Abstract
Borax and its calcined derivatives were used in pyrolytic valorization of tire tube rubber to produce high value oil and gases. This work involved the preparation and characterization of catalysts and optimization of the reaction parameters for the catalytic and non-catalytic pyrolysis of waste tire tube rubber. The derivatives of borax catalyst were prepared by calcining it at 600 °C, 800 °C and 1000 °C. The borax derivatives were labeled as borax-600, borax-800 and borax-1000 and used for the pyrolysis of waste rubber. The outcomes of the catalytic and thermal pyrolysis processes were compared to check the effectiveness of the borax derivatives as a catalyst. Both processes produced different quantities of oil, gas and char specifically in case of borax catalyzed and borax-1000 catalyzed reactions. The oil product of thermal pyrolysis was composed of 15 compounds while borax catalyzed pyrolysis produced oil with 14 compounds. On the other hand, all borax derivatives produced oil having nine compounds. The oil contained aliphatic and cyclic hydrocarbons along with small quantities of sulfur and phosphorous containing organic compounds. The results of distillation showed that thermal pyrolysis produced 83.80% volatile oil while borax catalyzed pyrolysis produced 83.84% volatile oil. The oil content decreased to 66.34% with an increase in borax calcination temperature. Similarly, thermal pyrolysis produced 12.56% residue while borax catalyzed reaction produced 16.10% residue. The residue decreased slightly with a rise in calcination temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rubber is one of the life supporting materials. Its applications range from household items to vehicles and heavy industries. It is included in the polymers of choice used by the human being. Its production has been increased many times since its discovery. It is estimated by the International Rubber Study Group (IRSG) in 2017 that the consumption of rubber is 28.05 million tonnes [1]. Rubber is characterized for its excellent mechanical properties, stability and resistance to the attack of corrosive chemicals like acids in addition to its resistance to wear and tear. Its excellent properties are becoming a set back to the disposal of its waste. Its waste is bulky and occupying large spaces in the landfills. The resistance to biodegradation is a reason for its very long life leading to the environmental issues. There are a variety of methods for waste rubber disposal and pyrolysis-based tertiary recycling is one of the most effective methods. Pyrolysis of waste rubber is an environmentally beneficial and cost-effective technique of disposal of wastes that results in the recovery of a wide spectrum of hydrocarbons, such as aromatics, paraffin, cyclic hydrocarbons and olefins [2, 3].
Pyrolysis is a process of cracking of macromolecular skeleton of the rubber resulting in the formation of gases, combustible liquids and byproducts [4]. The thermal degradation of a material in the absence or with a limited supply of air is known as pyrolysis. Pyrolysis is used to convert the waste into smaller compounds through the breaking of carbon–hydrogen bonds, carbon–carbon bonds and sulfur–carbon bonds. Pyrolysis also involves recombination and quenching events through aromatization, cyclization and the creation of multiple bond functions, in addition to cracking [5, 6]. The pyrolysis is triggered by a rise in the process temperature, which results in the formation of a wide variety of chemicals. The conventional pyrolysis is often found ineffective for recovering value-added compounds from waste rubber and other materials, such as plastics and even biomass. One of the hottest areas in pyrolysis research is the valorization and upgradation of the pyrolysis products. A variety of strategies are used to improve the pyrolysis yield as well as the quality of the pyrolysis products. The reactor design, catalyst type, heating rate, process temperature, and other chemical additives influence these strategies [6]. Catalytic pyrolysis has been found to minimize the range of pyrolysis products or raise the concentration of specific molecules, making the recovery of valuable chemicals from the feedstock easier. The use of catalyst improves the yield and economy of the process [7, 8]. Shah et al. [9] converted rubber into a mixture of aliphatic and non-aliphatic hydrocarbons using silica and alumina as catalysts. Yu et al. [10] used modified zeolites and mesoporous catalyst impregnated with metals as a catalyst for the pyrolysis of rubber. They converted rubber into gaseous and liquid products [11,12,13,14].
Most of the pyrolysis reactions are commonly carried out using zeolites as a catalyst [15,16,17]. Zeolites involve carbo-cationic mechanism for the cracking of feedstock. Such mechanism not only minimize the reaction activation energy but also ensures uniform heat transfer. This catalyst also provides a feasible environment for obtaining specific type of products due to characteristic nature of the reaction. Borax has been used as catalyst for the pyrolysis of biomass to produce upgraded bio-oil by a number of workers [18,19,20,21]. Borax is characterized for the presence of two types of active sites, namely sodium cations and boron oxygen poly ions. The use of alkali metal ions as catalyst for pyrolysis has been reported by a number of research groups [22,23,24]. Therefore, the use of sodium ion of borax as catalyst may be explained based on the earlier reported work. In addition to the presence of active sodium ions, borax is characterized for the presence of boron atoms having empty orbitals. The presence of anionic and cationic sites in the borax facilitates pyrolysis reaction through interactive ions and empty orbitals, which destabilize the polymeric backbone through attraction toward the bonded pairs of electrons in a state of vibration at the high temperature. It is also believed that presence of boron atoms may stabilize the anionic and free radical moieties through cyclization. However, borax itself is thermally unstable and may pass through a number of transitions on heating. It has been reported by Waclawska [25] that thermal decomposition of borax involves dehydration, internal structure reconstitution, amorphization, gradual dehydroxylation and crystallization.
This work was conducted on the conversion of waste rubber into oil and fuel gas products using borax and calcined borax catalysts. This work also aimed to investigate transformations of catalytic activity under thermal treatment. One of the ideas behind these investigations was to test an effective and low-cost catalyst for enhancing the yield and quality of the pyrolysis products. Borax is a material characterized for different types of active sites. It has very active cation and anion combinations, which offer highly reactive ionic sites. It is also characterized by the presence of water of hydration, which offers unique chemical environment when attached to the lattice. In case of borax, the boron atoms offer OH as well as empty orbitals for catalysis. The exciting feature of borax is the presence of boron atoms, which have empty orbitals and di-oxygen linkages. This results in multifunctional catalytic sites and catalytic activities, which facilitate the formation of more than one type of bonds [26]. It also facilitates the condensation reactions and formation of several bonds in one step [27]. Borax is also used as a catalyst for the hydrothermal treatment and liquefaction of wood [18].
Unlike the previous work, the present work explores the catalytic activity of the thermally treated borax. It has been reported that borax may pass through a number of changes under the action of thermal energy. These transformations include the dehydration, internal structure reconstitution, loss of crystallinity, de-hydroxylation and crystallization of Na2O2B2O3 [28]. These transformations and rearrangements may be responsible for the change in catalytic behavior. To the best of our knowledge, the catalytic activity of the thermally treated borax for valorization of polymers has never been explored. The basicity of borax may increase the production of liquid products while structural and morphological characteristics may improve the product quality. The goal of this work is to improve the yield as well as the nature of the pyrolysis products.
Materials and methods
In this work, inner tube of the tire was pyrolyzed using borax and its calcined derivatives. The rubber samples were collected from a local tire repair shop in Mardan City of Pakistan. The rubber samples were washed, cleaned with detergent and dried in an oven. The dried large rubber pieces were cut into smaller dimensions for the pyrolysis.
Preparation of the catalyst
The borax of analytical grade was purchased from a local supplier of Merck and used as catalyst after grinding into fine powder using a custom-made crusher. The powder was sieved by using a 200 μm mesh. This powder was used as a starting material for the preparation of thermally transformed or calcined borax catalyst. Three types of the thermally treated catalyst were prepared by heating borax at 600 °C, 800 °C and 1000 °C in separate experiments. Each experiment was carried out in the pyrolysis furnace for 1 h. It was observed that borax forms a fused mass on heating and results in a reduction in the mass. The fused and calcined material was crushed and grinded into fine powder followed by sieving through 200 μm mesh. The prepared catalyst samples were characterized for surface morphology and functional groups by producing SEM micrographs and FTIR spectra.
Pyrolysis reactor
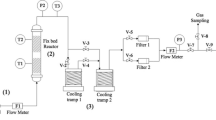
The pyrolysis of waste rubber was carried out in a custom-made reactor. This reactor is cylindrical in shape and made of stainless steel. The height of this batch reactor is 13.97 cm, and the internal diameter is 6.7 cm. The reactor has a lid which is associated with a side tube for the vapors produced during the pyrolysis. The length of the tube is 3.81 cm. The pyrolysis of rubber was carried out by placing the reactor in a pre-heated furnace at the set temperature. The furnace was a top loaded custom-made furnace powered by the temperature controller and equipped with a temperature sensor for obtaining set temperatures for the pyrolysis of rubber.
Pyrolysis procedure
The pyrolysis experiments were carried out using as supplied and thermally treated borax. Waste rubber was also pyrolyzed in the absence of a catalyst for comparison and evaluation of the catalytic activity of the catalysts under investigation. All these experiments were carried out in a batch type stainless steel reactor and under the distillation mode without using a carrier or inert gas. However, the pressure created in the reactor due to the vapors was utilized as a carrier. The catalytic pyrolysis was carried out by heating the mixture of catalyst and fine pieces of the waste rubber in a preheated furnace. At the start of the process, the reactor had some entrapped air. However, this air is replaced by vapors and gaseous products of the pyrolysis of waste rubber. Pyrolysis products are distilled out from the reactor via the side tube and condensed by using condensers and cold traps.
The reaction line also contains chemical traps for the qualitative analysis of the gaseous product of pyrolysis. The vapors are condensed while the gaseous products are allowed to escape after analysis. The chemical and cold traps are made of Pyrex glass tubing and properly connected with each other and reactor through the exits and inlets. The temperature of cold traps was kept below the ice temperature using freezing mixture of salt, calcium chloride and ice. The leak proof connection between the reactor, condenser and traps was ensured by the use of Teflon tubes. An aqueous ammoniacal solution of copper I chloride was used in the chemical trap next to the cold traps. This solution is used as spot test to analyze 1-alkynes and acetylene. Next to the alkyne trap was placed the trap for analysis of alkenes. This trap was filled with Bayer’s solution. The total gases product was determined from the difference while the amount of oil and solid products was weighed using analytical balance. This procedure was applied for optimizing the temperature for pyrolysis, heating time optimization and the relative weight of waste rubber and catalyst. Each of these experiments was conducted three times and the average of the three is reported in this paper.
Results and discussion
Analysis of the catalyst
The micrographs of borax, borax-600, borax-800 and borax-1000 are given in Fig. 1. Drastic changes can be seen in the morphology of borax with variation in the calcination temperature. Borax-600 can be seen in Fig. 1B, which is composed of a lump of irregular particles having pores of various sizes. Borax-800 is composed of particles of various sizes and wide pores while borax-1000 is composed of semi-crystalline material having pores of various sizes. This difference in morphology and porosity can be attributed to the extensive dehydration and bonds rearrangement specifically bonds of the boron atom. Furthermore, the difference in morphology and or chemical bonding is responsible for variation in the catalytic properties of these materials.
The chemical changes caused by the heat treatment of the catalyst at various temperatures were investigated using FTIR analysis of the borax. Table 1 summarizes the major FTIR peaks of borax catalysts. The number of peaks in borax-600 and untreated borax are almost same, however the position of peaks is significantly different. It shows changes in the bonding and relative groups. In case of borax-800 and borax-1000, there is a change in both the position and number of peaks which is the indicator of drastic changes in chemical composition.
Temperature optimization for thermal cracking of waste rubber
Almost all the pyrolysis reactions of the rubber are inspired by the temperature. In pyrolysis of rubber both the maximum conversion and formation of the useful oil and gas fraction are desirable and the amount of each of these products greatly dependents on the optimum temperature and rate of heating. It has been reported by the earlier workers that slow heating of the rubber and other polymers at relatively low temperature results the formation of large quantity of char and oil in comparison to the gases [29, 30]. In case of high temperature and fast rate of heating, large quantity of gaseous products is obtained. In this case, the reactions were carried out in a preheated furnace and the rate of heating was not considered. Temperature optimization studies are also important for assessment of the heat economy in order to ensure the low-cost of the process and maximum conversion rate. However, selection of the optimum temperature is based on the amount of char or residual solids, which is an indicator of conversion. The large quantity of residue refers to the less conversion. It can be observed from Fig. 2 that percentage conversion significantly changes with a change in temperature for the pyrolysis reaction. It can also be seen that percentage conversion varies with variation in the degree of dehydration of the catalyst. The FTIR spectra confirmed that untreated borax, borax-600 and borax-800 have ring structure although a difference in relative position of peaks suggested different compositions and sizes of the ring. The dehydration of dehydroxylation is also observed on heating. Motivated from this factor, this study is focused on the product distribution of the pyrolysis of waste rubber at different temperatures. It can further be observed from the results in Fig. 2 that the amount of product and percentage conversion remained lower for both catalyzed and un-catalyzed pyrolysis processes. This is a normal trend and is according to the previous reports on pyrolysis of rubber [22,23,24,25].
Almost all catalyzed pyrolysis processes are effective in terms of percentage conversion at optimum temperature. The untreated borax catalyzed process gives the highest oil yield. The thermal pyrolysis, borax-600 catalyzed pyrolysis and borax-800 catalyzed process give almost similar percentage oil yield, which is lower than borax and borax-1000 catalyzed reactions. The thermal pyrolysis of the rubber produces minimum gases while borax catalyzed process produces highest percentage of gases. It is also observed that the thermal pyrolysis and borax-600 catalyzed processes produce highest quantity of the solid residue. On the other hand, borax-1000 catalyzed process gives the lowest residue followed by borax catalyzed reaction and borax-800. This is due to effective heat transfer and the presence of active sites on the catalyst, which help lowering activation energy of the catalyzed reaction by ensuring better conversion efficiency.
In the case of dehydrated and calcined catalysts, some changes occur in the structure, porosity and even bonding of the catalyst. These changes are the reason for difference in heat transfer activity and the amount of the catalytic sites. The difference in the amount of residue is less at lower process temperatures except for borax catalyzed reactions. The reason for this difference is that borax is a hydrated compound and the water of hydration play role in catalyzing the reaction and conversion of waste rubber into oil and gas. The residue decreases with a decrease in the amount of water and an increase in the calcination temperature. It can be observed that the amount of residue for dehydrated borax is relatively greater while that for highly dehydrated borax and calcined borax is lesser. The reason for this trend may be the faster and effective heat transfer capacity of the calcined borax. The residue amount was used as an indicator for the efficiency of pyrolysis in terms of percentage conversion as:
Pyrolysis time optimization
The time optimization for a pyrolysis reaction is important, especially under optimum temperature condition. It is directly related to the power and time economy of the process. The data obtained may also be utilized for the determination of the kinetics of the reaction. Miranda and coworker determined the possible routes for the pyrolysis reactions of waste rubber using the reaction temperatures and times [32, 33]. Shaaban et al. [33] looked at how residence time and temperature affect the surface functional groups and porosities of biochar produced from sawdust and rubber pyrolysis. Time optimization studies may also determine the relative amount of the product fraction. Several researchers have studied the effect of reaction duration on the yield and product fraction of pyrolysis [33,34,35]. Pyrolysis at low temperatures for prolonged periods of time has been observed to cause secondary and tertiary cracking processes, resulting in the generation of gases and low molecular weight volatile chemicals. It also increases the amount of char. However, reactions carried out at high temperatures and for short periods of time may promote the formation of waxes and non-volatile compounds with a high molecular weight. In borax catalyzed pyrolysis, the cracking speed is believed to be faster and the temperature needed for the cracking may also come to a smaller value. In the present study, the pyrolysis reaction duration was investigated in the range of 15–75 min and the results are presented in Fig. 3. In this study, four types of the borax catalysts were used for the catalytic pyrolysis of waste rubber in separate experiments. The percentage conversion of the process increased with an increase in heating time from 15 to 30 min. The small difference between the catalytic and non-catalytic process was observed when temperature was high.
Catalyst weight optimization
For several processes including pyrolysis, the catalyst weight optimization is a critical parameter. In addition to cost, reactor load and reaction time economy, the optimal amount of catalyst ensures uniform heat transfer, selection of the maximum amount of the product fraction, selection of the particular chemical nature of the product such as aromatic and non-aromatic nature, and selection of the particular chemical nature of the product [35]. The present work is aimed to compare the catalytic activity of borax transformed into dehydrated form through thermal treatment. It was assumed that the number of water molecules present for hydration in the borax may play role in fixing its catalytic behavior. It has been reported that dehydration of borax is responsible for structural and chemical changes and consequently the reactivity and catalytic activity of the resulting dehydrated catalyst [25]. The relative weight of each catalyst for the catalytic pyrolysis will be different according to the nature of the catalyst. In this study, the relative weight of borax-based catalysts was varied according to the weight of the rubber. Each reaction was conducted at 500 °C for 30 min. The results of this study are presented in Fig. 3. Each fraction of the products of pyrolysis is given, however the residue or solid product was considered as an indicator for the progress of the pyrolysis. A change in the relative ratio of each catalyst is responsible for variation in quantity of the residue of the reaction. It can be seen from the results that when the weight of catalyst and rubber are in 1:1 ratio, the amount of residue is the lowest for borax catalyzed reaction (39.5%). The residue was highest for the borax calcined at 600 °C followed by the borax calcined at 800 °C and 1000 °C. The increased quantity of the residue is not due to the incomplete reaction but is because of the faster pyrolysis and char formation, which is greater for the borax-600 due to the dehydration and less changes in chemical composition. The amount of residue was found to decrease till 1:6 ratios. However, a decrease in weight of residue was small in the case of borax-600 catalyzed reaction. The same pattern was observed in the case of borax-800 (Table 2).
Fig. 4 shows the distribution of gas for various catalysts with variations in the amount of the catalyst. It can be seen that the amount of gases increases with an increase in the calcination temperature. In case of borax catalyst, the amount of gases was increased for 3:1 ratio. This tendency can be attributed to the number of particles of catalyst in contact with the feed and the extent of cracking reactions. When the quantity of borax in relation to feed is greater, the extent of cracking is greater but there is formation of solids as well. However, at decreasing concentration of catalyst, the rate of cracking of vapors rather than the feed is greater since the catalyst remains suspended. A further decrease in the amount of catalyst leads to a decrease in percentage conversion due to the non-availability of appropriate number of catalyst sites. In case of dehydrated catalysts, the case was reversed because of the role of hydration of borax in cracking process. It can also be seen that the amount of gases goes to highest ratio when the ratio of feed to catalyst is 8:1 for borax-600 although almost equal to the borax-800 and borax-1000. The amount of gases was found highest for more dehydrated catalyst at larger ratios of feed to catalyst.
Distribution of gases with a change in catalyst weight. The weight of the uncalcined borax, borax-600, borax-800 and borax-1000 was varied by fixing the pyrolysis temperature and time at 500 °C and 30 min (Table 5)
Distillation of pyrolysate of rubber
Using a laboratory scale distillation device, the oil recovered from each pyrolysis reaction was fractionated. The fractional distillation of each sample was carried out using 100 g of the oil sample. It was observed that each sample gives distillates in the boiling point range of 38–188 °C. Table 3 lists the boiling points and proportional amounts of each fraction. The oily produce was separated into liquid fractions, combustible gases and residue. The liquid products of condensed vapors were collected by allowing the gases to escape. The residue was tarry materials, which solidify into wax like material on cooling. Further it was observed that residues of all the samples were dark brown in color. The liquid product of non-catalyzed reaction contained 83.80% volatile oil, the borax catalyzed process gives 83.84%, the borax-600 gives 67.37%, borax-800 gives 66.34% and borax-1000 °C gives 85.23% volatile oil. The amount of residue for non-catalyzed process was 12.56%, 16.10% for borax-600, 32.57% for borax-800, and 14.72% for borax-1000. The difference in residue of catalyzed and non-catalyzed process can be attributed to the stability of the oil samples produced by the catalytic activity of the borax. The amount of dissolved gases are greater for the non-catalytic process than the catalytic process due to the presence of free radical species in the oil of non-catalytic process, which are responsible for the secondary and tertiary reactions during distillation to form gaseous product. The oil of catalyzed process contained greater fractions of volatiles as compared to the non-catalytic process. These results show that borax and its derivatives are effective for the cracking of rubber in terms of producing more stable and volatile fraction of oil product.
GC–MS analysis of oil
The oil product of catalytic pyrolysis reactions was compared with non-catalytic pyrolysis in terms of the chemical composition. The chemical composition of oils was determined using GC–MS analysis. The results of this study are presented in Tables 4 and 5 for catalytic and non-catalytic pyrolysis processes. The oil samples were composed of a major fraction of hydrocarbon in addition to small quantities of sulfur and phosphorous containing compounds. It was observed that the number of compounds is greater in the oil product of non-catalyzed process due to randomness of the cracking reaction. The nature of six most abundant compounds is the same for non-catalytic and catalytic pyrolysis with slight variations in concentration. The difference in concentration is most prominent for oil obtained from the borax-600 catalyzed reaction. The oil obtained by non-catalytic process is composed of 15 compounds while the borax catalyzed process contains 14 compounds. In case of the borax-600 and borax-800 catalyzed reactions, the oil is composed of nine compounds. However, the nature of some of the compounds differs. The borax-1000 catalyzed reaction produced 10 compounds.
The oil from non-catalytic pyrolysis is composed of alkanes, cycloalkane and different alkyl acids, alkyl sulfides and few alkyl phosphate groups. The borax catalyzed reactions produced oil composed of alkanes, cycloalkanes, alkyl alkenes, alkyl sulfide, and cyclic alkyl phosphate and cyclic organic alkyl acids. Oil product of the borax-600 catalyzed reactions contains more compounds of alkanes and cycloalkanes, few alkenes, cyclic alkyl organic acids and cyclic alkyl phosphate groups. However, the alkenes number is greater than alkane. In case of borax-800, the oil is composed of alkane, cycloalkane, cyclic alkyl sulfide, cyclic alkyl phosphate, and cyclic alkyl organic acids. The oil product of borax-1000 is composed of alkanes, cycloalkanes, alkene, cyclic alkyl phosphate, and cyclic alkyl organic acids. It can further be seen that the composition of each oil is similar in terms of the presence of six same compounds. These are mainly C6 and C7 compounds. It was also observed that the contribution of 1–6 compounds is slightly different from each other i.e. 94.06% for thermal, 94.15% for the borax catalyzed, 96.21% for borax-600, 96.8% for borax-800 and 95.27% for borax-1000 catalyzed process. The presence of sulfur containing compounds in the oil is due to the sulfur of rubber used for the cross linking of rubber while that of phosphorous is due to the additives of rubber. Unlike all the previous works, this oil was found to contain fuel products rather than the monomers.
Conclusions
Borax was converted into structurally, morphologically and chemically different materials by varying the calcination temperature. The calcined products were obtained by heating borax at 600 °C, 800 °C and 1000 °C. The SEM and FTIR data showed that these materials have prominent difference in their chemical nature and morphology. FTIR data suggested ring structure for borax, borax-600 and borax-800 samples while borax-1000 did not contain ring of oxy-borons. The catalytic behavior of the borax-1000 was significantly different from the rest of the catalyst samples. The SEM analysis also showed difference in crystallinity of the catalyst samples. The use of borax and its derivatives as a catalyst was successfully explored for the pyrolysis of tube rubber. Each catalyst showed different activity in terms of the relative quantities of oil, gas and residue. Borax catalyzed process produced highest percentage of oil. The borax-1000 also performed reasonably good to produce oil due to its crystalline nature. In case of borax-600 and borax-800, the amount of oil was almost similar due to similarity in their solid structure. It was also noted that non-catalytic pyrolysis produces smaller quantities of gases while borax catalyzed process produces highest percentage of gases. The borax-600 catalyzed process gave highest quantity of the solid residue while borax-1000 catalyzed process yielded lowest residue. It is also noticed that the catalyst lowers the reaction temperature and time. However, these parameters were kept according to those optimized for the non-catalytic pyrolysis. The results from fractional distillation demonstrate that as the catalyst's calcination temperature changes, the relative amount of the volatile fraction changes as well. Differences were also observed in the chemical composition and number of compounds in oil products for the non-catalytic and catalytic pyrolysis. It was observed that liquid product of non-catalytic process contained 15 compounds. Similarly, the borax catalyzed process produced oil with 14 compounds, borax-600 and borax-800 catalyzed reactions produced liquid of 9 compounds. However, some compounds differ in nature. For borax–1000, the oil contains 10 compounds. Differences were also observed in the relative concentration and nature of compounds of each of the oil. These observations support the idea of changing the catalytic activity with variation in calcination temperature.
Data availability
The data used in the paper is available from the corresponding author on request.
References
Nuzaimah M, Sapuan SM, Nadlene R, Jawaid M (2018) Recycling of waste rubber as fillers: a review. Mater Sci Eng 368:012016. https://doi.org/10.1088/1757-899X/368/1/012016
Ding K, Zhong Z, Zhang B, Wang J, Min A, Ruan R (2016) Catalytic pyrolysis of waste tire to produce valuable aromatic hydrocarbons: an analytical Py-GC/MS study. J Anal Appl Pyrolysis 122:55–63. https://doi.org/10.1016/j.jaap.2016.10.023
Rathsack P, Riedewald F, Sousa-Gallagher M (2015) Analysis of pyrolysis liquid obtained from whole tyre pyrolysis with molten zinc as the heat transfer media using comprehensive gas chromatography mass spectrometry. J Anal Appl Pyrolysis 116:49–57. https://doi.org/10.1016/j.jaap.2015.10.007
Yang Q, Yu S, Zhong H, Liu T, Yao E, Zhang Y, Zou H, Du W (2021) Gas products generation mechanism during co-pyrolysis of styrene–butadiene rubber and natural rubber. J Hazard Mater 401:123302. https://doi.org/10.1016/j.jhazmat.2020.123302
Milani G, Milani F (2018) Quasi-analytical kinetic model for natural rubber and polybutadiene rubber blends. Reac Kinet Mech Cat 123:351–365
Isahak WNRW, Hisham MW, Yarmo MA, Hin TYY (2012) A review on bio-oil production from biomass by using pyrolysis method. Renew Sustain Energy Rev 16(8):5910–5923. https://doi.org/10.1016/j.rser.2012.05.039
Luo S, Feng Y (2017) The production of fuel oil and combustible gas by catalytic pyrolysis of waste tire using waste heat of blast-furnace slag. Energy Convers Manag 136:27–35. https://doi.org/10.1016/j.enconman.2016.12.076
Duan D, Zhang Y, Wang Y, Lei H, Wang Q, Ruan R (2020) Production of renewable jet fuel and gasoline range hydrocarbons from catalytic pyrolysis of soap stock over corn cob-derived activated carbons. Energy 209:118454. https://doi.org/10.1016/j.energy.2020.118454
Shah J, Jan MR, Mabood F (2009) Recovery of value-added products from the catalytic pyrolysis of waste tyre. Energy Convers Manag 50(4):991–994. https://doi.org/10.1016/j.enconman.2008.12.017
Yu J, Liu S, Cardoso A, Han Y, Bikane K, Sun L (2019) Catalytic pyrolysis of rubbers and vulcanized rubbers using modified zeolites and mesoporous catalysts with Zn and Cu. Energy 188:116117. https://doi.org/10.1016/j.energy.2019.116117
Qu B, Li A, Qu Y, Wang T, Zhang Y, Wang X, Gao Y, Fu W, Ji G (2020) Kinetic analysis of waste tire pyrolysis with metal oxide and zeolitic catalysts. J Anal Appl Pyrolysis 152:104949. https://doi.org/10.1016/j.jaap.2020.104949
Hijazi A, Boyadjian C, Ahmad MN, Zeaiter J (2018) Solar pyrolysis of waste rubber tires using photoactive catalysts. Waste Manag 77:10–21. https://doi.org/10.1016/j.wasman.2018.04.044
Singh MV (2020) Conversions of waste tube-tyres (WTT) and waste polypropylene (WPP) into diesel fuel through catalytic pyrolysis using base SrCO3. Eng Sci 13:87–97. https://doi.org/10.30919/es8d1158
Demirbas A, Al-Sasi BO, Nizami AS (2016) Conversion of waste tires to liquid products via sodium carbonate catalytic pyrolysis. Energy Source A 38(16):2487–2493. https://doi.org/10.1080/15567036.2015.1052598
Wang J, Jiang J, Wang X, Liu P, Li J, Liu G, Wang K, Li M, Zhong Z, Xu J, Ragauskas AJ (2019) Catalytic conversion of rubber wastes to produce aromatic hydrocarbons over USY zeolites: effect of SiO2/Al2O3 mole ratio. Energy Convers Manag 197:111857. https://doi.org/10.1016/j.enconman.2019.111857
Salmasi SSZ, Abbas-Abadi MS, Haghighi MN, Abedini H (2015) The effect of different zeolite based catalysts on the pyrolysis of poly butadiene rubber. Fuel 160:544–548. https://doi.org/10.1016/j.fuel.2015.07.091
Elordi G, Olazar M, Aguado R, Lopez G, Arabiourrutia M, Bilbao J (2007) Catalytic pyrolysis of high density polyethylene in a conical spouted bed reactor. J Anal Appl Pyrolysis 79(1–2):450–455. https://doi.org/10.1016/j.jaap.2006.11.010
Tekin K, Akalın MK, Bektaş S, Karagöz S (2013) Hydrothermal wood processing using borax decahydrate and sodium borohydride. J Anal Appl Pyrolysis 104:68–72. https://doi.org/10.1016/j.jaap.2013.09.008
Yüceda E, Durak H (2019) Bio-oil and bio-char from Lactuca serriola: significance of catalyst and temperature for assessing yield and quality of pyrolysis. Energy Source A. https://doi.org/10.1080/15567036.2019.1645765
Wang Z, Qu L, Qian J, He Z, Yi S (2019) Effects of the ultrasound-assisted pretreatments using borax and sodium hydroxide on the physicochemical properties of Chinese fir. Ultrason Sonochem 50:200–207. https://doi.org/10.1016/j.ultsonch.2018.09.017
Durak H (2016) Pyrolysis of Xanthium strumarium in a fixed bed reactor: effects of boron catalysts and pyrolysis parameters on product yields and character. Energy Source A 38(10):1400–1409. https://doi.org/10.1080/15567036.2014.947446
Xu Q, Ma X, Yu Z, Cai Z (2014) A kinetic study on the effects of alkaline earth and alkali metal compounds for catalytic pyrolysis of microalgae using thermogravimetry. Appl Therm Eng 73(1):357–361. https://doi.org/10.1016/j.applthermaleng.2014.07.068
Jeong K, Jeong HJ, Lee G, Kim SH, Kim KH, Yoo CG (2020) Catalytic effect of alkali and alkaline earth metals in lignin pyrolysis: a density functional theory study. Energy Fuels 34(8):9734–9740. https://doi.org/10.1021/acs.energyfuels.0c01897
Giudicianni P, Gargiulo V, Grottola CM, Alfè M, Ragucci R (2018) Effect of alkali metal ions presence on the products of xylan steam assisted slow pyrolysis. Fuel 216:36–43. https://doi.org/10.1016/j.fuel.2017.11.150
Waclawska I (1995) Thermal decomposition of borax. J Therm Anal Calorim 43(1):261–269. https://doi.org/10.1007/BF02635993
He L, Szopinski D, Wu Y, Luinstra GA, Theato P (2015) Toward self-healing hydrogels using one-pot thiol–ene click and borax-diol chemistry. ACS Macro Lett 4(7):673–678. https://doi.org/10.1021/acsmacrolett.5b00336
Molla A, Hussain S (2014) Borax catalyzed domino reactions: synthesis of highly functionalised pyridines, dienes, anilines and dihydropyrano [3, 2-c] chromenes. RSC Adv 4(56):29750–29758. https://doi.org/10.1039/C4RA03627A
Sahin O, Bulutcu AN (2003) Evaluation of thermal decomposition kinetics of borax pentahydrate using genetic algorithm method by isothermal analysis. Turk J Chem 27:197–207
Mkhize NM, Van der Gryp P, Danon B, Görgens JF (2016) Effect of temperature and heating rate on limonene production from waste tyre pyrolysis. J Anal Appl Pyrolysis 120:314–320. https://doi.org/10.1016/j.jaap.2016.04.019
Barbooti MM, Mohamed TJ, Hussain AA, Abas FO (2014) Optimization of pyrolysis conditions of scrap tires under inert gas atmosphere. J Anal Appl Pyrolysis 72(1):165–170. https://doi.org/10.1016/j.jaap.2004.05.001
Miranda M, Pinto F, Gulyurtlu I, Cabrita I (2013) Pyrolysis of rubber tyre wastes: a kinetic study. Fuel 103:542–552. https://doi.org/10.1016/j.fuel.2012.06.114
Shaaban A, Se SM, Dimin MF, Juoi JM, Husin MHM, Mitan NMM (2014) Influence of heating temperature and holding time on biochars derived from rubber wood sawdust via slow pyrolysis. J Anal Appl Pyrolysis 107:31–39. https://doi.org/10.1016/j.jaap.2014.01.021
Inguanzo M, Domınguez A, Menéndez JA, Blanco CG, Pis JJ (2002) On the pyrolysis of sewage sludge: the influence of pyrolysis conditions on solid, liquid and gas fractions. J Anal Appl Pyrolysis 63(1):209–222. https://doi.org/10.1016/S0165-2370(01)00155-3
Mohamed BA, Ellis N, Kim CS, Bi X (2019) Microwave-assisted catalytic biomass pyrolysis: effects of catalyst mixtures. Appl Catal B 253:226–234. https://doi.org/10.1016/j.apcatb.2019.04.058
López A, De Marco I, Caballero BM, Laresgoiti MF, Adrados A, Aranzabal A (2011) Catalytic pyrolysis of plastic wastes with two different types of catalysts: ZSM-5 zeolite and Red Mud. Appl Catal B 104(3–4):211–219. https://doi.org/10.1016/j.apcatb.2011.03.030
Acknowledgements
The authors would also like to acknowledge the efforts of King Khalid University, Saudi Arabia (Deanship of Scientific Research) for support through the Research Groups Project under the Grant Number (R.G.P.2/169/42).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest regarding publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, A., Hussain, Z., Hussain, K. et al. Evaluation of the catalytic activity of borax and its calcined derivatives for pyrolytic valorization of waste tire tube rubber for production of oil and gases. Reac Kinet Mech Cat 134, 883–901 (2021). https://doi.org/10.1007/s11144-021-02108-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02108-3