Abstract
Y2O3 nanorods were prepared via a hydrothermal method. A series of Sr-modified Y2O3 nanorods (Sr–Y2O3–NR) with a Sr/Y molar ratio of 0.02–0.06 were synthesized by an impregnation method, and studied with respect to their performance in the oxidative coupling of methane (OCM). The structural and physicochemical properties of these catalysts were characterized by means of XRD, N2 adsorption, SEM, TEM, XPS, O2-TPD and CO2-TPD. Y2O3 nanorods exhibit higher CH4 conversion and C2–C3 selectivity relative to Y2O3 nanoparticles, which could link with the fact that Y2O3 nanorods predominantly expose (440) and (222) planes. The addition of a small amount of Sr to Y2O3 nanorods enhances the activation of oxygen, the ratio of (O− + O2−)/O2− and amount of moderate basic sites for the Sr–Y2O3-NR catalysts, thus promoting the OCM performance. The best 0.04Sr–Y2O3-NR catalyst with a Sr/Y molar ratio of 0.04 can give a 23.0% CH4 conversion with 50.2% C2–C3 selectivity at 650 °C. We found that the C2–C3 yield achieved on the Y2O3-based catalysts correlated well with the amount of moderate basic sites present on the catalysts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Catalytic conversion of methane to value added products has attracted much attention in the past few decades [1,2,3,4,5,6,7,8,9,10,11,12,13]. The proven reserve of natural gas, with its major component CH4, has increased markedly from 1996 to 2016, and therefore providing great motivation in methane utilization. There is no doubt that oxidative coupling of methane (OCM) is one of the most prospective directions among the various conversion of methane, since Keller et al. [14] first reported this technology in 1982. The main products of OCM reaction are ethane and ethylene. Ethylene, one of the chemical products with the largest output in the world, has been regarded as one of the important indicators to measure the development level of a country’s petrochemical industry [15]. Hence, a wide range of catalysts have been attempted on the OCM reaction [1, 2, 7, 10].
Recently, researchers have shifted the focus of study to the OCM process at relatively low temperatures. It is worth noting that rare earth oxide catalysts with special morphologies (e.g. nanorods, nanobelts and nanowires) such as La2O3 [16, 17], Sm2O3 [18] and CeO2 [19] can effectively catalyze low-temperature OCM reaction at 500–650 °C. To improve the C2 selectivity of OCM reaction, mixed or doped oxides with enhanced basicity such as alkali-rare earth oxides [20] and alkaline earth-rare earth oxides [21,22,23] were used. In addition to the basicity, introducing the low-valence metal into high-valence metal oxides can produce the surface defects to form electrophilic oxygen species such as O− and O2− which are conductive to improving the C2 selectivity. More recently, we have found that Er2O3 nanorods, Ho2O3 nanosheets and their Sr-promoted forms can act as effective catalysts for low-temperature OCM process [24, 25].
Takenaka et al. found that Li-added Y2O3 was the most effective catalyst for the OCM reaction among various basic metal oxide catalysts (MgO, Y2O3, La2O3, Gd2O3, Sm2O3, Eu2O3 and CeO2) modified with Li [20]. Although Y2O3-based catalysts used in the OCM reaction were reported, their catalytic performance at relatively low temperature was not satisfactory [20, 26,27,28]. Inspired by the aforementioned research results [16,17,18,19, 24, 25], in the present work we have developed Y2O3 and Sr-modified Y2O3 nanorods used as efficient catalysts for low-temperature OCM process. The catalytic performance of these catalysts was correlated with their characterization results.
Experimental
Catalyst preparation
Y2O3 nanorods (named as Y2O3-NR) were synthesized by a hydrothermal method. In a typical procedure, 3.83 g of Y(NO3)3·6H2O was dissolved in 100 mL deionized water. 5 mL aqueous ammonia (25–28 wt%) was then added dropwise to Y(NO3)3 solution under stirring. The resulting suspension was transferred into a Teflon-lined stainless autoclave, followed by being placed in an oven setting at 200 °C for 12 h. Y2O3 nanoparticles (labelled as Y2O3-NP) were synthesized by a conventional precipitate method. 3 mL aqueous ammonia (25–28 wt%) was added dropwise to 100 mL Y(NO3)3 solution (0.1 M) under stirring. All the obtained precipitates were fully washed with deionized water, dried at 80 °C for 12 h. Finally, the dried Y(OH)3 samples were calcined at 750 °C in air for 4 h to obtain Y2O3 nanorods and nanoparticles.
Sr-modified Y2O3 nanorods were synthesized by an incipient wetness impregnation method. Different amounts of Sr(NO3)2 were dissolved in deionized water, and then a certain amount of dried Y(OH)3 nanorods were added. After drying under an infrared lamp, the sample was dried at 80 °C for 12 h, then calcined at 750 °C in air for 4 h. The obtained catalysts were designated as xSr–Y2O3-NR, which x represents the Sr/Y molar ratio (x = 0.02, 0.04 and 0.06).
Catalyst characterization
X-ray diffraction (XRD) patterns were recorded on a D2 PHASER X-ray diffractometer using nickel-filtered Cu Kα radiation at 30 kV and 10 mA. The BET surface areas of the catalysts were measured by N2 adsorption at − 196 °C using a Micromeritics Tristar 3000 instrument. X-ray photoelectron spectroscopy (XPS) analyses were carried out with a Perkin–Elmer PHI 5000C spectrometer. All binding energy values were calibrated using the C 1 s peak at 284.6 eV. Field-emission scanning electron microscopy (FESEM) images were taken using a Hitachi S-4800 instrument. Transmission electron microscopy (TEM) images were recorded on an FEI Tecnai G2 F20 S-TWIN instrument. Fourier transform infrared (FTIR) spectra were measured on a Nicolet Avatar 360 spectrometer. 20 mg of spent catalyst and 200 mg of KBr were first mixed uniformly. 30 mg of mixture was then pressed into a self-supporting disk.
The amount and strength of basic sites were measured by CO2 temperature programmed desorption (CO2-TPD) using a Micromeritics AutoChem II analyzer. 0.2 g of catalyst (40–60 mesh) was preheated at 750 °C for 1 h under He (30 mL/min), followed by cooling down to 80 °C. CO2 adsorption was conducted at this temperature, then purged with He (30 mL/min) for 2 h. Finally, the temperature was raised from 80 to 950 °C at a ramping rate of 10 °C/min. O2 temperature programmed desorption (O2-TPD) was measured on the same instrument. 0.2 g of catalyst (40–60 mesh) was preheated at 750 °C for 1 h under He (30 mL/min), followed by cooling down to 50 °C. O2 adsorption was conducted at this temperature, then purged with He (30 mL/min) for 2 h. Finally, the temperature was then raised from 50 to 700 °C at a ramping rate of 10 °C/min. The desorbed CO2 and O2 were detected with a thermal conductivity detector (TCD).
Oxidative coupling of methane
The oxidative coupling of methane reaction was performed with a fixed-bed quartz tube reactor (internal diameter 6 mm) at atmospheric pressure. 0.2 g of catalyst (40–60 mesh) was loaded in the middle of reactor, with the downstream of the catalyst fixed with quartz wool. The catalytic performance was investigated using a gas mixture of methane and oxygen (CH4/O2 = 4/1 molar ratio). The total flow rate of 60 mL/min, corresponding to a gas hourly space velocity (GHSV) of 18,000 mL/(g h). Prior to the reaction, the catalyst was pretreated at 750 °C in Ar (30 mL/min) for 1 h. The reaction temperature (actually the catalyst bed temperature) was monitored by a thermocouple placed in the middle of the catalyst bed. The reaction products were analyzed by an on-line GC equipped with a TCD and a 2-m Shincarbon ST packed column (for separation of H2, O2, CO, CH4 and CO2), and by another on-line GC equipped with an FID and a 50-m PoraPLOT Q capillary column (for separation of CH4, C2H4, C2H6, C3H6 and C3H8). Before analyzing by TCD, the products were passed through a cold trap at -3 °C to remove most of water generated during the reaction. The CH4 conversion and C2-C3 selectivity were calculated using the standard normalization method based on carbon atom balance.
Results and discussion
Catalyst characterization
The XRD patterns of Y2O3 nanoparticles, nanorods and Sr-modified nanorods are shown in Fig. 1. All catalysts display similar characteristics of diffraction peaks belonging to the cubic Y2O3 phase (PDF #74–1828). The diffraction peaks at ca. 2θ = 21°, 29°, 34°, 36.1°, 40°, 44°, 49°, 54°, 58° and 59° are ascribed to the (211), (222), (400), (411), (332), (134), (440), (611), (622) and (136) planes of cubic Y2O3 phase. As the Sr/Y molar ratio is increased to 0.04, a small amount of SrCO3 phase appeared, which might be produced during the calcination of Sr(NO3)2-Y(OH)3 through the combination of SrO with CO2 in air [22, 29]. Table 1 shows that introducing a small amount of Sr into Y2O3 nanorods improves the lattice parameter from 1.0546 nm (Y2O3-NR) to 1.0569 nm (0.06Sr–Y2O3-NR). Taking into account the larger ionic radius of Sr2+ (0.118 nm) than Y3+ (0.090 nm), this result reveals that Sr is doped into the crystal lattice of Y2O3, albeit Sr was incorporated into Y2O3 nanorods via a simple impregnation method [22, 29].
From the SEM images of Y2O3-NR (Fig. 2a) and 0.04Sr–Y2O3-NR (Fig. 2b), one can see that both catalysts show the nanorod shape. The average length and width of Y2O3-NR nanorods are 1.11 μm and 191 nm, respectively. Obviously, the introduction of a small amount of Sr exerts a bit influence on the nanorod size (Table 1). The TEM image shown in Fig. 3 indicates that Y2O3-NP has irregular particle shape with a mean size of 17 nm. As demonstrated in Figs. S1 and S2, the HR-TEM images combined with a fast Fourier transform (FFT) analysis disclose that Y2O3-NR and 0.04Sr-Y2O3-NR nanorods predominantly expose (440) and (222) planes.
The BET specific surface areas of Y2O3 nanoparticles, nanorods and Sr-modified nanorods are between 20.0 and 25.4 m2/g (Table 1), which are low and typical for the OCM catalysts. Y2O3-NR presents a slightly higher surface area than Y2O3-NP (25.4 vs 20.0 m2/g). Modification of Y2O3-NR with a small amount of Sr brings about a slight decrease in surface area.
The XPS spectra of O 1 s on the Y2O3-based catalysts are shown in Fig. S3. The O 1 s spectrum of each catalyst can be deconvoluted into four peaks associated with three kinds of oxygen species: lattice oxygen (O2−, ∼ 529.4 eV), chemisorbed oxygen species (O−, ∼ 530.6 eV and O2−, ∼ 532.6 eV) and carbonate (CO32−, ∼ 531.8 eV) [16, 30,31,32,33]. The XPS data are presented in Table 1. It was reported that the chemisorbed oxygen species, i.e. surface electrophilic oxygen species O− and O2−, were responsible for the generation of C2 product in the OCM process, while the lattice oxygen favored deep oxidation of CH4 to form CO and CO2 [16, 17, 22, 32, 34]. Hence, the ratio of (O− + O2−)/O2− was found to correlate positively with C2 selectivity in the OCM reaction [17, 22, 25, 29, 34]. A comparison of Y2O3-NR with Y2O3-NP indicates that the former catalyst affords a higher ratio of (O− + O2−)/O2− than the latter one (1.3 vs 1.0). Introducing Sr into Y2O3-NR increases the (O− + O2−)/O2− ratio, and the 0.04Sr-Y2O3-NR catalyst exhibits the highest value (1.8). Apparently, the value of (O− + O2−)/O2− for all the Y2O3-based catalysts follows the sequence of 0.04Sr–Y2O3-NR > 0.06Sr–Y2O3-NR > 0.02Sr–Y2O3-NR > Y2O3-NR > Y2O3-NP.
A previous theoretical study has revealed that the energy required to produce oxygen vacancies over CeO2 is lower on the plane of (110) than (111) and (310) [35]. That is to say, oxygen vacancies are more readily to generate on the (110) plane of CeO2. Oxygen vacancies can interact with O2 to form the chemisorbed oxygen species such as O− and O2−. Hou et al. pointed out that, among the exposed facets for the La2O2CO3 catalysts, the (110), (1 \(\overline{2}\) 0) and (2 \(\overline{1}\) 0) facets had relatively loose atomic configurations, and these facets favored the formation of the chemisorbed oxygen species [33]. Thus, we consider that the higher (O− + O2−)/O2− ratio for Y2O3-NR than Y2O3-NP could be caused by the fact that the former catalyst predominantly exposes (440) and (222) planes, as revealed by the HR-TEM result.
The activation of oxygen will play an important role in the OCM reaction. To further study the oxygen activation on the Y2O3-based catalysts, O2-TPD experiments were performed. The results are given in Fig. 4 and Table 2. The Y2O3-NP catalyst gives two desorption peaks of oxygen, which are located at 93 °C and 526 °C, respectively. The low-temperature and high-temperature peaks are assigned to molecular and chemisorbed oxygen species, respectively [36]. The other Y2O3-based catalysts display only one peak of oxygen desorption located at 370–507 °C, which corresponds to the desorption of chemisorbed oxygen species [36]. These chemisorbed oxygen species originate from the interaction between O2 and the Y2O3-based catalysts, and may be O−, O2− and O2− [29, 33]. It is widely accepted that the chemisorbed oxygen species are helpful for CH4 activation and C2 selectivity in the OCM reaction [16, 33, 36, 37]. In comparison with Y2O3-NP, Y2O3-NR displays a higher amount of chemisorbed oxygen species (25.8 vs 21.0 μmol/g). Compared with Y2O3-NR, the Sr–Y2O3-NR catalysts possess a higher amount of chemisorbed oxygen species (36.3–43.7 vs 25.8 μmol/g) and lower peak temperature of the chemisorbed oxygen species desorption (370–403 °C vs 507 °C). The 0.04Sr–Y2O3-NR catalyst affords the highest amount of chemisorbed oxygen species (43.7 μmol/g). This finding suggests that the incorporation of a small amount of Sr into Y2O3 nanorods enhances the oxygen activation over the catalysts. Doping low-valence Sr into high-valence Y2O3 can improve the number of oxygen vacancies [37,38,39], thus enhancing the activation of oxygen. Consequently, a higher amount of chemisorbed oxygen species on the Sr–Y2O3-NR catalysts than Y2O3-NR can be observed.
Surface basic sites were also considered to play a key role in the OCM reaction [21, 40]. These basic sites could be O−, O2− and O2− oxygen species [21, 37, 41, 42]. The basic sites with medium strength are considered to be more favorable for forming C2 product in the OCM reaction [16, 17, 25, 33, 40, 43,44,45,46]. The CO2-TPD profiles of Y2O3 nanoparticles, nanorods and Sr-modified nanorods are depicted in Fig. 5. There are three peaks of CO2 desorption from the surfaces of Sr-modified Y2O3 nanorods, which are located at ∼ 150 °C, ∼ 340 °C and above 750 °C, corresponding to weak, moderate and strong basic sites of the catalysts [22, 29, 37, 47]. Both Y2O3-NR and Y2O3-NP catalysts have only weak and moderate basic sites, giving the peak temperature of CO2 desorption at ∼ 150 °C and ∼ 340 °C. The CO2-TPD data (Table 2) show that the amount of moderate basic sites is higher over Y2O3-NR than Y2O3-NP. The Sr–Y2O3-NR catalysts possess more basic sites with medium strength than Y2O3-NR, and 0.04Sr–Y2O3-NR has the greatest amount of moderate basic sites. The amount of moderate basic sites present on all the Y2O3-based catalysts decreases in the order of 0.04Sr–Y2O3-NR > 0.06Sr–Sr–Y2O3-NR > 0.02Sr–Y2O3-NR > Y2O3-NR > Y2O3-NP.
FTIR spectra can provide the information on structure of the catalysts. To gain insight into the impact of introducing excessive Sr on the Sr–Y2O3-NR catalysts for the OCM process, the used 0.06Sr–Y2O3-NR and 0.04Sr–Y2O3-NR catalysts after the OCM reaction at 600 °C for 1 h were recorded and compared in Fig. S4. The peaks located at 3445 and 1637 cm−1 are attributed to the stretching and bending vibrations of O–H groups in H2O [48]. The peaks centered at 1442 and 861 cm−1 are assigned to the asymmetric stretching and bending vibrations of CO32− groups [49, 50] which originate from combination of the Sr–Y2O3-NR catalysts with CO2 produced in the OCM process. Judging from the peak intensity, there are more surface carbonate species on the spent catalysts of 0.06Sr–Y2O3-NR than 0.04Sr–Y2O3-NR.
Catalytic performance
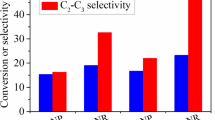
To explore the morphology effect of the Y2O3 catalysts, we first tested the catalytic performance of Y2O3 nanorods and nanoparticles. With an increase of the reaction temperature from 600 to 750 °C, the CH4 conversion increases slightly (Fig. 6A), while the selectivity toward C2–C3 (ethylene, ethane, propylene and propane) increases significantly (Fig. 6B). Accordingly, the C2–C3 yield rises with the reaction temperature (Fig. 6C). Whether CH4 conversion, C2–C3 selectivity or C2–C3 yield, Y2O3-NR performs better than Y2O3-NP. For example, Y2O3-NR affords a 21.9% CH4 conversion, 42.3% C2–C3 selectivity and 9.3% C2–C3 yield at 700 °C, whereas Y2O3-NP gives a 17.9% CH4 conversion, 22.9% C2–C3 selectivity and 4.1% C2–C3 yield. A higher C2–C3 selectivity achieved on Y2O3-NR than Y2O3-NP is caused by a higher (O− + O2−)/O2− ratio obtained on the former catalyst. The occurrence of more chemisorbed oxygen species and moderate basic sites on Y2O3-NR than Y2O3-NP is responsible for a higher CH4 conversion and C2–C3 yield achieved on the former catalyst. The La2O3, Sm2O3, Er2O3 and Ho2O3 rare earth oxide catalysts were also found to display shape effects on the OCM reaction [16,17,18, 24, 25].
Then we tested the catalytic performance of Sr–Y2O3-NR catalysts to get a better understanding of the influence of Sr modification. Table 3 shows the typical product distribution over the Y2O3-NR and Sr–Y2O3-NR catalysts at 650 °C. In addition to C2H4 and C2H6, small amounts of C3H6 and C3H8 were also produced. As to the by-products, the selectivity is higher for CO2 than CO. Compared with the Y2O3-NR catalyst, the addition of a small amount of Sr slightly improves the CH4 conversion (Fig. 7A), and obviously enhances the C2–C3 selectivity (Fig. 7B) and yield (Fig. 7C). With an increase of the Sr/Y molar ratio from 0 to 0.06, the CH4 conversion, C2–C3 selectivity and yield first increase and then decrease. The best catalytic performance is achieved on the 0.04Sr–Y2O3-NR catalyst, which affords a 23.0% CH4 conversion and 50.2% C2-C3 selectivity at 650 °C. Even at a low temperature of 600 °C, this catalyst still gives a 21.4% CH4 conversion and 41.8% C2–C3 selectivity. In contrast, the Y2O3-NR catalyst only affords a 21.5% CH4 conversion and 34.5% C2–C3 selectivity at 650 °C. In combination with the above XPS, O2-TPD and CO2-TPD results, the better OCM performance of the Sr–Y2O3-NR catalysts than Y2O3-NR can be attributed to an increased (O− + O2−)/O2− ratio and number of moderate basic sites, as well as enhanced activation of oxygen. The best 0.04Sr–Y2O3-NR catalyst display the highest ratio of (O− + O2−)/O2− as well as the most chemisorbed oxygen species and moderate basic sites. As revealed in Fig. 8, there exists a good correlation between the C2–C3 yield achieved on the Y2O3-based catalysts at 700 °C and the number of moderate basic sites present on the catalysts. This finding further demonstrates that the presence of moderate basic sites on the OCM catalysts is conducive to improving the C2 yield [16, 17, 25, 33, 40, 43,44,45,46].
Effect of Sr/Y molar ratio on the catalytic behavior of Sr‐modified Y2O3 nanorods at different temperatures: A CH4 conversion, B C2–C3 selectivity and C C2–C3 yield. (inverted triangle) 600 °C, (triangle) 650 °C, (filled circle) 700 °C, (filled square) 750 °C. Reaction conditions: 0.2 g catalyst, 60 mL/min flow (molar ratio CH4/O2 = 4/1)
A bit lower CH4 conversion and C2–C3 selectivity can be found on 0.06Sr–Y2O3-NR than 0.04Sr–Y2O3-NR at 750 °C and 700 °C, which could link with the blockage of some active sites upon the addition of excessive Sr. An interesting observation is that the former catalyst displays obviously worse OCM performance than the latter one at 650 °C and 600 °C, especially at a low temperature of 600 °C. This can be attributed to the blockage of more active sites by carbonate, since more surface carbonate species are formed on the spent 0.06Sr–Y2O3-NR catalyst (Fig. S4). Reportedly, there existed the optimal Li and Ba contents for Li–MgO and Ba–La2O3 catalysts used in the OCM process [51, 52].
We chose the best 0.04Sr–Y2O3-NR catalyst to investigate the lifetime for the OCM reaction performed at 650 °C. As seen in Fig. S5, the 0.04Sr–Y2O3-NR catalyst displays good stability during 60 h of reaction, maintaining around 23% CH4 conversion and 50% C2–C3 selectivity. As demonstrated in Fig. S6, the HR-TEM images combined with a fast Fourier transform (FFT) analysis indicate that the predominantly exposed surface facets observed for 0.04Sr–Y2O3-NR after the stability test are not altered. After the stability test, the SEM image of 0.04Sr–Y2O3-NR displays the nanorod shape with an average length of 1.10 μm and width of 190 nm (Fig. S7). The 0.04Sr–Y2O3-NR catalyst possesses a surface area of 19.9 m2/g and 43.4 μmol/g of chemisorbed oxygen species. The amount of weak, moderate and strong basic sites of spent 0.04Sr-Y2O3-NR is 3.3, 47.6 and 90.7 μmol/g. The characterization data obtained for 0.04Sr–Y2O3-NR after the stability test are equivalent to those of the fresh catalyst, indicating the maintenance of the catalyst structure during the reaction.
We compared catalytic performance of our catalyst 0.04Sr–Y2O3-NR and three reference catalysts, i.e. 0.04Ba–Y2O3-NR nanorods, 0.04Sr–La2O3-NF nanofibers [22] and 0.04Sr–Sm2O3-NB nanobelts [18], under our reaction conditions. As shown in Fig. S8. Our catalyst 0.04Sr–Y2O3-NR displays higher methane conversion than 0.04Sr–La2O3-NF, and lower conversion than 0.04Ba–Y2O3-NR and 0.04Sr–Sm2O3-NB. However, 0.04Sr–Y2O3-NR nanorods exhibit a bit greater C2–C3 yield than three reference catalysts at 600–750 °C. Recently, Sollier et al. has reported that Sr–La–Ce oxide fibers reached a C2 yield of 21.7% at 600 °C [53]. In our future work we will study the effect of Ce doping on the Sr–Y2O3-NR nanorods.
Conclusions
In this work, we have developed Y2O3 and Sr–Y2O3 nanorods as new catalysts for low-temperature OCM process. The HR-TEM images reveal that Y2O3 and Sr–Y2O3 nanorods preferentially expose (440) and (222) facets. The superior OCM performance of Y2O3 nanorods to their nanoparticles counterpart could be associated with the predominantly exposed (440) and (222) facets on the surface of Y2O3 nanorods. The XPS and CO2-TPD results indicate that the addition to a small amount of Sr to Y2O3 nanorods enhances the ratio of (O− + O2−)/O2− and amount of moderate basic sites. The O2-TPD result suggests that the Sr addition promotes the activation of oxygen on the Sr–Y2O3-NR catalysts. This enhancement and promotion lead to an improved catalytic performance of Y2O3 nanorods upon the introduction of Sr. The optimal 0.04Sr–Y2O3-NR nanorods with a Sr/Y molar ratio of 0.04 afford a 23.0% CH4 conversion and 50.2% C2–C3 selectivity at 650 °C. This catalyst displays good stability for 60 h of OCM reaction. We found that there existed a good correlation between the C2–C3 yield achieved on the Y2O3-based catalysts and the number of moderate basic sites present on the catalysts.
References
Lee J, Oyama S (1988) Oxidative coupling of methane to higher hydrocarbons. Catal Rev Sci Eng 30(2):249–280
Arndt S, Laugel G, Levchenko S, Horn R, Baerns M, Scheffler M, Schlögl R, Schomäcker R (2011) A critical assessment of Li/MgO-based catalysts for the oxidative coupling of methane. Catal Rev Sci Eng 53(4):424–514
Ge XM, Yang LC, Sheets JP, Yu ZT, Li YB (2014) Biological conversion of methane to liquid fuels: status and opportunities. Biotechnol Adv 32(8):1460–1475
Tang P, Zhu QJ, Wu ZX, Ma D (2014) Methane activation: the past and future. Energy Environ Sci 7(8):2580–2591
Taifan W, Baltrusaitis J (2016) CH4 conversion to value added products: potential, limitations and extensions of a single step heterogeneous catalysis. Appl Catal B 198:525–547
Zakaria Z, Kamarudin SK (2016) Direct conversion technologies of methane to methanol: an overview. Renew Sust Energ Rev 65:250–261
Galadima A, Muraza O (2016) Revisiting the oxidative coupling of methane to ethylene in the golden period of shale gas: a review. J Ind Eng Chem 37:1–13
Han B, Yang Y, Xu Y, Etim UJ, Qiao K, Xu B, Yan Z (2016) A review of the direct oxidation of methane to methanol. Chin J Catal 37(8):1206–1215
Schwach P, Pan XL, Bao XH (2017) Direct conversion of methane to value-added chemicals over heterogeneous catalysts: challenges and prospects. Chem Rev 117(13):8497–8520
Gambo Y, Jalil AA, Triwahyono S, Abdulrasheed AA (2018) Recent advances and future prospect in catalysts for oxidative coupling of methane to ethylene: a review. J Ind Eng Chem 59:218–229
Zhao GY, Drewery M, Mackie J, Oliver T, Kennedy EM, Stockenhuber M (2020) The catalyzed conversion of methane to value-added products. Energy Technol 8(8):1900665
Sun LL, Wang Y, Guan NJ, Li LD (2020) Methane activation and utilization: current status and future challenges. Energy Technol 8(8):1900826
Arinaga AM, Ziegelski MC, Marks TJ (2021) Alternative oxidants for the catalytic oxidative coupling of methane. Angew Chem Int Ed 60(19):10502–10514
Keller GE, Bhasin MM (1982) Synthesis of ethylene via oxidative coupling of methane I Determination of active catalysts. J Catal 73(1):9–19
Ren T, Patel M, Kornelis B (2006) Olefins from conventional and heavy feedstocks: energy use in steam cracking and alternative processes. Energy 31(4):425–451
Huang P, Zhao YH, Zhang J, Zhu Y, Sun YH (2013) Exploiting shape effects of La2O3 nanocatalysts for oxidative coupling of methane reaction. Nanoscale 5(22):10844–10848
Jiang T, Song JJ, Huo MF, Yang NT, Liu JW, Zhang J, Sun YH, Zhu Y (2016) La2O3 catalysts with diverse spatial dimensionality for oxidative coupling of methane to produce ethylene and ethane. RSC Adv 6(41):34872–34876
Fu B, Jiang T, Zhu Y (2018) Structural effect of one-dimensional samarium oxide catalysts on oxidative coupling of methane. J Nanosci Nanotechnol 18(5):3398–3404
Sun YN, Shen Y, Song JJ, Ba RB, Huang SS, Zhao YH, Zhang J, Sun YH, Zhu Y (2016) Facet-controlled CeO2 nanocrystals for oxidative coupling of methane. J Nanosci Nanotechnol 16(5):4692–4700
Takenaka S, Kaburagi T, Yamanaka I, Otsuka K (2001) Oxidative coupling of methane over Li+-added Y2O3 catalyst prepared from Y(OH)3. Catal Today 71(1–2):31–36
Papa F, Luminita P, Osiceanu P, Birjega R, Akane M, Balint I (2011) Acid-base properties of the active sites responsible for C2+ and CO2 formation over MO–Sm2O3 (M = Zn, Mg, Ca and Sr) mixed oxides in OCM reaction. J Mol Catal A 346(1–2):46–54
Song JJ, Sun YN, Ba RB, Huang SS, Zhao YH, Sun YH, Zhu Y (2015) Monodisperse Sr–La2O3 hybrid nanofibers for oxidative coupling of methane to synthesize C2 hydrocarbons. Nanoscale 7(6):2260–2264
Ferreira VJ, Tavares P, Figueiredo JL, Faria JL (2012) Effect of Mg, Ca, and Sr on CeO2 based catalysts for the oxidative coupling of methane: Investigation on the oxygen species responsible for catalytic performance. Ind Eng Chem Res 51(32):10535–10541
Fan YQ, Sun MX, Miao CX, Yue YH, Hua WM, Gao Z (2021) Morphology effects of nanoscale Er2O3 and Sr-Er2O3 catalysts for oxidative coupling of methane. Catal Lett 151(8):2197–2206
Fan YQ, Miao CX, Yue YH, Hua WM, Gao Z (2021) Nanosheet-like Ho2O3 and Sr-Ho2O3 catalysts for oxidative coupling of methane. Catalysts 11(3):388
Haneda M, Tanaka M, Doi Y, Bion N (2018) Oxidative coupling of methane over Ba-doped Y2O3 catalyst-Similarity with active site for direct decomposition of NO. Mol Catal 457:74−81
Long RQ, Wan HL (1997) Oxidative coupling of methane over SrF2/Y2O3 catalyst. Appl Catal A 159(1–2):45–58
Heneda M, Katsuragawa Y, Nakamura Y, Towata A (2018) Promoting effect of cerium oxide on the catalytic performance of yttrium oxide for oxidative coupling of methane. Front Chem 6:581
Zhao MQ, Ke SC, Wu HQ, Xia WS, Wan HL (2019) Flower-like Sr-La2O3 microspheres with hierarchically porous structures for oxidative coupling of methane. Ind Eng Chem Res 58(51):22847–22856
Kharas KCC, Lunsford JH (1989) Catalytic partial oxidation of methane over barium metaplumbate BaPbO3: possible involvement of peroxide ion. J Am Chem Soc 111(6):2336–2337
Peng XD, Richards DA, Stair PC (1990) Surface composition and reactivity of lithium-doped magnesium oxide catalysts for oxidative coupling of methane. J Catal 121(1):99–109
Ding WP, Chen Y, Fu XC (1994) Oxidative coupling of methane over Ce4+-doped Ba3WO6 catalysts: investigation on oxygen species responsible for catalytic performance. Catal Lett 23(1–2):69–78
Hou YH, Han WC, Xia WS, Wan HL (2015) Structure sensitivity of La2O2CO3 catalysts in the oxidative coupling of methane. ACS Catal 5(3):1663–1674
Bai Y, Xia WS, Weng WZ, Lian MS, Zhao MQ, Wan HL (2018) Influence of phosphate on La-based catalysts for oxidative coupling of methane. Chem J Chin Univ Chin 39(2):247–254
Sayle TXT, Parker SC, Sayle DC (2005) Oxidising CO to CO2 using ceria nanoparticles. Phys Chem Chem Phys 7(15):2936–2941
Spinicci R, Tofanari A (1990) Characterization of catalysts for methane-coupling by means of temperature programmed desorption. Catal Today 6(4):473–479
Xu J, Zhang Y, Xu X, Fang X, Xi R, Liu Y, Zheng R, Wang X (2019) Constructing La2B2O7 (B = Ti, Zr, Ce) compounds with three typical crystalline phases for the oxidative coupling of methane: The effect of phase structures, superoxide anions, and alkalinity on the reactivity. ACS Catal 9(5):4030–4045
McFarland EW, Metiu H (2013) Catalysis by doped oxides. Chem Rev 113(6):4391–4427
Liang Q, Wu X, Weng D, Xu H (2008) Oxygen activation on Cu/Mn−Ce mixed oxides and the role in diesel soot oxidation. Catal Today 139(1–2):113–118
Elkins TW, Roberts SJ, Hagelin-Weaver HE (2016) Effects of alkali and alkaline-earth metal dopants on magnesium oxide supported rare-earth oxide catalysts in the oxidative coupling methane. Appl Catal A 528:175–190
Driscoll DJ, Martir W, Wang JX, Lunsford JH (1985) Formation of gas-phase methyl radicals over MgO. J Am Chem Soc 107(1):58–63
Bernal S, Blanco G, El Amarti A, Cifredo G, Fitian L, Galtayries A, Martín J, Pintado JM (2006) Surface basicity of ceria-supported lanthana. Influence of the calcination temperature. Surf Interface Anal 38(4):229–233
Peng L, Xu J, Fang X, Liu W, Xu X, Liu L, Li Z, Peng H, Zheng R, Wang X (2018) SnO2 based catalysts with low-temperature performance for oxidative coupling of methane: Insight into the promotional effects of alkali-metal oxides. Eur J Inorg Chem 17:1787–1799
Xu J, Peng L, Fang X, Fu Z, Liu W, Xu X, Peng H, Zheng R, Wang X (2018) Developing reactive catalysts for low temperature oxidative coupling of methane: on the factors deciding the reaction performance of Ln2Ce2O7 with different rare earth A sites. Appl Catal A 552:117–128
Xu J, Zhang Y, Liu Y, Fang X, Xu X, Liu W, Zheng R, Wang X (2019) Optimizing the reaction performance of La2Ce2O7-based catalysts for oxidative coupling of methane (OCM) at lower temperature by lattice doping with Ca cations. Eur J Inorg Chem 2:183–194
Wang Z, Zou G, Luo X, Liu H, Gao R, Chou L, Wang X (2012) Oxidative coupling of methane over BaCl2-TiO2-SnO2 catalyst. J Nat Gas Chem 21(1):49–55
Cheng F, Yang J, Yan L, Zhao J, Zhao HH, Song HL, Chou LJ (2018) Impact of chloride ions on the oxidative coupling of methane over Li/SnO2 catalyst. React Kinet Mech Catal 125(2):675–688
Bernal S, Botana FJ, Garcia R, Rodiguez-Izquierdo JM (1987) Behaviour of rare earth sesquioxides exposed to atmospheric carbon dioxide and water. React Soliak 4(1–2):23–40
Djerdj I, Garnweitner G, Su DS, Niederberger M (2007) Morphology-controlled nonaqueous synthesis of anisotropic lanthanum hydroxide nanoparticles. J Solid State Chem 180(7):2154–2165
Farrukh MA, Imran F, Ali S, Khaleeq-ur-Rahman M, Naqvi II (2015) Micelle assisted synthesis of La2O3 nanoparticles and their applications in photodegradation of bromophenol blue. Russ J Appl Chem 88(9):1523–1527
Ito T, Wang JX, Lin CH, Lunsford JH (1985) Oxidative dimerization of methane over a lithium-promoted magnesium oxide catalyst. J Am Chem Soc 107(18):5062–5068
Yamashita H, Machida Y, Tomita A (1991) Oxidative coupling of methane with peroxide ions over barium-lanthanum-oxygen mixed oxide. Appl Catal A 79(2):203–214
Sollier BM, Bonne M, Khenoussi N, Michelin L, Miró EE, Gómez LE, Boix AV, Lebeau B (2020) Synthesis and characterization of electrospun nanofibers of Sr-La-Ce oxides as catalysts for the oxidative coupling of methane. Ind Eng Chem Res 59(25):11419–11430
Acknowledgements
Financial support of this work was provided by the National Key R&D Program of China (No. 2017YFB0602200), the National Natural Science Foundation of China (No. 91645201), the Science and Technology Commission of Shanghai Municipality (No. 19DZ2270100), and the Shanghai Research Institute of Petrochemical Technology SINOPEC (No. 33750000-19-ZC0607-0005).
Author information
Authors and Affiliations
Contributions
WH, CM: Conceptualization; WH, YY: Methodology; YF: Formal analysis and investigation; YF: Writing—original draft preparation; WH, ZG: Writing—review and editing; WH, CM:Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Data availability
The datasets of current study are available from the corresponding authors on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fan, Y., Miao, C., Yue, Y. et al. Oxidative coupling of methane over Y2O3 and Sr–Y2O3 nanorods. Reac Kinet Mech Cat 134, 711–725 (2021). https://doi.org/10.1007/s11144-021-02085-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02085-7