Abstract
Iron tetranitrophthalocyanine (FePc) was modified and immobilized on carbon fiber (FePc(NO2)3–CF) by covalent bond to obtain a supported heterogeneous catalyst for the removal of dibenzothiophene (DBT) in tridecane. In the supported catalytic system, the catalyst exhibited an excellent catalytic performance without any sacrificial agents, and the conversion of DBT could reach 92 % at 130 °C and 0.2 MPa of initial dioxygen pressure for 3 h. Compared to unsupported FePc, the introduction of carbon fiber dramatically improved the catalytic activity of FePc and facilitated the reuse of catalysts. The amount of FePc(NO2)3–CF, temperature and the initial pressure of molecular oxygen were also studied in detail to optimize the reaction conditions. The removal of DBT significantly increased with the increasing of concentration of DBT in model oil. Finally, a mechanism involving high-valent iron oxo species was proposed for the oxygenation. This study provides new insights into industrial desulfurization systems using carbon fiber as catalyst carrier.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deep desulfurization from fuel oils has attracted wide concerns due to increasingly prominent environmental pollution, thus many governments develop more stringent fuel standards. However, the conventional catalytic hydrodesulfurization (HDS) technology has difficulties in removing dibenzothiphene (DBT) and its derivatives efficiently from diesel [1]. Therefore, an urgent demand for developing feasible non-HDS methods exists to achieve green fuel. Many alternative desulfurization technologies have been attempted based on the principles of adsorption [2], extraction [3], biodesulfurization [4], alkylation [5], oxidation (including photocatalytic [6] and electrochemical oxidation [7] etc.) and their appropriate combinations. With the great advantages of mild reaction conditions, uncomplicated operation and low equipment investment, oxidative desulfurization (ODS) is considered one of the most promising process to dispel the refractory sulfur compounds.
Many catalysts have been developed in several ODS methodologies, such as polyoxometalates [8–10], ionic liquids [11–13], metal oxide [14–16], organic acid [17] and Ti-containing mesoporous molecular sieves [18, 19]. In ODS, hydrogen peroxide and molecular oxygen are most frequently used as oxidizing agents owing to their low-cost and environmental harmony. Despite great recent progress with catalytic oxidation desulfurization technologies, the development of an uncomplicated, low-cost and high-efficiency ODS technology under mild reaction conditions has yet to be achieved. The challenges are to achieve molecule-level dispersion and maximum interfacial interaction between the catalyst and the carrier at low loading.
Metallophthalocyanine (MPc) complexes with a macrocyclic structure resembling that of porphyrin complexes are widely used as catalysts for oxidation by nature in the active sites of oxygenase enzymes, which have been extensively employed in the degradation of organic contaminants, such as dyes [20, 21], pesticides [22], phenols [23, 24] and sulfides [25, 26]. Furthermore, they show better catalytic performance in heterogeneous reaction when supported on carrier materials as fibers [20, 21, 24, 26]. However, there are only a few works concerning metal porphyrin [27–29] and phthalocyanine [30, 31] complexes as effective catalysts for oxidation of thiophenic sulfur to sulfone in oxidative desulfurization. DBT could be removed by iron porphyrin with H2O2 or O2, and two key intermediates (hydroperoxideiron (III) species and porphyrin oxoiron (IV) cation radical) were considered to play a key role in the product selectivity [28], but a mechanism involving a two-step nucleophilic addition rather than a free radical reaction was proposed in the oxygenation of DBT [29]. Iron phthalocyanine (FePc) also showed good catalytic performance in ODS when supported on macroporous polyacrylic cationic exchange resin of D113 [31], while a heavy dosage of the catalyst was used.
The aim of the present work is to develop a promising oxidative desulfurization system in a model diesel. Iron phthalocyanine with amino (FePc(NO2)3NH2) was immobilized on carbon fiber (FePc(NO2)3–CF) according to the reference method that substituted aniline compounds were grafted onto single-walled carbon nanotubes by covalent bond [32], and FePc(NO2)3–CF was employed as the catalyst for the direct oxidation of DBT with O2 in hydrocarbon solvent. As a result, the introduction of the carbon fiber as carrier significantly improved the catalytic performance so that the extremely low level of FePc could convert DBT to DBT sulfone (DBTO2) efficiently without any sacrificial agents. The effects of different conditions such as temperature, initial O2 pressure and the concentration of catalyst were investigated, and the possible reaction mechanism was discussed.
Experimental
Materials
DBT (analytical grade reagent, AR) was purchased from J&K Scientific Ltd. Tridecane (AR) and hexadecane (chromatographic grade) were obtained from Aladdin Industrial Co. Carbon fiber was obtained from Ningbo Institute of Materials Technology & Engineering, Chinese Academy of Science. Dioxygen was provided by Zhejiang Hangzhou Dazhong Zhiyang Qiti Co., Ltd. Other reagents were of AR and used without further purification.
Preparation of FePc(NO2)3–CF
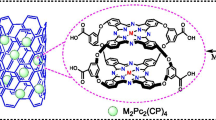
Iron tetranitrophthalocyanine (FePc) was synthesized from 4-nitrophthalic acid, iron dichloride tetrahydrate and urea following the procedures described in Ref. [33]. In a similar way that metal tetraamine phthalocyanines were done by reduction of the nitro derivatives using sodium sulfide reported previously in the literature [34], FePc(NO2)3NH2 was prepared by using one-fourth of stoichiometric amount of sodium sulfide for partial reduction of FePc(NO2)4 [31] in dimethyl formamide at 60 °C for 24 h. With minor modification of the method in Ref. [32], the chemical grafting process of FePc(NO2)3NH2 loaded by carbon fiber was carried out as follows (Fig. 1): FePc(NO2)3NH2 (0.06 g) dissolved in dimethyl sulfoxide and carbon fiber (2 g) were added to a flask with condenser and stirrer in oil bath, then heated to 85 °C. After 30 min, 1 ml isoamyl nitrite was added, retained stirring with N2 inlet for 24 h in 85 °C. The solution was cooled to room temperature and filtered, then the carbon fiber was washed by dimethylformamide and deionized water until the filtrate was fully clear, and dried at 80 °C overnight.
Characterization of catalyst
The infrared spectrum of catalyst, diluted with KBr and pressed into a pellet, was recorded on a Nicolet 5700 FTIR spectrometer. IR (ν/cm−1): (a) FePc(NO2)4, 733 and 761 cm−1 (Pc ring), 1,098 cm−1 (C–H, Pc), 1,143 cm−1 (Fe–N, Pc), 1,254 and 1,614 cm−1 (C=N, Pc), 1,335 and 1,546 cm−1 (N=O, NO2); (b) FePc(NO2)3NH2, 749 cm−1 (Pc ring), 1,094 cm−1 (C–H, Pc), 1,337 cm−1 (N=O, NO2), 1,610 cm−1 (C=N, Pc), 3,442 cm−1 (N–H, NH2). The content of FePc loaded on CF was given by atomic absorption spectrometer (Sollar M6) with recording the concentration of metal ion, and the final graft rate was calculated to be 0.23 wt%.
Typical procedure of oxidation desulfurization
The adsorption experiments were investigated at room temperature, the detection with GC–MS demonstrated no reduce for the concentration of DBT. Then the typical oxidative desulfurization experiments were carried out in a 100 mL closed reaction axe with magnetic stirrer, a pressure gage and a gas three-way valve, 0.25 g of catalyst and 25 mL model oil containing 500 μg/g DBT and 500 μg/g hexadecane (as internal standard for GC quantifying) were added. The autoclave was closed and the dioxygen flow was continued for 2 min, deflated and repeated the operation for three times to remove the air in autoclave completely, then heated to 130 °C and the initial oxygen pressure was set at 0.2 MPa. Stirring at 300 rpm was continued during 1–3 h at 130 °C. After the completion of the reaction, the autoclave was cooled to ambient temperature in water bath, depressurized slowly to atmospheric pressure, and then opened. The catalyst was filtered in vacuum, washed with anhydrous ethanol and then dried.
Analysis of sulfur compounds
The identification and quantification of sulfur compounds were analyzed by gas chromatography/mass spectrometry (MS: Agilent 5973i; GC: Agilent 6,890 N equipped with an OV1701 capillary column (30 m × 0.25 mm × 0.25 μm). Analysis conditions were as follows: injection port temperature, 280 °C; detector temperature, 250 °C; oven temperature program, 295 °C, hold for 3 min; split ratio, 1/100; carrier gas, ultra-purity nitrogen; column flow, 1.0 mL/min; reagent gases, air flow of 104 mL/min, hydrogen flow of 75 mL/min; the injection volume of sample was 1 μL.
Results and discussion
Catalytic performance of FePc supported by CF
Oxidation of DBT in tridecane using O2 in the presence of FePc(NO2)3–CF was investigated at 130 °C and 0.2 MPa of initial dioxygen pressure, the peak of DBT gradually stepped down and a new peak appeared in the high retention time (Fig. 2), the conversion of DBT in 3 h reached 92 %. The result indicates that the oxidation of DBT can be effectively performed using FePc(NO2)3–CF and dioxygen. Meanwhile, the conversion of DBT with 10 g/L different catalysts (FePc(NO2)3–CF (containing FePc, 0.23 wt%), FePc(NO2)4, FePc(NO2)3NH2) were given in Fig. 3. It is clear that the catalytic activity of FePc(NO2)3–CF is apparently higher than the unsupported catalyst of FePc(NO2)4 and FePc(NO2)3NH2, and the activity of catalyst decreases in the order of FePc(NO2)3–CF > FePc(NO2)4 > FePc(NO2)3NH2. It is known that the nitro substituent is an electron acceptor group, and the electron density of Pc ring is less than the Pc with amino group so that it is more convenient for the electron transfer. Furthermore, the dosage of FePc in heterogeneous supported system was almost 1/400 of that in unsupported system. Obviously, the introduction of carbon fiber significantly improved the catalytic activity of FePc, it seems that the method of preparation of the supported catalyst has influenced the catalyst structure in terms of distribution of the active sites on the surface and their accessibility and of the state of the complex [35]. Here, FePc on a molecular level is well dispersed on carbon fiber by covalent bond, and the large specific surface area of carbon fiber dramatically increases the number of catalytic active sites. In addition, we note that both carbon fiber and FePc have unique conjugated π electron structures, which facilitates the electron transfer in the catalytic reaction process. Therefore, FePc(NO2)3–CF presented well catalytic performance in the oxidative desulfurization of DBT.
Effect of catalyst concentration, reaction temperature, initial oxygen pressure
The oxidative desulfurization experiment without catalyst was performed before examining the efficiency of catalyst. As shown in Fig. 4, the conversion of DBT was 26 %. When 2.5 g/L of FePc(NO2)3–CF was added to the ODS system, it indicated an excellent catalytic performance that the conversion of DBT remarkably increased to 69.3 %, continued to increase the dosage of catalyst to 10 g/L and the removal of DBT reached 92 %. However, the conversion of DBT moved upward slightly with the increase of the catalyst, it might be due to the features that fiber was lightweight and unconsolidated. Because of the stirring in the reaction process, the fiber became fluffier and part of them was exposed to the surface of solution, as a matter of fact, not all of the catalyst could be fully utilized, while appropriate stirring could promote the flow of solution and accelerate the catalytic reaction. Therefore, the concentration of catalyst was set at 10 g/L and stirring speed was 300 rpm in the text, taking into account of efficiency and utilization rate of catalyst.
The removal of DBT from model diesel from 60 to 140 °C are shown in Fig. 5, a sharp increase of DBT conversion appeared when changing the temperature from 120 to 130 °C, and the conversion of DBT was 24 and 92 %, respectively. When the temperature was increased to 140 °C, the conversion of DBT could reached 100 %, whereas the stability of solvent was to be challenged that infinitesimal alkanes were oxidized, and the peak of oxidation product closed to internal standard substance. Besides, FePc might tend to be destabilized at a high temperature. Therefore, the appropriate reaction temperature employed for the subsequent studies was set at 130 °C.
Air as oxidant was considered before investigating the effect of initial oxygen pressure. As shown in Fig. 6, the data at pressure of zero indicated the conversion of DBT was 6 % in air. Once the inflow of pure oxygen started, the conversion of DBT rose to 59 % spectacularly and reached to 92 % with increasing the pressure to 0.2 MPa, then climbed slowly to 100 % at 0.5 MPa. These results indicate that the oxygen plays an important role in the reaction and efficient desulfurization can be achieved with the low concentration of oxygen. From the viewpoint of efficiency and safety, 0.2 MPa was adopted as an optimum pressure.
Even though the reaction was performed in alkane solution at 130 °C and 0.2 MPa oxygen pressure, there was no need to be anxious about the security issues. Only a few very small peaks of the GC–MS spectra displayed for the possible oxidation products of tridecane, and the amount of these products were too small to calculate the ratio of the concentration exactly. The reaction conditions were moderate relatively corresponding to the severe conditions of the exploration trial (160 °C, 7 MPa) to confirm the stability of decalin as the solvent by Zhou et al. [29], and tridecane was more stable apparently than decalin in model oil according to our comparison experiments. It seems to be acceptable to the multitudinous high temperature and pressure reactions in chemical industries.
Oxidative desulfurization in model oil with different DBT concentration
In actual diesel, the concentration of sulfur compounds has a wide range and it is higher than 500 μg/g in most cases. In this work, oxidative desulfurization was investigated in the range of concentration of DBT from 100 to 2,000 μg/g. The curve presented in Fig. 7 showed that the conversion of DBT declined with an increase of substrate concentration, while the removal value of DBT was on the rise, it implied more DBT molecules involved in reaction. The reason is easy to understand: the substrate molecule has more opportunity to contact with the catalyst in a higher concentration solution and it is better utilization efficiency for the catalyst.
Regeneration of catalyst
The principal motivation for the preparation of supported catalysts is the possibility of their easy separation from the reaction system and their reuse for successive reactions, provided that the catalysts retain their catalytic properties as well. So the stability and reusability of catalyst are an indispensable part to consider in catalytic reaction, carbon fiber as carrier of metallophthalocyanine is a superduper solution for this problem in our work. After some simple procedures like filtration, washing and drying, carbon fiber-supported FePc could be reused to remove DBT. FePc(NO2)3–CF was performed 4 runs in oxidative desulfurization as shown in Fig. 8, correspondingly, the conversion of DBT was 92, 88, 87 and 78 %. The slight decrease of the catalyst activity may be due to the weakened complexation of the center metal iron and Pc ring at the high reaction temperature. As a result, it becomes difficult to form intermediates and active species for FePc interaction with oxygen that its catalytic performance weakens gradually, nevertheless, FePc(NO2)3–CF still could maintain an impressive removal for DBT.
The possible mechanism
Some mechanisms based on different key intermediates have been proposed for the oxidation hydrocarbon compounds with molecular oxygen catalyzed by mononuclear FePc complexes, many of them tend to produce peroxide iron (PcFe–O–OR) that homolytic and heterolytic cleavages of the peroxide O–O bond are possible ways to form the intermediates:PcFeIV=O, PcFeV=O [36–38]. In this paper, FePc supported on CF by covalent bond was used as catalyst without any peroxide existing in the reaction system, it was hard to form the intermediates as the analogous way. Hence, the oxidation of DBT to DBTO2 with O2 catalyzed by FePc(NO2)3–CF was speculated on radical-free high valent iron-oxo mechanism (Fig. 9). With the activation at a high temperature, molecular oxygen is favored to interact with FePc to form the peroxideiron radical (PcFe–O–O•−), it is desired to capture oxygen from PcFe–O–O•− for the sulfur atom of DBT with two lone pair electrons. Since the bond energy of O–S is much higher than the O–O bond, the weakened O–O bond breaks up into PcFeV=O and DBT sulfoxide (DBTO). While DBTO cannot exist stably with high activity intermediate PcFeV=O, so sulfoxide is oxidized to sulfone rapidly, the oxygenation is a consecutive process, this might illuminate why the peak of DBTO could not be found in the GC–MS detection.
To confirm the radical-free high valent iron-oxo mechanism for the present oxygenation, a radical inhibitor (hydroquinone) was added to model oil before reaction, only infinitesimal DBTO2 was detected and there was not any DBTO in the oxidative products. These results provides direct evidence for the formation of high valent iron oxo free radical species. In the oxygenation of DBT, the species is speculated on high valent iron oxo anion free radical species, which accepts the electron from the sulfur atom of DBT and then turns into unstable intermediate (PcFeV=O), these are different from the high-valent iron oxo phthalocyanine cation radical species that can be stabilized and characterized at very low temperatures [39]. Combining the formation of species with the convinced oxidative process in many work that DBT was converted to DBTO and further DBTO2 [6, 16, 29], the speculated involvement of Fe(V)=O species shown in Fig. 9 seems to be supported.
Conclusion
In this paper, an efficient ODS process using carbon fiber-supported FePc as catalysts in the presence of molecular oxygen has been studied. It was found that the oxidative desulfurization of DBT could be achieved with the catalyst of FePc(NO2)3–CF in the simple ODS system. Compared with FePc(NO2)4, the use of carbon fiber as carrier vastly reduced the dosage of FePc, while the reactivity of the catalyst increased. FePc(NO2)3–CF is easily separated from the reaction system and can be recycled without obvious inactivation. A mechanism, involving a high-valent iron oxo phthalocyanine radical species, has been proposed. It may be useful to improve the graft rate of metallophthalocyanine on carbon fiber further and explore the reinforced mechanism of carbon fiber. In summary, this process offers a novel strategy for deep desulfurization.
Abbreviations
- FePc:
-
Iron tetranitrophthalocyanine
- FePc(NO2)3NH2 :
-
Iron amino trinitro phthalocyanine
- CF:
-
Carbon fiber
- FePc(NO2)3–CF:
-
Carbon fiber supported iron phthalocyanine
- HDS:
-
Hydrodesulfurization
- ODS:
-
Oxidative desulfurization
- DBT:
-
Dibenzothiophene
- DBTO:
-
Dibenzothiophene sulfoxide
- DBTO2 :
-
Dibenzothiophene sulfone
References
Babich IV, Moulijn JA (2003) Science and technology of novel processes for deep desulfurization of oil refinery streams: a review. Fuel 82:607–631
Yang RT, Hernández-Maldonado AJ, Yang FH (2003) Desulfurization of transportation fuels with zeolites under ambient conditions. Science 301:79–81
Wang XM, Wan H, Han MJ, Gao L, Guan GF (2012) Removal of thiophene and its derivatives from model gasoline using polymer-supported metal chlorides ionic liquid moieties. Ind Eng Chem Res 51:3418–3424
Guchhait S, Biswas D, Bhattacharya P, Chowdhury R (2005) Bio-desulfurization of model organo-sulfur compounds and hydrotreated diesel—experiments and modeling. Chem Eng J 112:145–151
Liu Y, Yang BL, Li SS (2012) Process simulation based on experimental investigations for 3-methylthiophene alkylation with isobutylene in a reactive distillation column. Ind Eng Chem Res 51:9803–9811
Zhang J, Zhao DS, Yang LY, Li YB (2010) Photocatalytic oxidation dibenzothiophene using TS-1. Chem Eng J 156:528–531
Lam V, Li GC, Song CJ, Chen JW, Fairbridge C, Hui R, Zhang JJ (2012) A review of electrochemical desulfurization technologies for fossil fuels. Fuel Process Technol 98:30–38
Lü HY, Gao JB, Jiang ZX, Yang YX, Song B, Li C (2007) Oxidative desulfurization of dibenzothiophene with molecular oxygen using emulsion catalysis. Chem Commun 2:150–152
Tang NF, Zhang YN, Lin F, Lü HY, Jiang ZC, Li C (2012) Oxidation of dibenzothiophene catalyzed by [C8H17N(CH3)3]3H3V10O28 using molecular oxygen as oxidant. Chem Commun 48:11647–11649
Zhang M, Zhu WS, Xun SH, Li HM, Gu Q, Zhao Z, Wang Q (2013) Deep oxidative desulfurization of dibenzothiophene with POM-based hybrid materials in ionic liquids. Chem Eng J 220:328–336
Zhu WS, Wu PW, Yang L, Chang YH, Chao YH, Li HM, Jiang YQ, Jiang W, Xun SH (2013) Pyridinium-based temperature-responsive magnetic ionic liquid for oxidative desulfurization of fuels. Chem Eng J 229:250–256
Zhang C, Pan XY, Wang F, Liu XQ (2012) Extraction-oxidation desulfurization by pyridinium-based task-specific ionic liquids. Fuel 102:580–584
Gao HS, Guo C, Xing JM, Zhao JM, Liu HZ (2010) Extraction and oxidative desulfurization of diesel fuel catalyzed by a Brønsted acidic ionic liquid at room temperature. Green Chem 12:1220–1224
Prasad VVDN, Jeong KE, Chae HJ, Kim CU, Jeong SY (2008) Oxidative desulfurization of 4,6-dimethyl dibenzothiophene and light cycle oil over supported molybdenum oxide catalysts. Catal Commun 9:1966–1969
Sundararaman R, Ma XL, Song CS (2010) Oxidative desulfurization of jet and diesel fuels using hydroperoxide generated in situ by catalytic air oxidation. Ind Eng Chem Res 49:5561–5568
Bakar WAWA, Ali R, Kadir AAA, Mokhtar WNAW (2012) Effect of transition metal oxides catalysts on oxidative desulfurization of model diesel. Fuel Process Technol 101:78–84
Otsuki SO, Nonaka T, Takashima N, Qian WH, Ishihara A, Imai T, Kabe T (2000) Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction. Energy Fuels 14:1232–1239
Jin CZ, Li G, Wang XS, Zhao LX, Liu LP, Liu H (2007) Synthesis, characterization and catalytic performance of Ti-containing mesoporous molecular sieves assembled from titanosilicate precursors. Chem Mater 19:1664–1670
Wang Y, Li G, Wang XS, Jin CZ (2007) Oxidative desulphurization of 4,6-dimethyldibenzothiophene with hydrogen peroxide over Ti-HMS. Energy Fuels 21:1415–1419
Chen WX, Lu WY, Yao YY, Xu MH (2007) Highly efficient decomposition of organic dyes by aqueous-fiber phase transfer and in situ catalytic oxidation using fiber-supported cobalt phthalocyanine. Environ Sci Technol 41:6240–6245
Drozd D, Szczubiałka K, Łapok Ł, Skiba M, Patel H, Gorun SM, Nowakowska M (2012) Visible light induced photosensitized degradation of acid orange 7 in the suspension of bentonite intercalated with perfluoroalkyl perfluorophthalocyanine zinc complex. Appl Catal B 125:35–40
Silva M, Calvete MJF, Goncalves NPF, Burrows HD, Sarakha M, Fernandes A, Ribeiro MF, Azenha ME, Pereira MM (2012) Zinc(II) phthalocyanines immobilized in mesoporous silica Al-MCM-41 and their applications in photocatalytic degradation of pesticides. J Hazard Mater 233–234:79–88
Marais E, Klein R, Antunes E, Nyokong T (2007) Photocatalysis of 4-nitrophenol using zinc phthalocyanine complexes. J Mol Catal A 261:36–42
Lu WY, Chen WX, Li N, Xu MH, Yao YY (2009) Oxidative removal of 4-nitrophenol using activated carbon fiber and hydrogen peroxide to enhance reactivity of metallophthalocyanine. Appl Catal B 87:146–151
Zanjanchi MA, Ebrahimian A, Arvand M (2010) Sulphonated cobalt phthalocyanine-MCM-41: an active photocatalyst for degradation of 2, 4-dichlorophenol. J Hazard Mater 175:992–1000
Yao YY, Chen WX, Lu SS, Zhao BY (2007) Binuclear metallophthalocyanine supported on treated silk fibres as a novel air-purifying material. Dyes Pigment 73:217–223
Pires SMG, Simões MMQ, Santos ICMS, Rebelo SLH, Pereira MM, Neves MGPMS, Cavaleiro JAS (2012) Biomimetic oxidation of organosulfur compounds with hydrogen peroxide catalyzed by manganese porphyrins. Appl Catal A 439–440:51–56
Zhou XR, Chen X, Jin YQ, Markó EI (2012) Evidence of two key intermediates contributing to the selectivity of P450-biomimetic oxidation of sulfides to sulfoxides and sulfones. Chem Asian J 7:2253–2257
Zhou XR, Lv S, Wang H, Wang XN, Liu JH (2011) Catalytic oxygenation of dibenzothiophenes to sulfones based on FeIII porphyrin complex. Appl Catal A 396:101–106
Zhang YP, Wang D, Zhang RL, Zhao JS, Zheng Y (2012) ZSM-5-Ln(Pc)2 catalyzed oxygen oxidation of thiophene. Catal Commun 29:21–23
Zhou XR, Li J, WangN X, Jin K, Ma W (2009) Oxidative desulfurization of dibenzothiophene based on molecular oxygen and iron phthalocyanine. Fuel Process Technol 90:317–323
Price BK, Tour JM (2006) Functionalization of single-walled carbon nanotubes “On Water”. J Am Chem Soc 128:12899–12904
Achar BN, Fohlen GM, Parker JA, Keshavayya J (1987) Synthesis and structural studies of metal (II) 4,9,16, 23-phthalocyanine tetraamines. Polyhedron 6:1463–1467
Achar BN, Lokesh KS (2004) Studies on tetra-amine phthalocyanines. J Organ Chem 689:3357–3361
Sorokin AB (2013) Phthalocyanine metal complexes in catalysis. Chem Rev 113:8152–8191
Moore KT, Horváth IT, Therien MJ (2000) Mechanistic studies of (porphinato) iron-catalyzed isobutane oxidation. Inorg Chem 39:3125–3139
Sorokin AB, Mangematin S, Pergrale C (2002) Selective oxidation of aromatic compounds with dioxygen and peroxides catalyzed by phthalocyanine supported catalysts. J Mol Catal A 182–183:267–281
Sorokin AB, Kudrik EV (2011) Phthalocyanine metal complexes: versatile catalysts for selective oxidation and bleaching. Catal Today 159:37–46
Afanasiev P, Kudrik EV, Albrieux F, Briois V, Koifman OI, Sorokin AB (2012) Generation and characterization of high-valent iron oxo phthalocyanines. Chem Commun 48:6088–6090
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 51133006 and 51103133), Textile Vision Science & Education Fund, and Science Foundation of Zhejiang Sci-Tech University (ZSTU, No. 1001803-Y).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, S., Lu, W., Yao, Y. et al. Oxidative desulfurization of dibenzothiophene with molecular oxygen catalyzed by carbon fiber-supported iron phthalocyanine. Reac Kinet Mech Cat 111, 535–547 (2014). https://doi.org/10.1007/s11144-013-0661-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-013-0661-3