the selectivity was improved by reducing the quantity of hydrogen adsorbed on the surface. The properties of the bi metallic catalyst depend on the preparation method, the support alumina, the impregnation sequence and other processing steps [2]. The alumina can be prepared in different phases, γ, δ, ρ, α. The different alumina phases have different properties and different stability. Palladium catalyst on different alumina phases were made and the properties of the catalyst was studied. Different alumina phases impart different characteristics to the catalyst and they influence the reaction yield, selectivity, the green oil formation etc. For the selective hydrogenation of acetylene, low surface area alumina supports are mostly used with the palladium content typically from 100–1000 ppm. Egg shell type catalysts are often used to avoid the mass transfer resistances in the catalyst bed [3]. In this paper, the impact of support properties on the catalyst performance and coke formation were studied.
Experimental
Preparation and characterisation of support and catalysts
Spheres of γ-Al2O3 and ρ-Al2O3 received from Sud-Chemie India (P) Ltd were used for the present study. γ-Al2O3 spheres were calcined at 900 °C and 1300 °C for 6 h to obtain δ-Al2O3 and α-Al2O3 spheres [4, 5]. The Al2O3 spheres were characterized using XRD (Bruker D2Phaser), surface area, pore volume and pore size (BET method—Micromeritics Tristar) and acidity measurements (ammonia TPD—Micromeritics Autochem II). The XRD scan was done at 2θ value ranging from 0 to 60. The results were evaluated using EVA software. The standard PDF files in repository were compared to identify the phases in each material using the Scan function [6,7,8,9,10]. For ammonia TPD measurements, 5% ammonia in helium was used as the analysis gas. The adsorption of ammonia on the alumina supports was done at 40 °C and the gas flow continued until there is no further adsorption of ammonia was observed. Purged with helium to drive out the gas from pores and the sample was heated in helium gas at a ramp rate of 10 °C/ min. The ammonia desorbed was measured using a TCD detector and quantified the total desorbed quantity of ammonia [11].
The catalyst preparation on different alumina supports were done using wet impregnation method [12, 13]. Palladium source used for the catalyst preparation was palladium nitrate solution purchased from Sigma Aldrich. The catalyst was dried at 80 °C and further calcined at 400 °C. [14,15,16]. The active metal loading ranged from 190 to 200 ppm on the catalyst as analyzed by ICP. Temperature programmed reduction (TPR) of the catalysts was done using Micrometrics Autochem II equipment using 5% hydrogen in nitrogen as the reducing gas. The TCD signals were recorded from 40 °C to 600 °C. The plot gives the reduction temperatures of each catalyst sample [17, 18]. Palladium metal penetration depth measurement was done using Olympus microscope and software. Different alumina supports have different pore size distributions and different pore structure; hence the penetration depth also varies. The catalyst was sliced into half using a special tools and the image of the palladium metal penetration was taken [19]. TEM analysis of the catalyst samples were done using HR TEM Joel/JEM 2100 microscope to understand about the Pd particle in the catalysts.
Catalyst performance evaluation
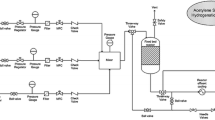
The performance evaluation was done in a fixed bed reactor with dimension 25 mm ID and 1000 mm length. The thermocouple was adjusted to measure the catalyst bed temperature. The reactor tube is made of SS 316 L. The gas flow through the reactor controlled using mass flow controllers and the pressure regulation of the reactor done using backpressure regulating valve [20].
The catalyst (10 cm3) was reduced with 5% hydrogen balance nitrogen at 5 bar pressure and 350 °C for 4 h. After catalyst reduction the reactor was cooled in nitrogen stream to 40 °C. The process gas containing 1% acetylene in nitrogen was passed through the reactor keeping the pressure at 5 bar g. The hydrogen to acetylene ratio is maintained 1.2 during the experiment. The reaction was done at different temperatures 60 °C, 70 °C, 80 °C and 90 °C at a GHSV of 3000 h−1. At each temperature, the conditions were kept constant and the reaction was allowed to stabilize for the initial 1 h and reactor outlet gas was collected at an interval for 30 min. The reaction temperature was changed after collecting 3 samples from each temperature. [21, 22].
The outlet gas analysis was done using an Agilent GC 7890B with CP Al2O3 column.
The spent catalyst from the reaction were collected and thermogravimetric analysis (TA Instruments SDT Q 600) along with mass spectroscopy (MKS-Cirrus 2) analysis was done to study the coke/green oil formation in each catalyst. The analysis was performed in air from 25 to 1000 °C at a ramp rate of 10 °C/ min.
Results and discussion
Support characterization
The XRD of the samples were done and were compared with the standard files in the data bank to identify the phases. The crystallinity was very high in α Al2O3 support whereas γ-, δ- and ρ-Al2O3 show amorphous XRD pattern as expected. [23, 24]. The XRD comparison of different Al2O3 phases is provided in the supplementary data. Acidity measurement of the supports was performed in Micrometrics Autochem-II with ammonia temperature programmed desorption. The ammonia desorption with the temperature shows the presence of weak and strong acid sites and the intensity varies in different phases of alumina (Fig. 1, Table 1).
The δ- and ρ-Al2O3 have relatively lower acidic sites compared to γ-Al2O3. The γ, δ- and ρ-Al2O3 has weak, medium and strong acid sites, the TPD peaks at temperatures above 200 °C indicates the presence medium and strong acid sites. In α-Al2O3, only very weak acid sites are present and the lower acidic sites help in lowering the alkylation reaction on alumina surface and consequently the polymerization and green oil formation [25, 26].
Different phases of Al2O3 exhibit different pore characteristics and SA (Table 2). The α-Al2O3 support has the lowest surface area and the lowest micro pore volume. The support mostly has macropores [27]. The γ-Al2O3 has the highest surface area and the highest nitrogen pore volume, indicating mostly micro and mesopores. The δ-Al2O3 also has lower nitrogen pore volume than γ-Al2O3 and lower surface area. The ρ-Al2O3 is entirely different type of alumina prepared by flash calcination and it has good surface area and relatively moderate pore volume [28].
Catalyst characterization
The catalyst properties like the metal content, surface area and pore volume are given in Table 3. No difference in surface area and pore volume was noticed for alumina before and after palladium impregnation. This indicates that the support is stable in palladium nitrate solution.
Temperature programmed reduction
The temperature-programmed reduction was performed using Micrometrics Autochem II instrument. The reductions were done using 5% hydrogen balance nitrogen gas with a temperature ramp of 2 °C/min and the resulting curve with the peaks were plotted.
The negative dip in the TPR plot was due to the reduction of the bulk PdO forming palladium β hydride, which forms at a low temperature around 20 to 25 °C. This palladium β-hydride decomposes at a higher temperature and shows a negative peak while decomposing. The position of the negative peak depends on the type of the hydride formation, which depends on the size of the Palladium molecule and the dispersion of the palladium on the alumina support. Larger sized PdO particles have the palladium hydride decomposition at lower temperature and the smaller size PdO particles have the Palladium hydride decomposition at higher temperature. The highly dispersed palladium molecule has high intensity decomposition peak and at a higher temperature. The α-Al2O3 support has the lowest surface area and hence lower Pd dispersion, the Pd particles will be the largest in this catalyst. Larger Pd species forms hydrides easily and these hydrides decomposes at temperatures below 25 °C. The TPR profile of Pd /γ—Al2O3, Pd/ δ-Al2O3, Pd/ρ-Al2O3 shows negative dip in the TPR at peaks at 62 °C, 63 °C and 94 °C attributed to the decomposition of palladium hydride. The high temperatures peaks in the TPR at 228 °C, 248 °C, 306 °C and 400 °C will be due to the reduction of smaller PdO particles and hydrogen spill over on the alumina surface hydroxyl groups [29,30,31] (Fig. 2)
.
Palladium metal penetration depth measurement
Palladium metal penetration depth measurement was done using Olympus microscope and software. Different alumina support has different pore size distribution and different pore structure hence the penetration depth varies too. The catalyst was sliced into half using a special tools and the image of the palladium metal penetration was taken. Palladium penetration depth was measured for twenty sphere from each catalyst sample. The microscopy measurement pictures are displayed in Fig. 3.
The active metal penetration depth is highest for the γ-Al2O3, hence the active metal will be highly dispersed, and the metal penetration depth was lowest for α-Al2O3. Pd/α-Al2O3 catalyst has the thinnest active metal shell. Table 4 gives a comparison of the penetration depth in the catalysts prepared with different supports (Fig. 3).
The selective hydrogenation reaction is a fast reaction and higher dispersion could make the catalyst more active and more prone to over hydrogenation [32, 33]. Hence better selectivity to ethylene can be expected for the catalyst with a typical egg-shell type palladium distribution.
TEM analysis of the catalysts
High-resolution transmission electron microscopy image were taken for the catalyst sample using Joel/JEM 2100 microscope. The images were taken at different resolutions and the particle size were analyzed (Fig. 4).
HR TEM analysis reveals Pd particles on the Pd/α-Al2O3 has larger size compared to other catalysts. The Pd particles on the Pd/γ-Al2O3 and Pd/ρ-Al2O3 catalyst are relatively smaller and most of the particles are below 5 nm in size.
The palladium particle size distribution on catalysts were analyzed using the J-image software (Fig. 5). Particle size distributions were done by analyzing 20 particles in each catalysts and were plotted in a bar chart. The average Pd particle size were 4.7, 8.9, 4.3 and 10 nm on Pd/γ-Al2O3, Pd/δ-Al2O3, Pd/ρ-Al2O3 and Pd/α-Al2O3.
Catalyst performance evaluation
All the catalysts were tested for the selective hydrogenation of acetylene. The catalyst (10 cm3) was loaded in the reactor and was reduced using 5% hydrogen in nitrogen at 370 °C and 5 bar gauge pressure for 3 h. The catalyst performance was evaluated at different temperatures 60–90 °C at a GHSV 3000 h−1 and an operating pressure of 5 bar gauge (Fig. 6).
Catalyst Pd/γ-Al2O3 showed very high acetylene conversion and low ethylene selectivity at all temperatures. Ethylene selectivity of these catalysts was calculated using the formula and it was found that the catalyst Pd/α-Al2O3 has the highest selectivity and Pd/γ-Al2O3 has the lowest selectivity (Table 5). The selectivity of all the catalysts reduces drastically with increase in the operating temperature. The increased temperature favors ethane formation on the catalysts. Catalyst Pd/ρ-Al2O3 support has a different behavior compared to the others. The catalyst Pd/ρ-Al2O3 exhibits improvement in selectivity as the temperature was increased. Reaction temperatures were not increased beyond 90 °C and hence a separate study has to be done to understand the behavior at high temperatures. The selectivity of the catalyst Pd/α-Al2O3 can be related to the low acidity and low palladium penetration depth of the catalyst. The lower acidity of the support in combination with lower metal penetration depth might be limiting the beta hydride formation in the catalyst surface hence lowering the chances of hydrogen over reaction with the ethylene molecule formed. The ρ-Al2O3 behaves differently at higher temperatures, the lower acidity of the support might be preventing the interaction with the metal alumina support and the combination of higher temperature might be causing faster desorption of the hydrogen molecule from the catalyst surface, showing improved selectivity at higher temperatures [34,35,36].
The ability of Pd to react with hydrogen and form the β-hydride phase may play an important role in these catalysts. It was shown that the β-hydride phase favors the conversion of acetylene into ethane, with small Pd particles being more selective for ethylene formation. During reaction, palladium may get converted to β-PdH and these PdH decomposes and release hydrogen resulting in over hydrogenation of the alkene leading to alkane formation and carbonaceous or hydrocarbon species may get deposited on the surface of the catalyst. These deposits also favors the increased hydrogenation resulting in the conversion to ethane. It is known from literature that the presence of hydrogen residing the palladium sub surface will cause over hydrogenation of acetylene resulting in ethane formation [15]. In certain studies, ethylene TPD on different alumina species were done, the results show that high temperature ethylene desorption peaks were predominant in the catalyst with high surface area similar to Pd/γ-Al2O3 and Pd/δ-Al2O3 and high temperature desorption peaks were almost nil in Pd/α-Al2O3 [22]. The low temperature peaks in the ethylene desorption studies were assigned to the π-bonded ethylene and the high temperature peaks are assigned to the di-σ bonded ethylene [37]. The low acidity of the Pd/α-Al2O3 might have led to predominant π-bonded ethylene intermediates, which dissociates easily from the catalyst site giving better selectivity. Studies have shown that the di-σ bonded ethylene formed on the catalyst site undergoes desorption slowly and it decomposes followed by reaction with the surface hydrogen to form ethylene and ethane [38]. Medium strength and high strength acid sites could give rise to green oil deposition during selective hydrogenation and these carbon deposits act as a hydrogen bridge for hydrogen spill over from the palladium to the support, facilitating ethylene hydrogenation to ethane.[39]. The palladium particle size analyzed using the TEM shows that Pd/α-Al2O3 catalyst has larger Pd particles compared to the other catalysts. Researches says that as the Pd particle size increases for the Pd on the surface, the selectivity increases [40]. The carbonaceous hydrocarbon deposits increase the mass transfer limitation on the catalyst surface limiting the dissociation mechanisms and increases the hydrogen concentration on the catalyst surface temporarily favoring over hydrogenation [41, 42].
Temperature programmed oxidation (TPO) of spent catalysts
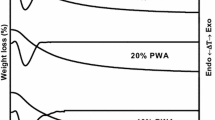
To study the TPO, the spent catalysts were powdered and subjected to thermogravimetric analysis in air with a ramp rate of 10 °C/ min and up to 1000 °C. The mass spectrometer was connected to TGA machine and the mass fractions corresponding to CO2 (44) and H2O (18 and 17) were monitored to understand the weight loss was due to coking or heavy hydrocarbon formation on the catalysts during the reaction (Fig. 7).
The weight loss in the thermogravimetric analysis for the spent catalyst could be due to the heavy hydrocarbon deposit on the catalyst. The mass spectroscopy data shows evolution of CO2 (mass number 44) and evolution of mass fraction corresponding to H2O (m/e 18). Figs. 8 and 9 indicate the evolution of CO2 and H2O for the different spent catalysts. Data show that combustion profiles are different and hence the nature of carbonaceous species formed on the catalysts with different supports are different in nature.
The weight loss due to heavy component formation can be directly correlated with the catalyst activity and catalyst selectivity. Catalyst with highest activity and the lowest selectivity has attributed to higher heavy formation (Table 6). The catalysts Pd/γ-Al2O3 and Pd/δ-Al2O3 has the highest weight loss in the region 200 to 600 °C. The catalyst, Pd on α-Al2O3, with the highest selectivity showed the lowest weight loss in the region 200 to 600 °C. The Pd/ρ-Al2O3 has lower acidity compared to the other supports contributing to low heavy formation compared to Pd /δ & Pd/γ-Al2O3 catalysts. The catalyst with high acidity has high carbon deposit and has lower ethylene selectivity as it favors over hydrogenation due to the hydrogen entrapped in the sub surface [43,44,45,46].
Conclusion
At temperatures below 100 °C, the catalyst prepared over α-Al2O3 observed to be more selective with the model feed. The better selectivity of the α-Al2O3 based catalyst could be due to the lower acidity of the support and lower penetration depth of the metal on the support. The beta hydride formation was found to be low at the operating temperature and hence better hydrogen dissociation could be achieved. The lower residence time for hydrogen on the support might have attributed to the higher selectivity. Higher temperature operation lowers the ethylene selectivity of the catalysts based on γ, δ and α-Al2O3, whereas the catalyst based on the ρ-Al2O3 support shows slightly better ethylene selectivity at higher temperature. The support acidity and palladium penetration depth of the catalyst plays vital role in the selectivity and conversion of the catalyst. The catalyst with the most dispersed active metal shows the highest conversion, here the γ-Al2O3 based catalyst has the most dispersed palladium, as it has the highest penetration depth. Thermogravimetric analysis of the spent samples were supporting the catalyst performance data, the highly active and less selective catalysts have higher weight loss at 200 to 600 °C. The mass spectroscopy data confirm the evolution of carbon dioxide at temperatures 200 to 600 °C, the higher weight loss was for the catalyst with low selectivity, the γ-Al2O3 and δ-Al2O3 based catalysts have higher weight loss at the mentioned temperature. The α-Al2O3 based catalyst has the lowest weight loss for the spent sample, which clearly supports the ethylene selectivity of the catalyst. The modern day bimetallic catalysts are based on the α-Al2O3 with Ag or Au added on to the catalyst for higher selectivity. The study done for acetylene hydrogenation in nitrogen environment gives information on the ideal behavior of the catalyst for selective hydrogenation of acetylene to ethylene.
References
Tiedtke DB, Bergmeister JJ, Cheung TP, Rhoades RA (2001) Progress in the development of E-Series catalyst technologies for the selective hydrogenation of acetylene in various hydrocarbon streams. Thai Olefins Ethylene Technology Forum
McCue AJ, Anderson JA (2015) Recent advances in selective acetylene hydrogenation using palladium containing catalysts. Front Chem Sci Eng 9:142–153. https://doi.org/10.1007/s11705-015-1516-4
Takht Ravanchi M, Fadaeerayeni S, Rahimi Fard M (2014) An egg-shell Pd-Ag/α-Al2O3 catalyst for tail-end acetylene selective hydrogenation. IJChE 11(1):42–54
Sivaraj CH, Contescue CR, Schwarz JA (1991) Effect of calcination temperature of alumina on the adsorption/impregnation of Pd(II) compounds. J Catal 132(2):422–431. https://doi.org/10.1016/0021-9517(91)90159-2
Hill MR, Bastow TJ, Celotto S, Hill AJ (2007) Integrated study of the calcination cycle from gibbsite to corundum. Chem Mater 19(11):2877–2883. https://doi.org/10.1021/cm070078f
Boumaza A, Favaro L, Lédion J et al (2009) Transition alumina phases induced by heat treatment of boehmite: An X-ray diffraction and infrared spectroscopy study. J Solid State Chem 182(5):1171–1176. https://doi.org/10.1016/j.jssc.2009.02.006
Takht Ravanchi M, Rahimi Fard M, Fadaeerayeeni S, Yaripour F (2015) Effect of calcination conditions on crystalline structure and pore size distribution for a mesoporous alumina. Chem Eng Commun 202(4):493–499. https://doi.org/10.1080/00986445.2013.850577
Chang PL, Yen FS, Cheng KC, Wen HL (2001) Examinations on critical and primary crystallite sizes during θ- to α-phase transformations of ultrafine alumina powders. Nano Lett 1(5):253–261. https://doi.org/10.1021/nl015501c
Iaponeide M, Macedo F, Aparecido Bertran C, Osawa CC (2007) Kinetic of the γ-to α-alumina transformation by quantitative X-ray diffraction. J Mater Sci 42:2830–2836. https://doi.org/10.1007/s10853-006-1364-1
Perander LM, Zujovic ZD, Groutso T, Hyland MM, Smith ME, O’Dell LA, Metson JB (2007) Characterization of metallurgical-grade aluminas and their precursors by 27Al NMR and XRD. Can J Chem 85(10):889–897. https://doi.org/10.1139/v07-106
Application note 134 https://www.micromeritics.com/Repository/Files/apnote134.pdf.
Gopinath R, Babu NS, Kumar JV, Lingaiah N, Prasad PS (2008) Influence of Pd precursor and method of preparation on hydrodechlorination activity of alumina supported palladium catalysts. Catal Lett 120:312–319. https://doi.org/10.1007/s10562-007-9287-2
Zhu Q, Gao J, Chen J, Wen L (2010) Selective hydrogenation of acetylene over egg-shell palladium nanocatalyst. J Nanosci Nanotechnol 10(9):5641–5647. https://doi.org/10.1166/jnn.2010.2472
Storozhenko PA, Aleshin AI, Douganyuk VF (2010) Selecting heat treatment conditions of alumina supports to improve the quality of selective hydrogenation palladium catalysts. Catal Ind 2:282–286. https://doi.org/10.1134/S207005041003013X
Khan NA, Shaikhutdinov S, Freund HJ (2006) Acetylene and ethylene hydrogenation on alumina supported Pd-Ag model catalysts. Catal Lett 108:159–164. https://doi.org/10.1007/s10562-006-0041-y
Ludwig W, Savara AA, Dostert K-H, Schauermann S (2011) Olefin hydrogenation on Pd model supported catalysts: new mechanistic insights. J Catal 284(2):148–156. https://doi.org/10.1016/j.jcat.2011.10.010
Paryjczak T, Rynkowski J (1984) Temperature-programmed reduction and temperature-programmed oxidation of nickel and copper-nickel catalysts with addition of palladium supported on alumina. React Kinet Mech Cat 24:187–191
Application note 120 “Temperature-Programmed Reduction Using the AutoChem” https://www.micromeritics.com/Repository/Files/appnote120.pdf
Sorbier L, Gay A-S, Fécant A, Moreaud M, Brodusch N (2013) Measurement of palladium crust thickness on catalysts by optical microscopy and image analysis. Microsc Microanal 19(2):293–299. https://doi.org/10.1017/S1431927612014316
Dehghani O, Rahimpour MR, Shariati A (2019) An experimental approach on industrial Pd-Ag supported α-Al2O3 catalyst used in acetylene hydrogenation process: mechanism, kinetic and catalyst decay. MDPI Process 27(3):136. https://doi.org/10.3390/pr7030136
Takht Ravanchi M, Fadaeerayeni S, Rahimi Fard M (2017) Acetylene selective hydrogenation: a technical review on catalytic aspects. Rev Chem Eng 34(2):215–237. https://doi.org/10.1515/REVCE-2016-0036
Komhom S, Mekasuwandumrong O, Praserthdam P, Panpranot J (2008) Improvement of Pd/Al2O3 catalyst performance in selective acetylene hydrogenation using mixed phases Al2O3 support. Catal Commun 10(1):86–91. https://doi.org/10.1016/j.catcom.2008.07.039
Taromi AA, Kaliaguine S (2017) Synthesis of ordered mesoporous γ-alumina: effects of calcination conditions and polymeric template concentration. Microporous Mesoporous Mater 248:179–191. https://doi.org/10.1016/j.micromeso.2017.04.040
Macêdo MIF, Bertran CA, Osawa CC (2007) Kinetics of the γ → α-alumina phase transformation by quantitative X-ray diffraction. J Mater Sci 42:2830–2836. https://doi.org/10.1007/s10853-006-1364-1
Ravanchi MT, Fadaeerayeni S, Fard MR (2016) The effect of calcination temperature on physicochemical properties of alumina as a support for acetylene selective hydrogenation catalyst. Res Chem Intermed 4(2):4797–4811. https://doi.org/10.1007/s11164-015-2320-y
Sung DM, Kim YH, Park ED, Yie JE (2010) Correlation between acidity and catalytic activity for the methanol dehydration over various aluminum oxides. Res Chem Intermed 36:653–660. https://doi.org/10.1007/s11164-010-0201-y
Standard Practice for Calculation of Pore Size Distributions of Catalysts and Catalyst Carriers from Nitrogen Desorption Isotherms, ASTM D4641-1. https://www.quantachrome.com/standards.html
Vaidya SD, Thakkar NV (2001) Effect of temperature, pH and ageing time on hydration of rho alumina by studying phase composition and surface properties of transition alumina obtained after thermal dehydration. Mater Lett 51(4):295–300. https://doi.org/10.1016/S0167-577X(01)00307-X
Bhogeswararao S, Srinivas D (2015) Catalytic conversion of furfural to industrial chemicals over supported Pt and Pd catalysts. J Catal 327:65–77. https://doi.org/10.1016/j.jcat.2015.04.018
Wongwaranom N, Mekasuwandumrong O, Praserthdam P, Panpranot P (2008) Performance of Pd catalysts supported on nanocrystalline α-Al2O3 and Ni-modified α-Al2O3 in selective hydrogenation of acetylene. Catal Today 131(1–4):553–558. https://doi.org/10.1016/j.cattod.2007.10.036
Duca D, Varga Z, La Manna G, Vidoczy T (2000) Hydrogenation of acetylene-ethylene mixtures on Pd catalysts: study of the surface mechanism by computational approaches, metal dispersion and activity of the catalyst. Theor Chem Acc 104:302–311. https://doi.org/10.1007/s002140000123
Komeilia S, Ravanchib MT, Taeb A (2015) The influence of alumina phases on the performance of the Pd–Ag/Al2O3catalyst in tail-end selective hydrogenation of acetylene. Appl Catal A 502:287–296. https://doi.org/10.1016/j.apcata.2015.06.013
Bos ANR, Westerterp KR (1993) Mechanism and kinetics of the selective hydrogenation of ethyne and ethane. Chem Eng Process 32(1):1–7. https://doi.org/10.1016/0255-2701(93)87001-B
Gislason J, Xia W, Sellers H (2002) Selective hydrogenation of acetylene in an ethylene rich flow: results of kinetic simulation. J Phys Chem 106(5):767–774. https://doi.org/10.1021/jp011238s
Battiston GC, Dalloro L, Tauszik GR (1982) Performance and aging of catalysts for the selective hydrogenation of acetylene: a micropilot-plant study. Appl Catal 2(1–2):1–17. https://doi.org/10.1016/0166-9834(82)80170-X
Ball MR, Rivera-Dones KR, Gilcher EB, Ausman SF, Hullfish CW, Lebrón EA, Dumesic JA (2020) AgPd and CuPd catalysts for selective hydrogenation of acetylene. ACS Catal 10(15):8567–8581. https://doi.org/10.1021/acscatal.0c01536
HeupMoon S-J (2011) Performance of Cu-promoted Pd catalysts prepared by adding Cu using a surface redox method in acetylene hydrogenation. Appl Catal A 401(1–2):12–19. https://doi.org/10.1016/j.apcata.2011.04.048
Zou S et al (2021) Grafting nanometer metal/oxide interface towards enhanced low-temperature acetylene semi-hydrogenation. Nat Commun 12:5570. https://doi.org/10.1038/s41467-021-25984-8
Feng J-T, Ma X-Y, Evans DG, Li D-Q (2011) Enhancement of metal dispersion and selective acetylene hydrogenation catalytic properties of a supported Pd catalyst. Ind Eng Chem Res 50(4):1947–1954. https://doi.org/10.1021/ie101508z
Ibhadon AO, Kansal SK (2018) The reduction of alkynes over Pd-based catalyst materials: a pathway to chemical synthesis. J Chem Eng Process Technol. https://doi.org/10.4172/2157-7048.1000376
Pradier CM, Mazina M, Berthier Y, Oudar J (1994) Hydrogenation of acetylene on palladium. J Mol Catal 89(1–2):20211–20220. https://doi.org/10.1016/0304-5102(93)E0323-9
Ravanchi MT, Sahebdelfar S, Komeili S (2018) Acetylene selective hydrogenation: a technical review on catalytic aspects. Rev Chem Eng 34(2):215–237. https://doi.org/10.1515/revce-2016-0036
Ravanchi MT, Sahebdelfar S, Fard MR, Fadaeerayeni S, Bigdeli P (2016) Pd-Ag/α-Al2O3 catalyst deactivation in acetylene selective hydrogenation. Chem Eng Process 39(2):301–310. https://doi.org/10.1002/ceat.201400526
Wang Z, Du W, Qian F, Tian L, Jiang D (2015) Improve acetylene hydrogenation selectivity using dynamic deactivation estimation. Hydrocarbon processing. Gulf Publishing Company, Houston
Kuhn M, Lucas M, Claus P (2015) Precise recognition of catalyst deactivation during acetylene hydrogenation studied with the advanced TEMKIN reactor. Catal Commun 72(5):170–173. https://doi.org/10.1016/j.catcom.2015.10.001
Samavatia M, Ebrahima HA, Dorjb Y (2018) Effect of the operating parameters on the simulation of acetylene hydrogenation reactor with catalyst deactivation. Appl Catal A 567:45–55. https://doi.org/10.1016/j.apcata.2018.06.038
Acknowledgements
The author (KR) acknowledges the support received from the Management and R&D colleagues of Sud-Chemie India (P) Ltd for all the support received in completing this study. The author acknowledges DST-SAIF Cochin for the microscopic data analysis.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
KR—main contributor for literature search, design, execution of the experiments and data interpretations involved in this study. GM—supervisor and research guide. VRR—contributed to the design and safety aspects of the test unit and the process. SR—contributed for the review of thermogravimetric studies and spent catalyst analysis.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Consent to participate
All authors participated in the creation of this manuscript.
Consent for publication
All authors agree to publish this manuscript in the journal.
Availability of data and materials
The authors confirm that the data and the findings mentioned in this study are available within the article and raw data and the derived data supporting the findings are available from the corresponding author on request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ravindran, K., Madhu, G., Renjith, V.R. et al. Influence of different alumina phases on the catalytic properties of palladium-alumina catalysts for selective hydrogenation of acetylene to ethylene. Reac Kinet Mech Cat 134, 867–882 (2021). https://doi.org/10.1007/s11144-021-02112-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02112-7