Abstract
In this work, new applications of one solid acid, nano silica boron sulfuric acid (NSBSA) as a dual Brønsted/Lewis acid in Baeyer–Villiger (B–V) oxidation of ketones with hydrogen peroxide has been introduced; which, because their very small size and large surface area, can improve the capability of this heterogeneous catalyst and promote the B–V oxidation process. The synthesized nano matter was characterized by X-ray diffraction, scanning electron microscopy, transmission electron microscopy and FT-IR. It was found that the H2O2/NSBSA system showed good efficiency in the B–V oxidation of alicyclic, aliphatic and aromatic ketones to either esters or lactones. The effects of reaction conditions, such as amount of catalyst, reaction temperature, reaction time and different solvents on the catalytic performance of NSBSA were also investigated. Notably, the process has advantages such as mild reaction conditions, simple operation and high product yield. To the best of our knowledge, the use of the H2O2/NSBSA system in B–V reactions has never been reported. It should be pointed out that H2O2, as an environmentally benign oxidant, produces water as the safest by-product and NSBSA can also be recycled and reused for several times without extremely loss of its catalytic activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Baeyer–Villiger (B–V) oxidation reaction as a long-known versatile chemical transformation of ketones to either an ester or a lactone, has attracted great interest as a tool for the preparation of pharmaceuticals such as antibiotics and also compounds of fine chemicals, agrochemical and intermediate industries [1–4]. Many traditional oxidation processes are associated with the use of the organic peracids and also stoichiometric high-oxidation state transition metals [4, 5]. Although more than a century has gone by since the discovery of B–V oxidation, it still suffers from several disadvantages. The use of an organic peracid, which is a well-known protocol, results in the formation of one equivalent of the corresponding carboxylic acid salt as the waste of the reaction, which has to be disposed or hardly recovered. Moreover, organic peracids are dangerously reactive, shock sensitive and also expensive materials. Consequently, a lot of efforts have been focused on finding out of less dangerous and greener alternatives such as oxygen/aldehydes, hydrogen peroxide/carboxylic acids, hydrogen peroxide/acids or bases and also bio-oxidants, etc. [6–14]. Hydrogen peroxide with a high content of oxygen (47 wt%) is a commercially available, inexpensive and environmentally benign reagent, which simplifies greatly the workup and, contrary to peracids, avoids the separation of the carboxylic acid salt as the by-product. It seems that hydrogen peroxide could be an ideal oxidant for developing a cleaner and more sustainable chemistry [4, 15, 16]. Nevertheless, hydrogen peroxide is one of the weakest oxidants of a wide range of the available peroxides and peracids used in B–V reaction [4, 15]. So, two more practical strategies applied to overcome its drawbacks: (i) applying an acidic catalyst to improve the electrophilicity of the carbonyl group of ketone to compensate the weakness of the nucleophilicity of hydrogen peroxide, (ii) using a base to deprotonate or polarized the O–H bonds of hydrogen peroxide to improve its nucleophilicity toward the carbonyl group of ketone. In recent years, heterogeneous catalysts, chiefly solid acids and especially those based on micelle-templated silica and other mesoporous high surface area support materials, play a more serious roles in acid-catalyzed chemical transformations because of their good catalytic activities, high efficiency and selectivity, reusability, simple separation from the reaction mixture and also less environmentally impacts, as important principles of green chemistry [16–22]. It is shown that nano silica boron sulfuric acid (NSBSA), which is considered as a dual Brønsted/Lewis acid and also an eco-friendly nano-heterogeneous catalyst, has an effective role in performing acid–base catalyzed organic transformations [22]. We report here the first catalytic B–V oxidation of alicyclic, aliphatic and aromatic ketones with a recyclable transition metal-free catalytic system; H2O2/NSBSA. Furthermore, we also studied the regioselectivity aspects of reaction in different ketones as well as some competitive chemoselective reactions between epoxidation of double bond and B–V oxidation of carbonyl group in (+)-carvone to elucidate the more active sites of molecule under the reaction condition.
Results and discussion
In this work, experiments have been done to investigate the catalytic performances of a mixed dual Brønsted/Lewis acid. Herein, we wish to report our results in preparation of aliphatic, aromatic and especially cyclic esters (lactones) in good to high yields, by successful application of hydrogen peroxide (35 %) and nano silica boron sulfuric acid (NSBSA), for the first time in B–V oxidation of ketones (Scheme 1).
The instrumental structure characterization of NSBSA
The structure of the prepared NSBSA was characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), patterned X-ray powder diffraction (XRD) and Fourier transform infrared spectroscopy (FT-IR), respectively. Based on the SEM image, it is clearly shown that the silica nanoparticles are regular in shape and arranged in approximately good orderly manner. In this work, the average diameter of the synthesized NSBSA was obtained ≈46 nm, and the SEM and TEM images also confirmed the nearly spherical morphology of catalyst (Figs. 1, 2).
To further explore the chemical modification of the silica support with boron sulfuric acid particles, the XRD technique was also used. A comparison between the XRD pattern of both nano silica and NSBSA shows that two significant peaks are positioned at 2θ = 25.09° and 27.91°, which is related to the presence of Si–O and B–O bonds in the crystalline structure of NSBSA, respectively (Fig. 3) [22].
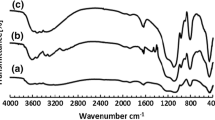
In agreement with the FT-IR spectra, the bands centered at ~588 and ~1635 cm−1 are related to the vibrational frequency of B–O–B and B–O bonds, respectively. The bands at ~459 and 1088 cm−1 were attributed to the Si–O–Si vibrations in NSBSA catalyst. The stretching vibration of B–OH band is also seen at ~1414 cm−1. The symmetric stretching vibration of O=S=O is seen at ~1168 cm−1. The very broad and intense O–H stretching absorption band appears in the region of 3366 cm−1 [22, 23]. As clearly seen, the FT-IR spectrum gives a good indication of the successful preparation of NSBSA (Fig. 4).
Effect of the amount of NSBSA
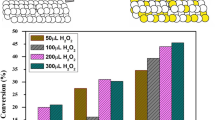
ɛ-Caprolactone is an important chemical compound, which is widely used in the synthesis of polyester [1, 5, 16, 24, 25]. Therefore, the B–V oxidation of cyclohexanone catalyzed by H2O2/NSBSA was chosen as a sample reaction to evaluate the effects of amount of catalyst, solvent and temperature. The B–V oxidation of cyclohexanone was conducted using different amounts of NSBSA at reflux condition (Table 1). It can be seen that the best results are obtained for the 0.01 g catalyst per 1.0 mmol of cyclohexanone (Entry 2). One can see that the cyclohexanone conversion increased with increasing of NSBSA until 0.01 g. So, it is clearly seen that NSBSA plays an important role in oxidation of the examined ketone. When the amount of catalyst exceeds 0.01 g, there is little increase in the conversion of cyclcohexanone. Therefore, it is indicated that the optimum amount of catalyst is about 0.01 g under the adopted reaction conditions. Notably, to obtain the same results, up to 0.05 g of silica boron sulfuric acid (SBSA), i.e. fivefold of NSBSA was required. This means more efficiency of NSBSA, which may be attributed to its more catalytic surface area and is obvious that enough active sites could be provided by the amount of 0.01 g catalyst.
Effect of solvent
During our optimization studies, different types of solvents, e.g. nonpolar, polar protic and polar aprotic were examined (Table 2). As one can see, the rate of conversion does not depend on the polarity of solvents; because the sample reaction in water, ethanol and DMF as high polar solvents (Entries 7–9), and also in acetic acid and ethyl acetate (Entries 4–5) as less polar solvents did not proceed and in most cases, no sight of any product detected. When non-coordinating solvents such as n-hexane, CH2Cl2 and CHCl3 (Entries 1–3) were used, where the nano-catalyst showed more reactivity, the conversion of cyclohexanone was fulfilled perfectly. So, the drastic reduction of the catalytic activity of NSBSA, especially in coordinating solvents, guided us to conclude that the catalytic activity of NSBSA depends on both sulfuric (OSO3H) and boron sites. Moreover, bonding of each boron atom with at least two electron withdrawing groups (OSO3H), may increase its Lewis acidity character more efficiently [22, 26]. Therefore, among a variety of examined solvents, based on the results of Table 2, chloroform was selected as the ideal solvent in these series because the B–V oxidation of ketones proceeded more smoothly. Consequently, these findings are in good agreement with the well-known B–V oxidation mechanism, which involves two steps; the addition step seems to be ionic and the migration step, which is rate-determining, uncatalyzed, non-ionic and fully concerted [27]. It should be also mentioned that, stirring and heating the reaction mixture for 9 h at 80 °C under solvent free conditions, was not complete at all (Entry 10). Notably, the reaction did not proceed remarkably at room temperature. So, refluxing the reaction mixture in chloroform at 80 °C was our best choice. Also, it should be noticed that hydrogen peroxide decomposes to oxygen and water at elevated temperature, and its decomposition rate increases with temperature at about 2.3 times per 10 °C rise. So, there is serious limitation in the length of heating the reaction because of the decomposition of hydrogen peroxide and also probable hydrolysis of ester to corresponding carboxylic acid at acidic conditions. Therefore, the need for using an excess of hydrogen peroxide is not surprising [1].
Analysis of catalytic functions of each component of NSBSA
To evaluate this multi-component nano catalyst in more detail, each part of the catalyst was carefully considered in the sample B–V oxidation of cyclohexanone (Table 3). As shown, each part of catalyst was entirely effective in the desired transformation, except single nano silica or B(OH)3, (Entries 5–6). Nano silica sulfuric acid (NSSA), silica boron sulfuric acid (SBSA) and silica sulfuric acid (SSA) promoted the reaction efficiently (Entries 2–4), but NSBSA shows higher reactivity, which enhances the reaction in shorter times and higher yields, comparably (Entry 1). So, we can conclude that the dual Brønsted/Lewis acid proeprties of the catalyst, in addition to its higher acidic surface due to presence of nano silica, are favorable. Additionally, a blank test in the absence of any catalyst showed totally no yield of ɛ-caprolactone (Entry 7).
Recyclability of NSBSA
The possibility of recycling the NSBSA was also examined using the reaction of cyclohexanone with hydrogen peroxide under optimized conditions. After the completion of the reaction and during the isolating steps of product, NSBSA was separated from the reaction mixture by simple filtration. To remove any solvent and for assurance, the recycled catalyst, before being subjected to another cycle of cyclohexanone oxidation, was put in an oven (at 100 °C for 2 h) and finally saved for the next operation. As the recycled catalyst was reused four times, a gradual decline in its catalytic activity and efficiency was observed. So, it can be considered as a weakly reusable catalyst (Table 4).
The generality and the scope of this protocol were considered for different kinds of commercially available aliphatic, aromatic and alicyclic ketones. Various esters were synthesized using NSBSA under the optimized reaction conditions (Table 5). Approximately, all the B–V reaction proceeded perfectly and the desired esters were obtained in good to high yields in an easy reaction procedure. Besides, the selectivity of the products exceeds 99 %, except for 4-methyl-benzaldehyde. In a coarse evaluation, we can conclude that cyclic ketones were reacted more rapidly in good to high yields, aromatic ketones were the slowest, and open-chain aliphatic ketones were in the middle of the list. The least reactivity of aromatic ketones could be attributed to electronic effects, due to their considerable resonance of the carbonyl group with phenyl rings, which decreases the electrophilicity of carbonyl group extremely and lowers the rate of reaction and also yields of products.
As clearly seen in Table 5, in contrast to aromatics, cyclic ketones are converted to their corresponding cyclic esters (lactones) in good to high yields in almost shorter reaction times (Entries 1–8). Although in the cases of the substituted-cyclohexanone, the substituents are far away from the reaction site, we can see that all of them are converted to their lactones in longer reaction time and lower yields in comparison to the parent cyclohexanone, which may be attributed to their more steric hindrance that may severe attaining the transition state of adduct (Entries 2–8).
So, it is may be an acceptable assessment that the steric approach controls the rate and possibility of establishing the cyclic Criegee transition state. However, Cavani and co-workers have reported that during the thermal, uncatalyzed oxidation of cyclohexanone with hydrogen peroxide, 6-hydroxyhexanoic acid and adipic acid have been observed, but based on chemical tests and instrumental analysis, no evidence of producing such by-products was detected in our oxidation system [1].
We can also notice that aromatic ketones have reacted slowly, but in phenyl rings, those containing EDGs (Entries 14, 15, 18), the yields of products are little enhanced, because of the electron donating character of the substituents to lessen the Criegee transition state energy level in comparison with rings without any substituents or vice versa rings containing EWGs, which is in agreement with previous observations and literature reports [4, 28].
Moreover, it was recognized that in competition between different groups for migration, in all cases, based on 1H and 13C NMR spectra analysis, rings involving more releasing groups entirely migrated and the other possibility had not distinguished at all (Entries 2, 7, 14–20, Scheme 2). A study of the regioselectivity of 4-methylbenzaldehyde (Entry 20) found out the position of the hydrogen in the migratory aptitude. Based on IR and NMR spectra, we isolated two different products; the minor one results from the migration of phenyl group, produced 4-methylphenyl methanoate (20a), and a major product from the migration of hydrogen atom; produced 4-methylbenzoic acid (20b see Scheme 2).
Notably, (+)-carvone as an important optically active compound, found in caraway seed, was also examined to investigate the chemoselectivity aspects of process under the reaction conditions, (Entry 8). Lei and et al got exclusively the product of the B–V oxidation of carvone with excellent chemoselectivity by H2O2/AlCl3 system [29]. Nevertheless, we obtained two different products based on TLC, GC, IR and NMR investigations. We think that due to the presence of two double bonds and also the availability of excess of hydrogen peroxide, one of the characterize products arose from the epoxidation of both double bonds in addition to B–V oxidation of the carbonyl group (8a) and in the other one, only the carbonyl group was oxidized (8b), which was the prevalent product (Scheme 3). Consequently, based on our results, the B–V oxidation of ketones shows higher regioselectivity than chemoselectivity in this reaction system.
We also proposed the following mechanism for the B–V oxidation of ketones by hydrogen peroxide in the presence of NSBSA on the basis of studying the related mechanisms reported in the literature and based on our observed results and knowledge (Scheme 4). Mechanistically, it seems plausible to suppose that the oxygen atom of the carbonyl group of ketone is hard basic, which can coordinate to the empty orbital of boron atoms or gets a proton of sulfuric unit that remarkably enhances its electrophilicity to compensate the lack of the reactivity of hydrogen peroxide. Now, hydrogen peroxide might attack the active carbonyl group, and through a Criegee intermediate, the insertion of oxygen atom from H2O2 and subsequently migration of a more electron donating alkyl or aryl group will happened. The rearrangement will end by releasing an ester, a water molecule and deliverance of catalyst for the next catalysis processes. Of course, tethering boron and sulfuric as Lewis and Brønsted acid units, respectively, with nano silica as a mesoporous high surface area, may play an important role in entrapping the reactants and active sites of catalyst in proximity of each other (Scheme 4). Moreover, it should be pointed out that, based on our observations, the oxidation reaction did not occur in the absence of the catalyst because hydrogen peroxide is a too weak nucleophile to attack directly the carbonyl group of ketone without any aim of an acidic catalyst. Of course, it is not the unique feature of occurrences and the reaction mechanism may be more complicated because we used a three-component nano catalyst that may have many active catalytic sites and another species may also exist.
Experimental
The boron sulfuric acid (BSA) and nano silica were synthesized according to the methods in previous litarture [30, 31]. The IR spectra (neat) were recorded on a St-Jean Baptist Ave Bomem 450 instrument. NMR spectra were recorded on a Bruker Avance DPX (400 MHz) instrument in CDCl3 and D2O with TMS as an internal standard for protons and solvent signals as internal standard for carbon spectra. Chemical shift values are reported in δ (ppm) and coupling constants are given in Hz. The progress of all reactions was monitored by TLC on 2 cm × 5 cm pre-coated silica gel-60 F-254 plates of thickness of 0.25 mm (Merck). The chromatograms were visualized under UV 254-336 nm or by immersion in tanks of common chemical visualizer such as DNP, H2SO4 (conc.), I2, etc.
The synthesis of NSBSA
A 500-mL suction flask was equipped with a constant pressure dropping funnel. The gas outlet was connected to a vacuum system through an adsorbing solution of alkali trap. Boric acid [B(OH)3] (25 mmol, 1.55 g) in CHCl3 (5 mL) was added to the suction flask. Then, chlorosulfonic acid (ClSO3H) (75 mmol, 8.74 g, 5 mL) in CHCl3 (15 mL) was added dropwise to the above flask, over a period of 30 min at room temperature. After the completion of the addition, the mixture was shaken for 4 h, while the residual HCl was eliminated by suction. Then, BSA was washed several times with dried CH2Cl2. Finally, BSA (4.62 g) was thoroughly mixed with nano silica (1:3) 13.86 g (Scheme 5).
General procedure for the oxidation of ketones to esters
In a typical procedure, ketone (1 mmol) and NSBSA (0.01 g) were dissolved in CHCl3 (5 mL) and stirred at room temperature for 30 min. Then, aqueous H2O2 35 % (20 mmol, 1.6 mL) was added to the reaction mixture and refluxed at 80 °C till reaction completion. The progress of reaction was followed by TLC n-hexane/ethyl acetate (5:1). The product was isolated by extraction with CH2Cl2 (3 × 10 mL) and washed with brine (2 × 10 mL). The extraction solvent was removed by a rotary evaporator and the prepared ester was identified by comparison of its physical properties with known compounds.
Conclusions
Many traditional oxidation processes are associated with the use of stoichiometric high oxidation-state transition metals and production of large amounts of toxic wastes. Therefore, there is an increasing interest in catalytic systems able to perform reactions in a more environmentally friendly ways, using less toxic reagents and oxidants [5]. In summary, NSBSA as a novel and effective dual Brønsted/Lewis acid is introduced to prepare esters from ketones during the well-known B–V oxidation process for the first time. This new approach was produced by moderate to good isolated yields, and the target products were obtained in acceptable reaction times. The efficiency, large catalytic surface of nano catalyst, easy workup and moderate reusability were also other advantages of NSBSA catalyst for this process. Compared with the others, although dioxygen/benzaldehyde is an interesting approach and supplies good yields and times, but this protocol needs at least two or three equivalents of aldehyde per one equivalent of starting ketone. In addition, this combination also produces a peracid in situ, and once again we encounter to the aforementioned drawbacks of peracids [12–14]. Moreover, according to our literature survey, though many of the reported oxidative catalytic system of ketones, are powerful and efficient in oxidation of cyclic ketones, but most of them are unsuccessful or very weak in the oxidation of aromatic or acyclic ketones, whereas this work is more successful and shows a good versatility [6, 12, 29, 32–34]. Consequently, we can claim that by using H2O2 with a high content of oxygen as an environmentally benign and commercially available, inexpensive and easy to handle oxidant with a heterogeneous Brønsted/Lewis acid catalyst allows a cleaner and more sustainable oxidation process of ketones to either esters or lactones, the oxidizing agent produces water as an environmentally friendly by-product and the catalyst could be easily removed and reused. It is envisaged that the H2O2/NSBSA system is potentially applicable in a number of chemical industries.
References
Cavani F, Raabova K, Bigi F, Quarantelli C (2010) Chem Eur J 16:12962–12969
Cavarzan A, Scarso A, Sgarbossa P, Michelin RA, Strukul G (2010) Chem Cat Chem 2:1296–1302
Brink GJT, Arends IWCE, Sheldon RA (2004) Chem Rev 104:4105–4123
Zárraga M, Salas V, Miranda A, Arroyo P, Paz C (2008) Tetrahedron 19:796–799
Steffen RA, Teixeira S, Sepulveda J, Rinaldi R, Schuchardt U (2008) J Mol Catal A 287:41–44
Kawabata T, Fujisaki N, Shishido T, Nomura K, Sano T, Takehira K (2006) J Mol Catal A 253:279–289
Xu S, Wang Z, Zhang X, Zhang X, Ding K (2008) Angew Chem 120:2882–2885
Bernini R, Coratti A, Fabrizi G, Goggiamani A (2003) Tetrahedron Lett 44:8991–8994
Kim TW, Hwang JY, Kim YS, Joo SH, Chang SC, Lee JS, Takatsuto S, Kim SK (2005) Plant Cell 17:2397–2412
Llamas R, Jiménez-Sanchidrián C, Ruiz JR (2007) React Kinet Catal Lett 90:309–313
Yakura T, Kitano T, Ikeda M, Uenishi JI (2002) Tetrahedron Lett 43:6925
Li YF, Guo MQ, Yin SF, Chen L, Zhou YB, Qiu RH, Au CT (2013) Reac Kinet Mech Cat 109:525–535
Nabae Y, Rokubuichi H, Mikuni M, Kuang Y, Hayakawa T, Kakimoto MA (2013) ACS Catal 3:230–236
Murahashi S, Oda Y, Naota T (1992) Tetrahedron Lett 33:7557–7560
Piscopo CG, Loebbecke S, Maggi R, Startori G (2010) Adv Synth Catal 352:1625–1629
Chen C, Peng J, Li B, Wang L (2009) Catal Lett 131:618–623
Renz M, Meunier B (1999) Eur J Org Chem 4:737–750
Maleki B, Shirvan HK, Taimazi F, Akbarzadeh E (2012) Int J Org Chem 2:93–99
Dabiri M, Salehi P, Baghbanzadeh M, Zolfigol MA, Agheb M, Heydari S (2008) Catal Commun 9:785–788
Zolfigol MA, Mirjalili BF, Bamoniri A, Zarchi MAK, Zarei A, khazdooz L, Noei J (2004) J Bull Korean Chem Soc 25:1414–1416
Zolfigol MA, Madrakian E, Ghaemi E (2002) Molecules 7:734–742
Khalafi-Nezhad A, Foroughi H, Doroodmand M, Panahi F (2011) J Mater Chem 21:12842–12851
Silverstein RM, Webster FX, Kiemle DJ (2005) Spectrometric identification of organic compounds, 7th edn. Wiley, New York
Changjiu X, Min L, Bin Z, Xing S (2012) Chin Pet Process Petrochem Tech 14:7–17
Bučko M, Schenkmayerová A, Gemeiner P, Vikart A, Mihovilovič M, Lacík DI (2011) Enzyme Microb Technol 49:284–288
Khalafi-Nezhad A, Foroughi HO, Panahi F (2013) Heteroatom Chem 24:1–8
Mora-Diez N, Keller S, Alvarez-Idaboy JR (2009) Org Biomol Chem 7:3682–3690
Strukul G (1998) Angew Chem Int Ed 37:1198–1209
Lei Z, Ma G, Wei L, Yang Q, Su B (2008) Catal Lett 124:330–333
Kiasat AR, Fallah-Mehrjardi M (2008) J Braz Chem Soc 19:1595–1599
Jafarzadeh M, Rahman IA, Sipaut CS (2009) J Sol–Gel Sci Technol 50:328
Naota T (1992) Tetrahedron Lett 33:7557–7560
Li C, Wang J, Yang Z, Hu Z, Lei Z (2007) Catal Commun 8:1202–1208
Olah GA, Wang Q, Trivedi NJ, Prakash GKS (1991) Synthesis 9:739–740
Acknowledgments
Authors thank the Shahid Chamran University of Ahvaz for their financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Javaherian, M., Doraghi, F. Nano silica boron sulfuric acid as a dual Brønsted/Lewis acid and a heterogeneous catalyst in Baeyer–Villiger oxidation of ketones with hydrogen peroxide. Reac Kinet Mech Cat 116, 235–248 (2015). https://doi.org/10.1007/s11144-015-0884-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-015-0884-6