Abstract
A series of tungsten modified CeAlOx catalysts prepared by the homogeneous co-precipitation method were used for the selective catalytic reduction NOx with NH3. The activity evaluation results exhibited that the activity of CeAlOx was enhanced by the addition of tungsten, and the CeWAlOx catalyst also showed high resistance to SO2 and H2O poisoning at 300 °C. The BET analysis results showed that the total pore volume and the average pore diameter of the CeAlOx catalyst was improved by the addition of WO3.The NH3-TPD, in situ DRIFTS, H2-TPR and XPS characterization results displayed that the introduction of tungsten could enhance the amount of acid sites, the adsorbability to the NH3, the redox ability and the amount of oxygen vacancies, which should be the critical factors for the CeWAlOx catalyst to achieve high NH3-SCR performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The emission of NOx gases produced from stationary sources has received tremendous attention because they can result in acid rain, ozone depletion, photochemical smog etc [1]. Selective catalytic reduction of NOx with NH3 (NH3-SCR) is a commercially proven technology for NOx abatement produced from power plant flue gases. Commercially available catalysts are made up of the anatase TiO2 carrier supporting the active components, i.e. V2O5–WO3/TiO2 or V2O5–MoO3/TiO2. However, some defects still remain in this catalyst, such as the narrow operational temperature window (350–400 °C), the toxicity of vanadium pentoxide to the environment and human health and poor N2 selectivity in the high temperature et al. [2–4]. Therefore, many researchers are focusing on developing environmental-friendly NH3-SCR catalysts with promising performance in a broad temperature scope.
Cerium based oxide catalysts with the superiority of unique oxygen storage capacity and excellent redox properties are thought to be the substitute for the commercial catalyst. Shen et al. developed a novel CeO2/Al2O3 catalyst supported on the activated-ceramics, which exhibited high activity in the temperature range from 250 to 350 °C [5]. Guo et al. investigated the relationship between the prepared method and the NH3-SCR catalytic activity for the CeO2–Al2O3 catalyst [6]. However, the anti- poisoning to the SO2 and the narrow reaction temperature window for the CeO2–Al2O3 catalyst are thought to be the key factors restrict its further industrial application. Molybdenum modified CeAlOx catalyst was reported by Li et al. [7], which exhibited high NO conversion at a broad temperature range from 200 to 400 °C, yet the N2 selectivity needed to be further improved in the high temperature. In addition, WO3 has been used as a promoter to enhance the NOx conversion in the V2O5–WO3/TiO2 catalyst for many years, which could strengthen acid sites on the catalyst surface. In this study, a series of tungsten doped CeAlOx catalysts were synthesized by the homogeneous co-precipitation method and used for the NH3-SCR reaction process, the corresponding substantial changes were characterized by the Brunauer–Emmett–Teller (BET), X-ray diffraction (XRD), NH3-temperature-programmed desorption (NH3-TPD), hydrogen-temperature-programmed reduction (H2-TPR), X-ray photoelectron spectroscopy (XPS) and the in situ diffuse reflectance infrared Fourier transform spectroscopy (in situ DRIFTS).
Experimental
Catalyst preparations
Tungsten doped CeAlOx catalysts were prepared by the homogeneous co-precipitation method using (NH4)10W12O41, Ce(NO3)3·5H2O and Al(NO3)3·9H2O as precursors. Certain amounts of (NH4)10W12O41 with an equal weight of H2C2O4·2H2O were added into deionized water. After the dissolution of (NH4)10W12O41, the solution of Ce(NO3)3·5H2O and Al(NO3)3·9H2O was added with the required molar ratio. Then excess urea aqueous solution was added into the mixed solution, with the urea/(Ce + W+Al) molar ratio being 15:1, the mixed solution was then heated at 90 °C for 12 h in the water bath. The precipitated solids were collected by filtration and washed with the deionized water, followed by drying at 105 °C for 12 h and subsequently calcined at 500 °C for 5 h. Finally, the catalysts were crushed and sieved to 40-60 mesh for test purposes.
Catalyst characterization
The specific surface area, pore volume and the average pore size of the samples were obtained by N2 adsorption/desorption at 77 K by using a Mircomeritics ASAP 2010 instrument. Prior to the N2 adsorption, the catalysts were degassed at 90 °C for 1 h and 300 °C for 4 h. The specific surface areas of the catalysts were determined by using the BET method under the 0.05–0.3 partial pressure range. The total pore volume and the average pore diameters were measured from the N2 desorption branches of the isotherms using the Barret–Joyner–Halenda (BJH) method.
The crystallinity of the catalysts were measured by means of powder XRD patterns obtained on a Rigaku D/max 2200 with Cu Kα radiation, scanning between 20° and 80° at a step of 5°/min.
Hydrogen-temperature-programmed reduction (H2-TPR) and NH3-temperature programmed-desorption (NH3-TPD) profiles were measured by using a chemisorption analyzer (Micromeritics, Chemisorb 2729 TPx). For the H2-TPR, in each experiment, about 50 mg sample was loaded into the quartz reactor and pretreated in He (50 mL/min) at 300 °C for 1 h. The sample was then cooled to room temperature under flowing N2. The sample was reduced starting at room temperature and increasing up to 800 °C in a gas mixture of 10 % H2/He at 10 °C/min. The consumption of H2 was monitored continuously by using a thermal conductivity detector. For the NH3-TPD experiments, after pretreatment in a He stream at 200 °C for 1 h, the catalysts were saturated with NH3 at a flow rate of 40 mL/min for 1 h. Desorption was carried out by heating the catalyst in He from 100 to 600 °C with a heating rate of 10 °C/min.
The in situ DRIFTS experiments were performed on a Fourier transform infrared (FT-IR) spectrometer (Nicolet Nexus 670) equipped with an in situ diffuse reflection chamber and high sensitivity mercury–cadmium–telluride (MCT) detector cooled by liquid nitrogen. About 50 mg sample was placed in the in situ chamber. The mass flow controllers and a temperature controller were used to simulate the reaction conditions. Prior to each experiment, the catalyst was heated at 500 °C for 30 min in a flow of 10 % O2/N2 and then cooled to 200 °C. The background spectrum was recorded and subtracted from the sample spectrum. All the spectra were collected with an accumulating 100 scans at a resolution of 4 cm−1.
Catalytic activity tests
The catalytic activity tests for the selective catalytic reduction of NO with NH3 were carried out in a fixed bed reactor. The simulated gas for these tests contained 1000 ppm NO, 1000 ppm NH3, 5 % O2, 8 % H2O (when used), 100 ppm SO2 (when used) and balance N2. Water vapor was generated by passing N2 through a heated gas-wash bottle containing deionized water. The catalytic reactions were carried out at temperatures from 100 to 400 °C under atmospheric pressure, with a total flow rate of 500 mL/min. The NO and NO2 concentrations before and after reaction were determined by using a NO–NO2–NOx analyzer (Thermo, Model 42i-HL). The N2O concentration was analyzed by a gas chromatograph (Agilent 6820) with a Porapak Q column. The SCR catalytic activity and N2 selectivity of the catalysts were expressed by the following equation:
Results and discussion
Catalytic activity evaluation
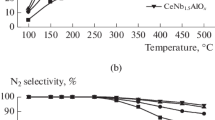
Fig. 1 shows the NO conversion and N2 selectivity over CeAlOx and W modified CeAlOx catalyst for the selective catalytic reduction of NO by NH3. Obviously, the CeAlOx catalyst showed relatively poor performance in the tested temperature range. However, the addition of W to the CeAlOx catalyst has remarkable influence on the NO conversion and N2 selectivity. When the W doped molar ratio reached 1.0, more than 95 % NO conversion was attained from 225 to 400 °C and almost no N2O could be detected in the tested condition. However, further increasing the W/Al molar ratio decreased the NO conversion slightly both at low and high temperatures, although the N2 selectivity showed no changes. The above results suggested that the CeWAlOx catalyst with W/Al molar ratio being 1:1 exhibited the best NH3-SCR performance.
In order to investigate the effects of SO2 and H2O on the NH3-SCR performance over the W doped CeAlOx catalyst, the CeWAlOx catalyst was selected to study the influences of 100 ppm SO2 or/and 8 % H2O on the performance at 300 °C and the corresponding results are illustrated in Fig. 2. It can be seen from the results that a slight decline in NO conversion occurred after the 100 ppm SO2 was added, after removing SO2 the NO conversion recovered immediately. When 8 % water vapor was introduced into the stream, the NO conversion was kept above 97 % during the tested period. When the 100 ppm SO2 and 8 % H2O were injected into the feed gases in the meanwhile, the NO conversion decreased much more severely compared with only adding SO2 or H2O. However, the conversion was still maintained at a relatively high level with more than 90 % NO conversion attained during the measured period. The above results suggested that the catalyst had better SO2/H2O durability.
Characterization of catalysts
XRD
The XRD patterns of the CeAlOx and W modified CeAlOx catalysts are shown in Fig. 3. Among all the catalysts, it can be found that there were some peaks appearing at 2θ values of 28.5°, 33.2°, 47.5° and 56.5°, which can be assigned to the cerianite CeO2, yet no diffraction peaks attributed to WO3 could be found, which indicated that WO3 existed mainly in the amorphous state or major in highly dispersed state on the Ce–W–AlOx catalysts. In the meanwhile, no alumina phase was detected in the all catalysts. As the W doped molar ratio increased from 0.2 to 2.0, almost no changes occurred in the intensities of the CeO2 peaks. Combining the XRD and NH3-SCR catalytic activity evaluation results, there must be some synergistic effect among Ce, W and Al oxides in the W modified CeAlOx catalyst.
BET
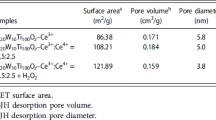
The BET results of the CeAlOx and W modified CeAlOx catalysts are summarized in Table 1. It can be seen that the introduction of W into the CeAlOx catalyst could lead the surface area lower at different degrees, while the total pore volume and the average pore diameter were enlarged at different extents. The surface area, pore volume and average pore diameter of CeAlOx were 158 m2/g, 0.18 cm3/g and 3.7 nm. When the W/Al molar ratio reached 1.0, the surface area of the CeWAlOx catalyst dropped to 108 m2/g, while the pore volume and average pore diameter increased to the maximum 0.28 cm3/g and 9.4 nm, respectively. However, the surface area, pore volume and average pore diameter decrease gradually with further doping tungsten on the CeAlOx catalyst. The above results indicated that the BET parameter was not the key factor to influent the NH3-SCR activity.
NH3-TPD
The amount of acid sites on the catalyst surface is crucial to the NH3-SCR reaction, which can be characterized by the NH3-TPD. The corresponding NH3-TPD curves of the CeAlOx and CeWAlOx catalysts are shown in Fig. 4. As can be seen from the figure, the desorption curve of the CeAlOx catalyst possessed three desorption peaks. The first peak at 184 °C can be assigned to the weakly bonded NH3, the peaks at 287 and 355 °C can be attributed to the strongly adsorbed NH3. The total acidity of the CeAlOx catalyst was about 554 μmol/g. For the CeWAlOx catalyst, the strongly adsorbed NH3 peak at 298 °C disappeared and the total acidity increased to around 763 μmol/g, which suggested that the surface acidity of the CeWAlOx catalyst was strengthened by doping with W. Combining the NH3-SCR activity results with the NH3-TPD curves indicates that the increase of the surface acidity was a key factor to enhance the catalytic activity.
H2-TPR
Apart from the surface acidity of the catalyst, the reducibility of the catalyst also plays an important role during the NH3-SCR reaction. The redox activity changes of the catalysts were investigated by H2-TPR and the corresponding profiles of the CeAlOx and CeWAlOx are displayed in Fig. 5. It can be seen from the figure that the CeAlOx profile showed one broad reduction peak from 300 to 600 °C due to the reduction of surface Ce4+ to Ce3+ [8]. For the CeWAlOx catalyst, the reduction peak shifted to around 526 °C, and the area of the reduction peak became larger, which indicates that the redox activity was enhanced. The above result suggested that the mobility of the surface oxygen was enhanced after introduction of W, which was beneficial to the NH3-SCR reaction.
XPS
It was reported that higher Ce3+/Ce4+ molar ratio on the catalyst surface is helpful to the NH3-SCR reaction [9]. The XPS results of the Ce on the CeAlOx and CeWAlOx surfaces are depicted in Fig. 6. The Ce 3d peaks were fitted by searching for the optimum combination of Gaussian bands. The sub-bands labeled u′ and v′ represent the 3d 104f 1 initial electronic state corresponding to Ce3+, and the sub-bands labeled u, u′′, u″′, v, v′′, and v′′′ represent the 3d 104f 0 state of Ce4+. The Ce3+ ratio, calculated by Ce3+/(Ce3++Ce4+) of CeWAlOx (38.9 %) was significantly higher than that of CeAlOx (27.8 %), indicating the presence of more surface oxygen vacancies on CeWAlOx. The above result was consistent with the Shan’s report [9].
In situ DRIFTS
In order to examine the changes of NH3 absorbed on the surface of the CeAlOx and CeWAlOx catalysts, the in situ DRIFTS of NH3 adsorption result over CeAlOx and CeWAlOx catalysts at 200 °C are presented in Fig. 7. As shown in the figure, the band at 1270 cm−1 is assigned to the symmetric and asymmetric deformation vibration of NH3 adsorbed on Lewis acid site [10]; the band at 1502 cm−1 can be attributed to ionic NH4 + species on Brønsted acid site [11]. In the meanwhile, the band at 1148 cm−1 is due to the scissoring vibration mode of NH2 species [12]; the bands at 3275 and 3380 cm−1 are attributed to N–H stretching vibration modes [13]; some negative bands at 3632 and 3792 cm−1 belong to the surface O–H stretching were also detected [14, 15]. After the addition of W, the band assigned to the scissoring vibration mode of NH2 species disappeared obviously. However, the band at 1284 cm−1 assigned to the Lewis acid site and the bands at 1178 and 1425 cm−1 corresponding to the Brønsted acid site were much stronger than that on the CeAlOx catalyst [16–18]. In the meantime, much stronger bands at 3178, 3266 and 3366 cm−1 attributed to N–H stretching vibration modes were also detected on the CeWAlOx catalyst [13]. The above analysis indicated that both the Lewis and Brønsted acid strength were increased by the introduction of W, according to some previous studies, the increased Lewis acid are mainly composed of unsaturated Wn+ cations [19], while the increased Brønsted acid are mainly composed of W-OH site arising from partially hydrated tungsten species, such as W=O, W–O–W and Ce–W–O [20–22]. The above results were also consistent with the NH3-TPD result.
Conclusions
A series of tungsten modified CeAlOx catalysts were prepared by the homogeneous co-precipitation method and used for the selective catalytic reduction of NO with NH3. It is concluded that the NH3-SCR catalytic activity of the CeAlOx could be markedly improved by the substitution with W. The CeWAlOx catalyst also showed high resistant to the SO2 and H2O poisoning in the tested condition. As can be seen from the characterization results of the BET, XRD, NH3-TPD, H2-TPR, XPS and in situ DRIFTS, the pore volume and the average pore diameter of CeAlOx can be increased by addition of W. The acidity, redox property and Ce3+/Ce4+ molar ratio were also enhanced by modification with W. The higher acidity, better redox ability, stronger adsorption capability to the NH3 and much more oxygen vacancies on the catalyst surface played important roles for the CeWAlOx catalyst to achieve high NH3-SCR performance.
References
Qi G, Yang RT (2003) Appl Catal B Environ 44(3):217–225
Zhang D, Zhang L, Shi L et al (2013) Nanoscale 5(3):1127–1136
Li X, Li Y (2014) React Kinet Mech Cat 112(1):27–36
Shan W, Liu F, He H et al (2011) Chem Commun 47(28):8046–8048
Shen Y, Zhu S, Qiu T et al (2009) Catal Commun 11(1):20–23
Guo R, Zhou Y, Pan W et al (2013) J Ind Eng Chem 19(6):2022–2025
Li X, Li Y (2014) J Mol Catal A: Chem 386:69–77
Murugan B, Ramaswamy AV (2008) J Phys Chem C 112(51):20429–20442
Shan W, Liu F, He H et al (2012) Appl Catal B Environ 115:100–106
Stoilova D, Cheshkova K (1998) Nickolov R. React Kinet Catal L 65(2):265–270
Oktar N, Mitome J, Holmgreen EM et al (2006) J Mol Catal A: Chem 259(1):171–182
Wang C, Yang S, Chang H et al (2013) J Mol Catal A: Chem 376:13–21
Busca G, Larrubia MA, Arrighi L et al (2005) Catal Today 107:139–148
Topsøe NY, Anstrom M, Dumesic JA (2001) Catal Lett 76(1–2):11–20
Huang SJ, Walters AB, Vannice MA (2000) Appl Catal B Environ 26(2):101–118
Sultana A, Nanba T, Haneda M et al (2010) Appl Catal B Environ 101(1):61–67
Liu F, He H (2010) Catal Today 153(3):70–76
Nicosia D, Czekaj I, Kröcher O (2008) Appl Catal B Environ 77(3):228–236
Busca G, Lietti L, Ramis G et al (1998) Appl Catal B Environ 18(1):1–36
Alemany LJ, Lietti L, Ferlazzo N et al (1995) J Catal 155(1):117–130
Mamede AS, Payen E, Grange P et al (2004) J Catal 223(1):1–12
Kobayashi M, Miyoshi K (2007) Appl Catal B Environ 72(3):253–261
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, P., Li, K. & Lei, Q. Enhanced activity of tungsten doped CeAlOX catalysts for the selective catalytic reduction of NOx with NH3 . Reac Kinet Mech Cat 116, 523–533 (2015). https://doi.org/10.1007/s11144-015-0925-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-015-0925-1